Abstract
Protection against oncogenic non-vaccine types (cross-protection) offered by human papillomavirus (HPV) vaccines may provide a significant medical benefit. Available clinical efficacy data suggest the two licensed vaccines [HPV-16/18 vaccine, GlaxoSmithKline Biologicals (GSK), and HPV-6/11/16/18 vaccine, Merck and Co., Inc.,] differ in terms of protection against oncogenic non-vaccine HPV types -31/45. The immune responses induced by the two vaccines against these two non-vaccine HPV types (cross-reactivity) was compared in an observer-blind study up to Month 24 (18 mo postvaccination), in women HPV DNA-negative and seronegative prior to vaccination for the HPV type analyzed [HPV-010 (NCT00423046)]. Geometric mean antibody titers (GMTs) measured by pseudovirion-based neutralization assay (PBNA) and enzyme-linked immunosorbent assay (ELISA ) were similar between vaccines for HPV-31/45. Seropositivity rates for HPV-31 were also similar between vaccines; however, there was a trend for higher seropositivity with the HPV-16/18 vaccine (13.0–16.7%) vs. the HPV-6/11/16/18 vaccine (0.0–5.0%) for HPV-45 with PBNA, but not ELISA . HPV-31/45 cross-reactive memory B-cell responses were comparable between vaccines. Circulating antigen-specific CD4+ T-cell frequencies were higher for the HPV-16/18 vaccine than the HPV-6/11/16/18 vaccine {HPV-31 [geometric mean ratio (GMR) = 2.0; p = 0.0002] and HPV-45 [GMR = 2.6; p = 0.0092]}, as were the proportion of T-cell responders (HPV-31, p = 0.0009; HPV-45, p = 0.0793). In conclusion, immune response to oncogenic non-vaccine HPV types -31/45 was generally similar for both vaccines with the exception of T-cell response which was higher with the HPV-16/18 vaccine. Considering the differences in cross-protective efficacy between the two vaccines, the results might provide insights into the underlying mechanism(s) of protection.
Key words: Cervarix®, cervical cancer, cross-protection, Gardasil®, HPV-31, HPV-45, human papillomavirus, immunogenicity
Introduction
Worldwide, nearly 530,000 new cases of cervical cancer are diagnosed annually, and 274,000 women die of the disease each year.1 Persistent infection with human papillomavirus (HPV) is the necessary cause for cervical cancer. Among the 15 oncogenic types identified, HPV-16 and HPV-18 are the most common and cumulatively account for approximately 70% of invasive cervical cancer cases.2,3 HPV-45 and -31 are the third and fourth most common oncogenic types globally, contributing to an additional 7% of cervical cancer cases.4 In addition to HPV-16 and -18, HPV-45 is associated with adenocarcinoma of the cervix, which is more difficult to detect.4
Two prophylactic HPV vaccines are currently licensed and available in more than 100 countries: Cervarix® (HPV-16/18 vaccine GlaxoSmithKline, Rixensart, Belgium) and Gardasil® (HPV-6/11/16/18 vaccine Merck, Whitehouse Station NJ USA). Both vaccines consist of virus-like particles (VLPs) of recombinant L1 capsid proteins from HPV-16 and -18. The HPV-6/11/16/18 vaccine also contains L1 VLPs from non-oncogenic types HPV-6 and -11, which are implicated in 75–90% of genital warts.5–7 The VLPs in the HPV-16/18 vaccine are formulated with the proprietary Adjuvant System 04 [AS04; 3-O-desacyl-4′-monophosphoryl lipid A (MPL) and aluminum hydroxide], which has been shown to induce higher cellular and humoral immune responses than VLPs formulated with aluminum salt alone.8,9 The VLPs in the HPV-6/11/16/18 vaccine are formulated with a proprietary amorphous aluminum hydroxyphosphate sulfate (AAHS) adjuvant.10,11 Both vaccines have demonstrated protection against infections and pre-cancerous lesions associated with oncogenic types HPV-16/18 in large, multicenter, randomized clinical trials.12–19
In an early study, the HPV-16/18 vaccine demonstrated broad protection against histopathological outcomes beyond that anticipated for HPV-16/18, and also against incident infection with HPV-31/45.15 This led to further investigation into the protection offered by the HPV-16/18 vaccine against non-vaccine oncogenic HPV types (cross-protection). The cross-protective efficacy of the HPV-16/18 vaccine was further demonstrated in the large Phase III clinical efficacy study (HPV-008; PATRICIA, NCT00122681) using both virological and histopathological endpoints.17 In the according-to-protocol cohort for efficacy (ATP-E; all evaluable women who received three vaccine doses, had normal or low-grade cytology at baseline, and were evaluable for efficacy), vaccine efficacy was 77.5% [96.1% confidence interval (CI): 68.3–84.4] against 6-mo persistent HPV-31 infection and 92.0% (66.0–99.2) against HPV-31 associated cervical intraepithelial neoplasia (CIN) 2+ lesions. Vaccine efficacy was 76.1% (59.1–86.7) against 6-mo persistent HPV-45 infection and 100% (−67.8–100) against HPV-45 associated CIN2+ lesions.17 Cross-protection against HPV-31/45 was also shown in the total vaccinated naive cohort (TVC-naive) and the total vaccinated cohort (TVC).17 Cross-protection was also observed against persistent infection and CIN2+ associated with HPV-33.17
Cross-protection has also been evaluated in efficacy studies with the HPV-6/11/16/18 vaccine. It should be noted that these cannot be directly compared with those for the HPV-16/18 vaccine due to differences in study design, immunological assays, endpoints and study populations. In a cohort of women who were HPV DNA negative for each of the 4 vaccine types and 10 non-vaccine HPV types, protection against HPV-31 was 46.2% (95% CI: 15.3–66.4) for persistent infection and 56.9% (28.6–74.8) for CIN1–3 or adenocarcinoma in situ. For the other types evaluated, including HPV-45, efficacy was not statistically significant.20
We previously presented results from study HPV-010 (NCT00423046), a comparative immunogenicity and safety study of the HPV-16/18 vaccine and the HPV-6/11/16/18 vaccine up to Month 24.21,22 At Month 7, the HPV-16/18 vaccine induced significantly higher anti-HPV-16/18 type-specific neutralizing antibody levels and significantly higher frequencies of HPV-16/18 type-specific circulating memory B cells than the HPV-6/11/16/18 vaccine. We now present data from a sub-analysis comparing the immune response induced by the HPV-16/18 vaccine and the HPV-6/11/16/18 vaccine against the oncogenic non-vaccine HPV types -31 and -45 (cross-reactivity). These immunological investigations were performed to provide insights into the potential underlying mechanism(s) of clinical cross-protection against HPV-31 and -45.
Results
Study population.
A total of 1,106 women (553 women in each vaccine group) stratified into three age groups (18–26 y, 27–35 y and 36–45 y) were vaccinated as described in Einstein et al. 2009.22 The ATP cohort for immunogenicity included all subjects who were HPV DNA-negative and seronegative prior to vaccination for the HPV type under analysis, received three vaccine doses and for whom immunogenicity endpoints were available (671 women at Month 24: 341 women in the HPV-16/18 vaccine group and 330 women in the HPV-6/11/16/18 vaccine group).
Serum antibody responses.
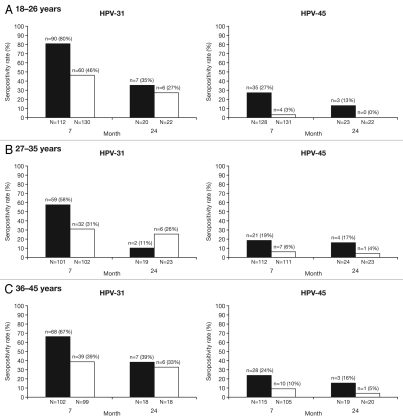
Seropositivity rates (Fig. 1) and geometric mean titers (GMTs) of neutralizing antibodies against HPV-31 and HPV-45 (Fig. 2) were measured by PBNA in the ATP cohort for immunogenicity, which was stratified into three age groups: 18–26 y, 27–35 y and 36–45 y. Across all age cohorts, one month after completion of the three-dose vaccination course (Month 7), 58.4–80.4% of women in the HPV-16/18 vaccine group were seropositive (defined as an antibody titer ≥40 ED50) for HPV-31 compared with 31.4–46.2% of women in the HPV-6/11/16/18 vaccine group; 18.8–27.3% of women in the HPV-16/18 vaccine group and 3.1–9.5% of women in the HPV-6/11/16/18 vaccine group were seropositive for HPV-45 (Fig. 1). At Month 24, 10.5–38.9% of women in the HPV-16/18 vaccine group compared with 26.1–33.3% of women in the HPV-6/11/16/18 vaccine group remained seropositive for HPV-31; in the HPV-16/18 vaccine and HPV-6/11/16/18 vaccine groups, 13.0–16.7% and 0.0.5.0% of women, respectively, remained seropositive for HPV-45 (Fig. 1). As anticipated and across all age groups, GMTs peaked at Month 7 for HPV-31 (HPV-16/18 vaccine: 102–206 ED50; HPV-6/11/16/18 vaccine, 41–50 ED50) and for HPV-45 (HPV-16/18 vaccine, 29–33 ED50; HPV-6/11/16/18 vaccine, 21–24 ED50). With both vaccines and across all age groups, HPV-31 PBNA GMTs decreased from Month 7 to Month 12 and plateaued through Month 24 to levels close to or below the assay cut-off (40 ED50); at Month 24, neutralizing antibody levels were 27–49 ED50 for the HPV-16/18 vaccine and 35–38 ED50 for the HPV-6/11/16/18 vaccine. HPV-45 neutralizing antibody GMTs decreased from Month 7 to Month 12 and then plateaued through to Month 24 (24–27 ED50 for the HPV-16/18 vaccine and 20–21 ED50 for the HPV-6/11/16/18 vaccine); at all timepoints, HPV-45 neutralizing antibody levels were below the PBNA limit of detection (Fig. 2).
Figure 1.
Seropositivity rates for anti-HPV-31 and anti-HPV-45 serum neutralizing antibodies measured by pseudovirion-based neutralization assay at Months 7 and 24 (ATP cohort for immunogenicity, seronegative and DNA-negative at baseline for HPV type analyzed). N, number of evaluable subjects; n, number of seropositive subjects, per vaccine, per timepoint. Percentages indicate seropositivity rates at each timepoint [seropositivity defined as neutralizing antibody titer ≥40 ED50 (the PBNA limit of quantification)]. Black bars, Human Papillomavirus Bivalent (Types 16 and 18) Vaccine (Recombinant, adjuvanted, adsorbed) (Cervarix®); white bars, Human Papillomavirus Quadrivalent (Types 6, 11, 16 and 18) Vaccine, Recombinant (Gardasil®). The ATP cohort for immunogenicity included all evaluable subjects who received three vaccine doses (i.e., those meeting all eligibility criteria and complying with the procedures defined in the protocol) for whom data concerning immunogenicity endpoint measures were available.
Figure 2.
Geometric mean titers (GMTs) for anti-HPV-31 and anti-HPV-45 serum neutralizing antibodies measured by pseudovirion-based neutralization assay at Months 7, 12, 18 and 24 (ATP cohort for immunogenicity, seronegative and DNA-negative at baseline for HPV type analyzed). ED50, effective dose producing 50% response; GMT, geometric mean titer. Black square line, Human Papillomavirus Bivalent (Types 16 and 18) Vaccine (Recombinant, adjuvanted, adsorbed) (Cervarix®); white square line, Human Papillomavirus Quadrivalent (Types 6, 11, 16 and 18) Vaccine, Recombinant (Gardasil®). Error bars denote 95% confidence intervals (CIs) of GMTs; dashed line, PBNA limit of detection (40 ED50). N, number of evaluable subjects. The ATP cohort for immunogenicity included all evaluable subjects who received three vaccine doses (i.e., those meeting all eligibility criteria and complying with the procedures defined in the protocol) for whom data concerning immunogenicity endpoint measures were available.
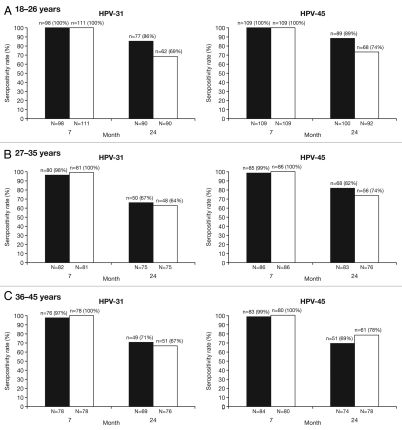
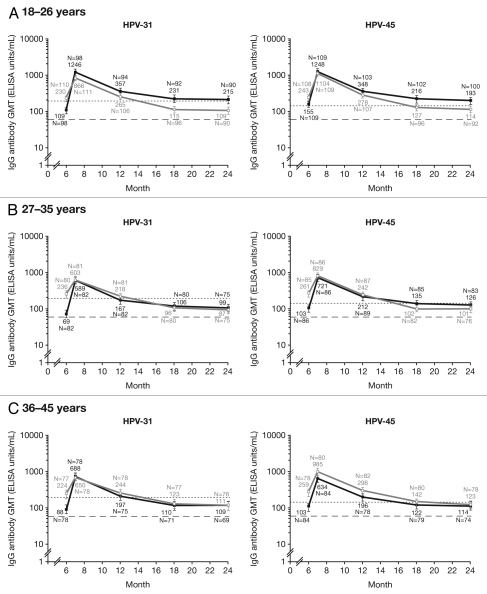
Seropositivity rates and vaccine-induced serum antibody titers against HPV-31/45, assessed by ELISA in the ATP cohort for immunogenicity are shown in Figures 3 and 4, respectively. At Month 7, seroconversion rates were similar for both vaccines against HPV-31/45 across all age groups (97.4–100% for the HPV-16/18 vaccine vs. 100% for the HPV-6/11/16/18 vaccine). At Month 24, 66.7–85.6% of women in the HPV-16/18 vaccine group and 64.0–68.9% in the HPV-6/11/16/18 vaccine group remained seropositive for HPV-31; 68.9–89.0% of women in the HPV-16/18 vaccine group compared with 73.7–78.2% of women in the HPV-6/11/16/18 vaccine group remained seropositive for HPV-45 (Fig. 3). GMTs in women aged 18–26 y were generally higher for the HPV-16/18 vaccine than the HPV-6/11/16/18 vaccine for both HPV-31 (215 and 109 ELISA units/mL, respectively) and HPV-45 (193 and 114 ELISA units/mL, respectively), and were similar with either vaccine in women aged 27–35 and 36–45 y (Fig. 4).
Figure 3.
Seropositivity rates for anti-HPV-31 and anti-HPV-45 IgG antibodies measured by enzyme-linked immunosorbent assay at Months 7 and 24 (ATP cohort for immunogenicity, seronegative and DNA-negative at baseline for HPV type analyzed). N, number of evaluable subjects; n, number of seropositive subjects, per vaccine, per timepoint. Percentages indicate seropositivity rates at each timepoint (seropositivity defined as IgG titer ≥59 ELISA units/mL [the ELISA limit of quantification]). Black bars, Human Papillomavirus Bivalent (Types 16 and 18) Vaccine (Recombinant, adjuvanted, adsorbed) (Cervarix®); white bars, Human Papillomavirus Quadrivalent (Types 6, 11, 16 and 18) Vaccine, Recombinant (Gardasil®). The ATP cohort for immunogenicity included all evaluable subjects who received three vaccine doses (i.e., those meeting all eligibility criteria and complying with the procedures defined in the protocol) for whom data concerning immunogenicity endpoint measures were available.
Figure 4.
Geometric mean titers (GMTs) for anti-HPV-31 and anti-HPV-45 IgG antibodies measured by enzyme-linked immunosorbent assay at Months 7, 12, 18 and 24 (ATP cohort for immunogenicity, seronegative and DNA-negative at baseline for HPV type analyzed). GMT, geometric mean titer; IgG, immunoglobulin G. Black square line, Human Papillomavirus Bivalent (Types 16 and 18) Vaccine (Recombinant, adjuvanted, adsorbed) (Cervarix®); white square line, Human Papillomavirus Quadrivalent (Types 6, 11, 16 and 18) Vaccine, Recombinant (Gardasil®). Errors bars denote 95% confidence intervals (CIs) of GMTs. Dashed line, ELISA limit of detection (59 ELISA units/mL). Dotted line, GMTs for natural infection antibody levels (183.5 ELISA units/mL for HPV-31 and 139.0 ELISA units/mL for HPV-45). N, number of evaluable subjects. The ATP cohort for immunogenicity included all evaluable subjects who received three vaccine doses (i.e., those meeting all eligibility criteria and complying with the procedures defined in the protocol) for whom data concerning immunogenicity endpoint measures were available.
Memory B-cell responses.
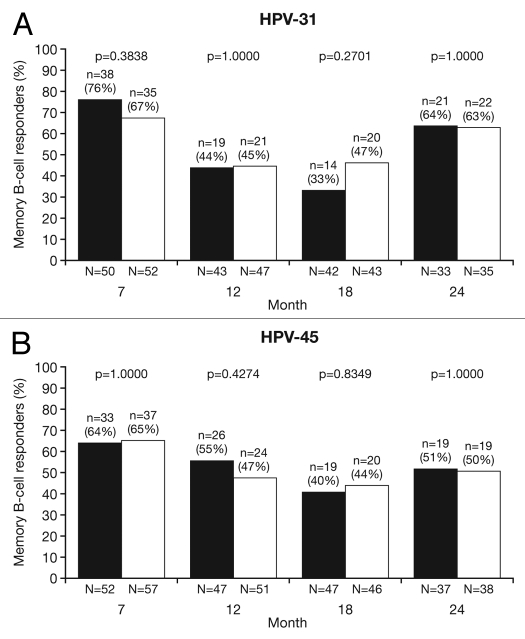
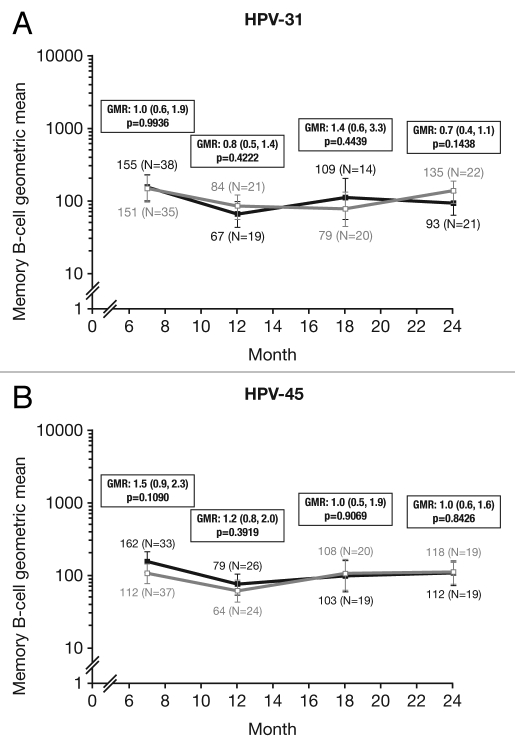
HPV cross-reactive memory B-cell responses were evaluated in a subset of women in the ATP cohort for immunogenicity who had no detectable B-cell response prior to vaccination. The geometric means (GMs) of circulating antigen-specific memory B-cell responses were measured only in ‘responders’ (defined as subjects with detectable HPV crossreactive memory B-cells, i.e., ≥1 specific memory B-cell/million memory B-cells; see methods) because data for all subjects did not follow a normal distribution. The highest percentage of memory B-cell responders was observed at Month 7 for HPV-31 (HPV-16/18 vaccine, 76.0%; HPV-6/11/16/18 vaccine, 67.3%: p = 0.3838) and HPV-45 (HPV-16/18 vaccine, 63.5%; HPV-6/11/16/18 vaccine, 64.9%: p = 1.0000). At Month 24, there were approximately equal proportions of responders between vaccine groups for both HPV-31 (HPV-16/18 vaccine, 63.6%; HPV-6/11/16/18 vaccine, 62.9%: p = 1.0000) and HPV-45 (HPV-16/18 vaccine, 51.4%; HPV-6/11/16/18 vaccine, 50%: p = 1.0000) (Fig. 5).
Figure 5.
Proportion of responders for (A) HPV-31- and (B) HPV-45-specific memory B-cell responses at Months 7, 12, 18 and 24 (ATP cohort for immunogenicity; seronegative, DNA-negative and with no detectable HPV cross-reactive B-cells prior to vaccination). N, number of subjects with available results; n, number of responders, per vaccine, per timepoint; %, percentage of responders, per vaccine, per timepoint. Black bars, Human Papillomavirus Bivalent (Types 16 and 18) Vaccine (Recombinant, adjuvanted, adsorbed) (Cervarix®); white bars, Human Papillomavirus Quadrivalent (Types 6, 11, 16 and 18) Vaccine, Recombinant (Gardasil®). Responders defined as subjects with detectable HPV cross-reactive memory B cells (≥1 cell/million cells). p-values were calculated using Fisher's exact test to compare proportion of responders.
Cross-reactive memory B-cell GMs at Month 24 (calculated only in responders) were comparable for both groups for HPV-31 and -45 (Fig. 6). GM ratios (GMRs) of the response to the HPV-16/18 vaccine vs. the HPV-6/11/16/18 vaccine for HPV-31 and -45 were 0.69 (95% CI: 0.41, 1.14; p = 0.1438) and 0.95 (0.55, 1.62; p = 0.8426), respectively.
Figure 6.
Geometric means (GM) and GM ratios (GMR) in responders only for (A) HPV-31- and (B) HPV-45-specific memory B-cells at Months 7, 12, 18 and 24 (ATP cohort for immunogenicity; seronegative, DNA-negative and with no detectable HPV cross-reactive B cells prior to vaccination). GMR, geometric mean ratio; N, number of responders [i.e., subjects with detectable HPV cross-reactive memory B cells (≥1 cell/million cells)]. Black square line, Human Papillomavirus Bivalent (Types 16 and 18) Vaccine (Recombinant, adjuvanted, adsorbed) (Cervarix®); white square line, Human Papillomavirus Quadrivalent (Types 6, 11, 16 and 18) Vaccine, Recombinant (Gardasil®). Error bars denote 95% confidence intervals of geometric means. Statistical comparison (GMR ANOVA p-value) was performed on B-cell responders because data for all subjects in the subset did not follow a normal distribution.
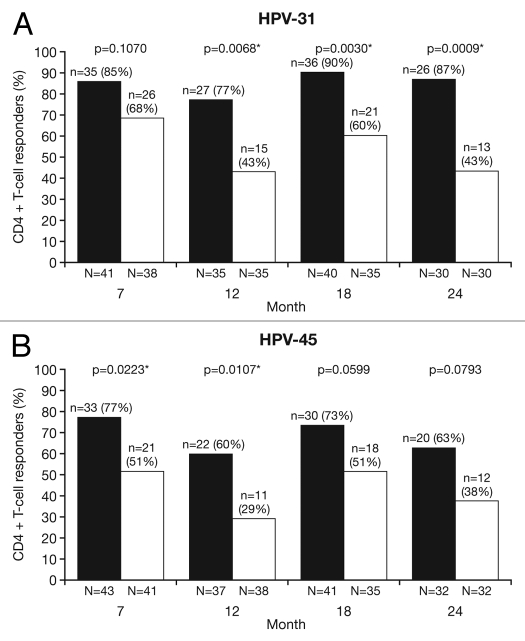
CD4+ T-cell responses.
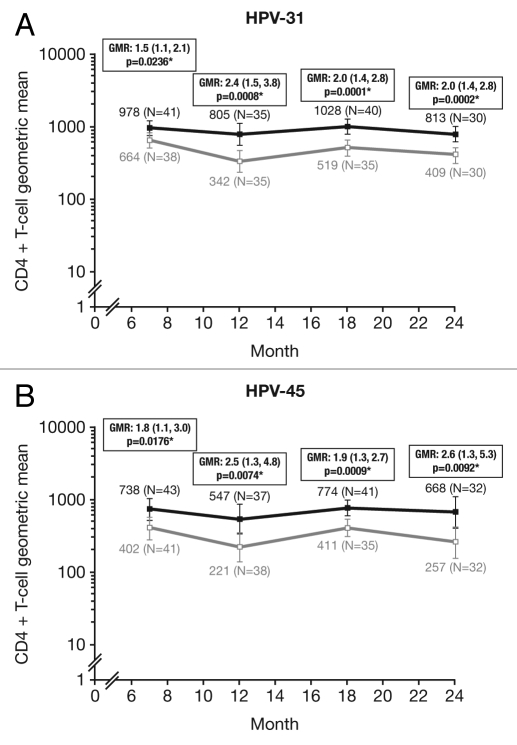
Cross-reactive CD4+ T-cell responses were evaluated in a subset of women in the ATP cohort for immunogenicity who were T-cell-negative prior to vaccination (<500 HPV cross-reactive CD4+ T-cells/million CD4+ T-cells at baseline). The proportion of T-cell responders [defined as subjects with ≥500 HPV cross-reactive CD4+ T-cells identified in vitro as expressing two or more of four immune markers (CD40L, IL-2, TNFα, IFNγ) per million cells] was analyzed between vaccine groups]. At Month 7, the proportion of T-cell responders were similar between vaccines for HPV-31 (HPV-16/18 vaccine, 85.4%; HPV-6/11/16/18 vaccine, 68.4%: p = 0.1070) and significantly higher for HPV-45 with the HPV-16/18 vaccine vs. the HPV-6/11/16/18 vaccine (76.6% vs. 51.2%, p = 0.0223). At Month 24, the proportion of T-cell responders was significantly higher in the HPV-16/18 vaccine group than the HPV-6/11/16/18 vaccine group for HPV-31 (86.7% vs. 43.3%, p = 0.0009), and higher in the HPV-16/18 vaccine group than the HPV-6/11/16/18 vaccine group for HPV-45 (62.5% vs. 37.5%, p = 0.0793) (Fig. 7). The GM of the frequency of circulating antigen-specific CD4+ T-cells in all subjects at Month 24 was significantly higher in the HPV-16/18 vaccine group than the HPV-6/11/16/18 vaccine group for both HPV-31 [GMR = 2.0 (GM: 813, 409); p = 0.0002] and HPV-45 [GMR = 2.6 (GM: 668, 257); p = 0.0092] (Fig. 8).
Figure 7.
Proportion of responders for (A) HPV-31- and (B) HPV-45-specific CD4+ T-cell response at Months 7, 12, 18 and 24 (ATP cohort for immunogenicity; seronegative, DNA-negative and with a HPV-specific CD4+ T-cell response below 500 cells per million cells prior to vaccination). N, number of subjects with available results; n, number of responders, per vaccine, per timepoint; %, percentage of responders, per vaccine, per timepoint. *p < 0.05. Black bars, Human Papillomavirus Bivalent (Types 16 and 18) Vaccine (Recombinant, adjuvanted, adsorbed) (Cervarix®); white bars, Human Papillomavirus Quadrivalent (Types 6, 11, 16 and 18) Vaccine, Recombinant (Gardasil®). Responders defined as subjects with ≥500 HPV cross-reactive memory CD4+ T-cells expressing at least two of four immune markers (CD40L, IL-2, TNFα, IFNγ) per million cells. p-values were calculated using Fisher's exact test to compare proportion of responders.
Figure 8.
Geometric means (GM) and GM ratios (GMR) for (A) HPV-31- and (B) HPV-45-specific CD4+ T-cell response at Months 7, 12, 18 and 24 in all subjects in the subset (ATP cohort for immunogenicity; seronegative, DNA-negative and with a HPV cross-reactive CD4+ T-cell response below 500 cells per million cells prior to vaccination). *p < 0.05. GMR, geometric mean ratio; N, number of subjects with available results. Black square line, Human Papillomavirus Bivalent (Types 16 and 18) Vaccine (Recombinant, adjuvanted, adsorbed) (Cervarix®); white square line, Human Papillomavirus Quadrivalent (Types 6, 11, 16 and 18) Vaccine, Recombinant (Gardasil®). Error bars denote 95% confidence intervals of geometric means. Statistical comparison (GMR ANOVA p-value) was performed on all subjects.
Discussion
The cross-protective efficacy of the HPV-16/18 vaccine against HPV-31/33/45 was demonstrated in an earlier large clinical study (HPV-008)17,23,24 in a cohort of women who were HPV DNA-negative for corresponding HPV type at baseline, regardless of serostatus.17 In the total vaccinated cohort for efficacy, vaccine efficacy against 6 mo persistent infection attributed to HPV-31, -33 and -45 increased over time from an interim analysis, performed at a mean follow-up of 14.8 mo [standard deviation (SD): 4.9 mo] after third vaccine dose (36.1%, 36.5% and 59.9%, respectively),24 through to analysis at 34.9 mo (SD: 6.4 mo) (66.9%, 42.2% and 71.6%, respectively).17 Recent results from the end-of-study analysis of this trial confirmed the cross-protective efficacy of the HPV-16/18 vaccine against these three HPV types through to Month 48.23 Together, HPV types -16, -18, -31, -33 and -45 account for approximately 82% of cervical cancers.25
Cross-protection results have also been published for the HPV-6/11/16/18 vaccine,20 although it should be noted that these should not be directly compared with the HPV-16/18 results, as the study designs of the HPV-16/18 vaccine and HPV-6/11/16/18 vaccine trials differ in HPV DNA- and immunological assays, endpoints and study populations. In a cohort of women who were seronegative and DNA-negative for HPV types in the HPV-6/11/16/18 vaccine, and DNA-negative for ten non-vaccine HPV types (including HPV-31/45), the HPV-6/11/16/18 vaccine demonstrated cross-protective efficacy against CIN1.3 or adenocarcinoma in situ associated with HPV-31; no cross-protection was shown against CIN1.3 or adenocarcinoma in situ associated with HPV-45.20
The current study was designed to directly compare the immune response to non-vaccine oncogenic HPV types elicited by the HPV-16/18 vaccine and the HPV-6/11/16/18 vaccine. Our sub-analysis of the HPV-010 study, along with the available data generated in clinical efficacy studies, was intended to provide insight into the potential mechanism(s) for cross-protection.
Our observations show that both the HPV-16/18 vaccine and the HPV-6/11/16/18 vaccine induced cross-reactive serologic responses against HPV-31 and HPV-45. Humoral immune responses for non-vaccine types HPV-31/45 (PBNA and ELISA) were much lower than those for vaccine types HPV-16/18.21,22 At Month 24, there were no significant differences between the HPV-16/18 vaccine and the HPV-6/11/16/18 vaccine in terms of HPV-31/45 circulating antibodies as measured by PBNA. It should be noted that circulating neutralizing antibodies were at levels close to or below the limit of detection of each assay. Similar levels of circulating antibodies (measured by ELISA) were observed between both vaccines in the 27–35 y and 36–45 y groups; for both vaccines, the highest levels of HPV-31 and HPV-45 circulating antibodies were reported in the 18–26 y group.
Previous results from the HPV-010 study demonstrated higher vaccine specific immune responses with the HPV-16/18 vaccine compared with the HPV-6/11/16/18 vaccine, as supported by higher levels of specific neutralizing antibodies, seropositivity rates and a higher frequency of HPV cross-reactive memory B cells and T cells.21,22 However, in our sub-analysis, the levels of neutralizing antibodies to HPV-31/45 were relatively low for both vaccines. There was also no significant difference in circulating HPV cross-reactive memory B-cell response for HPV-31 and HPV-45 with both vaccines, likely due to the fact that most memory B cells would have migrated to central lymphoid organs, such as the spleen, to be subsequently activated by the interaction of circulating antigen presenting cells such as CD4+ T cells.26
An earlier study from women immunized with the HPV-6/11/16/18 vaccine, investigating vaccine-induced antibody binding and neutralization with HPV-18/45 using PBNA, also demonstrated relatively low levels of vaccine-induced antibodies to cross-neutralize HPV-45 pseudovirions.27 Findings from a recent study by Kemp et al. suggest that low levels of cross-neutralizing antibodies may contribute to the mechanism of cross-protection observed with the HPV-16/18 vaccine.28 It may simply follow that the response to HPV-16 and -18 is higher due to the immunodominance of the type-specific epitopes, so only a relatively small proportion are shared with phylogenetic family members like HPV-31 and -45, respectively.
A separate study showed that a fourth dose of the HPV-16/18 vaccine induced an anamnestic response (evidenced by a rapid and strong increase of antibody titers) not only to the vaccine types HPV-16/18, but also to non-vaccine types HPV-31/45.29 The demonstration of immunologic memory for the HPV-31/45 response in women confirms that the HPV-16/18 vaccine does induce cross-reactive immune responses even though circulating neutralizing cross-reactive antibodies are at levels close to or below the limit of detection of the assays.30
The main immunological parameter that differentiates the immune response to HPV-31/45 is the CD4+ T-cell response. In our sub-analysis, CD4+ T-cell responses remained high from Month 7 to Month 24 and the HPV-16/18 vaccine gave higher CD4+ T-cell responses than the HPV-6/11/16/18 vaccine at all timepoints. Importantly, in contrast to the relatively low levels of cross-reactive antibodies, the frequency and quality of the crossreactive CD4+ T-cell responses to HPV-31 and -45 were similar to the specific responses to HPV-16 and -18.22 However, we note that, based on published data,17,20 there appear to be differences in the HPV types for which the vaccines confer cross-protective efficacy. This suggests that higher levels of CD4+ T-cell response may be necessary for cross-protection against certain HPV types, such as HPV-45, but that the requirements for cross-protection against other types such as HPV-31 might be different.
Of note, the cross-reactive T-cell response to HPV-31 and -45 was measured using pools of synthetic peptides spanning the truncated VLP L1 sequences of HPV-31 and HPV-45. We cannot exclude the possibility that the HPV-6/11/16/18 vaccine induced a cross-reactive T-cell response against the portion absent (approximately 30 amino acids) from the truncated HPV-31/45 L1 VLPs. However, such bias is unlikely given that the truncated portion is small and also the similarity of the HPV-31/45 T-cell responses to the specific responses to HPV-16/18 (obtained using a pool of peptides spanning the entire VLP L1 sequences of HPV-16/18).
Other assays used in the current study are not anticipated to have introduced a bias toward either vaccine. As discussed previously in references 21 and 22, no bias was observed for HPV-16/18 data obtained using the ELISA or memory B-cell data obtained using the ELISPOT assay. For the PBNA used in the present study, the amino acid sequence of the L1 protein and the cell-line used for the production of the pseudovirions, which contain L1 and L2, are different from those used in either vaccine. Importantly, PBNA and ELISA have also been shown to correlate with Merck's competitive Luminex immunoassay (cLIA).31,32 Moreover, none of the assays, nor the HPV-16/18 vaccine or the HPV-6/11/16/18 vaccine contain HPV-31/45 VLPs.
Adjuvant systems have been shown to enhance specific and cross-clade neutralizing antibody immunological responses in addition to T-cell responses.33–35 In the case of the HPV-16/18 vaccine, the AS04 adjuvant system,36 which contains MPL (50 µg) absorbed on aluminum salt (Al3+, 500 µg), may similarly enhance the immune responses. The AS04 component of the HPV-16/18 vaccine was shown to induce a higher frequency of type-specific memory B cells and antibody responses against HPV-16/18 compared with the same vaccine formulated with aluminum salt alone.8 The MPL component of the AS04 adjuvant binds and activates the Toll-like receptor-4 (TLR-4), which is present on key antigen-presenting cells that play an important role in the induction of innate and adaptive immune responses.37,38 Furthermore, the combination of an aluminum salt with MPL is thought to prolong cytokine responses at the injection site.36 Taken together, these factors may plausibly account for the higher CD4+ T-cell response against HPV-31/45 observed with the HPV-16/18 vaccine compared with the HPV-6/11/16/18 vaccine in this study.
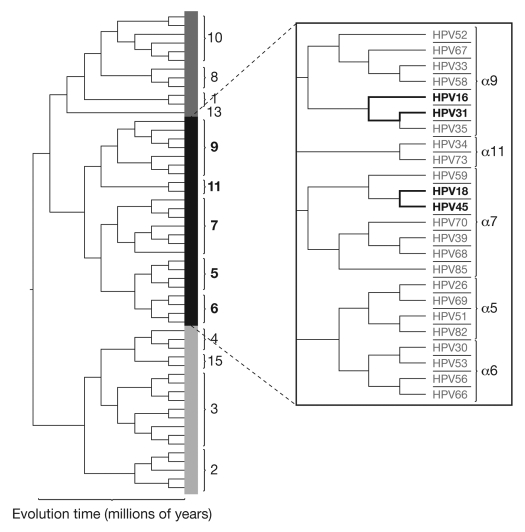
Although the mechanism(s) of cross-protection has not yet been fully elucidated, it is likely to be associated with the phylogenetic relationship of HPV types. HPV-16, -18, -31 and -45 belong to the genus α-papillomavirus, which is further classified by species and then type; the A7 species includes HPV types -18 and -45 and the A9 species includes HPV types -16 and -31 (Fig. 9). HPV types belonging to the same species are phylogenetically related;39,40 based on predicted L1 amino acid sequences, HPV-31 shares 83% homology with the L1 protein of HPV-16 and HPV-45 shares 88% homology with the L1 protein of HPV-18.20 The observed difference in the cross-protective efficacy for HPV-45 between the vaccines is probably not related to the primary sequence of the HPV-18 L1 VLPs, since both share a similar percentage of sequence homology with HPV-45. However, the truncation of ∼30 amino acids at the C-terminus of the HPV-16/18 vaccine's HPV-18 L1 VLPs41 might impact on the accessibility of epitopes that are shared between HPV-18 and HPV-45.
Figure 9.
Phylogenetic tree of anogenital human papillomavirus types (adapted from Schiffman and Wentzensen 2010 and Schiffman et al. 2005).54,55 This phylogenetic tree is based on the alignment of concatenated early and late open reading frames. The carcinogenicity of HPV types reflects viral evolution. The clade presented in detail in the figure above (α5, 6, 7, 9, 11) reflects the HPV types associated with cervical cancers and their precursors.54
Confirmation would require further biophysical structural characterization of HPV-18 L1 VLPs, the demonstration of differences in epitope recognition by antibodies induced by the two vaccines, or evaluation of the relative avidity of antibodies for the different epitopes. In addition, conformational differences in L1 VLP epitope exposure due to differences in vaccine production process and/or the adjuvant formulation (as discussed above) may also contribute to differences in the quality of cross-reactive neutralizing antibody responses, and may contribute to the differences in cross-protection observed between the vaccines.
Despite high levels of homology between HPV types of the same species, even if the conformational structures of L1 VLPs from different HPV types are very similar, the surface loops that contain neutralizing domains display significant amino acid heterogeneity. The unique features of these surface loops are the distinct surface immunodominant conformational epitopes that provide type-specific protection. There are high levels of conserved homology between HPV types that may represent subdominant cross-reactive epitopes.42 As cross-neutralization induced by L1 VLPs represents less than 1% of the type specific neutralizing activity induced by the immunodominant conformational epitopes, it is uncertain whether this is sufficient to offer cross-protection in vivo.43 The capacity of L1-VLP-induced antibodies to mediate type-specific and cross-protection against cervicovaginal challenge was recently demonstrated in a murine challenge model, following vaccination with HPV-16 VLPs (on alum) or following passive transfer of immune serum.44 A high level of specific protection was observed against HPV-16 and partial cross-protection was observed against HPV-31 challenge.44 Furthermore, in rabbits that received the HPV-16/18 vaccine, high levels of cross-protection against HPV-31/45 were observed after HPV quasivirion [virions with human HPV L1/L2 capsids and the cotton-tail rabbit papillomavirus (CRPV) genome, produced in 293TT cells] 45 challenge in the presence of no or low levels of neutralizing antibodies.46 Importantly these controlled experimental studies clearly demonstrate the capacity of L1 VLPs to induce cross-protective immunity and indicate that the cross-protection observed in clinical studies resulted from vaccine-induced immune responses.
Investigations into the mechanism of L1 VLP-induced prevention of HPV infection have led to the proposal of two distinct mechanisms of protection by L1 specific polyclonal antibodies: (1) high levels of antibodies result in an immunoglobulin-coated HPV capsid, which prevents interaction of the capsid with the cell surface, (2) a lower antibody level allows the capsid to associate with the cell surface but prevents the conformational changes required for virus entry.44,47 It has been suggested that cross-protection and type-specific long-term protection may be attributed to the second mechanism with a relatively low antibody to virus ratio and that PBNA may not be a suitable assay to assess cross neutralizing antibodies.44 The National Cancer Institute (United States) is currently investigating the possibility of developing more accurate methods to assess all sets of neutralizing antibodies.47
An alternative role of antibodies in mediating cross-protection follows from the observation that HPV suppresses Langerhans cell activation, resulting in local immune suppression. Interestingly, this suppression can be reversed when Langerhans cells encounter HPV virions or L1/L2 VLPs in the presence of antibodies.48,49 Thus, HPV infection in the presence of vaccine-induced antibodies could activate Langerhans cells, via the Fc receptor or other mechanisms, and lead to local inflammation that does not occur in the absence of antibodies. Importantly, the antibodies would not need to have the functional capacity to neutralize the virus.
Furthermore, a role for CD4+ T-cells in cross-protection in the presence of cross-reactive antibodies is proposed. It has been shown that CD4+ T-cells can activate NK cells in the presence of pro-inflammatory signals,50 suggesting that the combination of activated Langerhans cells, due to the presence of antibodies,46 and elevated frequencies of cross-reactive cytokine producing CD4+ T-cells could activate IFNγ producing NK cell responses.48 Thus both CD4+ T- and NK cells could contribute to cytokine-mediated reduction of viral replication and/or elimination of viral infected epithelial cells.
Assuming that CD4+ T-cells and NK cells have a role to play, one would expect to see better cross-protection for progressive disease efficacy endpoints such as CIN2+/3+, due to elimination of infected cells, than for incident/persistent infection efficacy parameters. Although there is some evidence of this pattern being observed from AS04-adjuvanted HPV-16/18 vaccine efficacy studies, it is not unequivocal and further investigation will provide more complete data to verify this hypothesis.17
Notably, CD4+ T-cell responses have been shown to be important in limiting the progression of cervical cancerous lesions51 and in the regression of genital warts.52 In a study examining the efficacy of a novel therapeutic vaccine containing HPV-16 E6 and/or E7 synthetic peptides, 60% (95% CI: 36–81) of patients with HPV-16-positive vulvar intraepithelial neoplasia had a clinical response three months after last vaccination; patients with a complete response had a significantly higher CD4+ T-cell response than patients without a complete response.51 Therefore, a CD4+ T-cell-mediated mechanism that eliminates HPV-infected cells is plausible.
However, our study assessed only immunological endpoints; whether or not the higher levels of cross-reactive CD4+ T cells observed with the HPV-16/18 vaccine compared with the HPV-6/11/16/18 vaccine correlate with enhanced protection against the progression of cervical lesions has not been assessed.
In conclusion, vaccination with the HPV-16/18 vaccine or the HPV-6/11/16/18 vaccine induces humoral responses to non-vaccine HPV types -31/45, albeit at generally low levels; in the case of the HPV-16/18 vaccine, this response has demonstrated immunological memory in other investigations. The HPV-16/18 vaccine induced relatively higher HPV-31/45-specific CD4+ T-cell responses compared with the HPV-6/11/16/18 vaccine, which may play a role in cross-protection; however, further studies are necessary to fully understand and elucidate the roles (and possible interactions) of both humoral and cell-mediated immunity in the HPV-16/18 vaccine and the HPV-6/11/16/18 vaccine for protection against cervical lesions caused by oncogenic non-vaccine types. A limitation of our analysis is that it only evaluated the immune response to two oncogenic non-vaccine HPV types. The assessment of a greater number of non-vaccine types associated with cross-protection or a lack of cross-protection would be valuable to identify other immunological markers associated with cross-protection. This may increase our understanding of the underlying mechanism(s) of cross-protection, and with further follow-up, help to determine the duration of cross-protection and perhaps subsequently facilitate the determination of valid and universally accepted correlates of protection.
Materials and Methods
Study design.
This was a sub-analysis performed at Month 24 of a Phase III trial conducted in 40 centers in the US (HPV-010; NCT00423046). The overall study design was described previously in references 21 and 22. Healthy women were stratified by age (18–25, 27–35 and 36–45 y) and randomized in a 1:1 ratio to receive the HPV-16/18 vaccine or the HPV-6/11/16/18 vaccine according to their recommended three-dose schedules (Months 0, 1, 6 or Months 0, 2, 6, respectively). To ensure the study was conducted in an observer-blind manner, a dose of placebo (aluminum hydroxide) was given at either Month 1 or 2 as appropriate; the study is ongoing and will be maintained blind through 48 mo of follow-up. The study was conducted in accordance with the Declaration of Helsinki and Good Clinical Practice guidelines.
Blood sampling and assays.
Analyses were performed on the ATP cohort for immunogenicity that included all women who met eligibility criteria, complied with the trial protocol, received all three doses of study vaccine and were DNA-negative and seronegative for HPV type considered at baseline, and for whom immunogenicity endpoint measures were available.
Blood sampling was performed as described previously in references 21 and 22. At Months 6, 7, 12, 18 and 24, additional samples were collected from a pre-specified subset of women in all age groups in both vaccine groups for additional immunological assessment, which included HPV cross-reactive memory B-cell and CD4+ T-cell responses. HPV-31/45 type-specific pseudovirion-based neutralization assays (PBNA), enzyme-linked immunosorbent assays (ELISA) and B-cell ELISPOT assays were performed in assays similar to those previously described for HPV-16/18.21,22 In brief, PBNA and ELISA were performed as described in Dessy et al.53 with minor amendments as follows: (1) For PBNA, HPV-31 or -45 pseudovirions were used instead of HPV-16 or -18 pseudovirions; (2) for ELISA, HPV-31 and -45 VLP were used as coating antigen; instead of using eight two-fold serial dilution starting at 1/100, sera were diluted 1/100 and 1/1,000; the cut-off for ELISA was established as the concentration, in EU/mL, equivalent to the upper limit of the one-sided 95% confidence interval of a population of 18–25-y-old women (who had no more than one lifetime sexual partner, whose cervix was free of cytological abnormalities and who tested DNA negative for high-risk HPV by PCR and seronegative for HPV-16 and -18 by ELISA). For each antigen, seropositivity was defined as a neutralizing antibody titer ≥40 ED50 (the assay threshold) for PBNA and an antibody titer ≥59 ELISA units/mL for ELISA. Responders for memory B cells were defined as subjects with detectable HPV cross-reactive memory B -cells (≥1 cell per million cells).
CD4+ T-cell responses to HPV-31/45 were evaluated in an assay similar to that previously described for HPV-16/18.22 Cross-reactive responses were evaluated in vitro by using a pool of 20-mer HPV peptides to stimulate peripheral blood antigen-specific CD4+ T-cells to produce cytokines. The 20-mer HPV synthetic peptides overlapped by ten amino acids and spanned the entire truncated sequences of the HPV-31/45 L1 VLPs. Intracellular cytokine staining, followed by flow cytometry, was used to quantify the number of cells producing immune markers (CD40L, IL-2, TNFα and IFNγ) in response to in vitro stimulation with four different peptide pools.22 Responders were defined as women who had ≥500 specific CD4+ T cells per million identified as producing two or more of the immune markers after in vitro stimulation.
Statistical analysis.
The primary exploratory objective of the HPV-010 study was to compare HPV-16/18 antibody titers induced by the HPV-16/18 vaccine and the HPV-6/11/16/18 vaccine in women 18–26 y of age as measured by PBNA at Month 7.21 Results from Month 24 have also been published in reference 22. Secondary objectives included evaluating serum immune responses to HPV-31/45 in the ATP cohort for immunogenicity of women aged 18–26, 27–35 and 38–45 y of age at Months 6, 7, 12, 18 and 24 by PBNA and ELISA. Secondary objectives also included evaluating the frequency of HPV-31/45-specific memory B-cells and cytokine-positive CD4+ T-lymphocytes in a pre-specified 30% of women in each vaccine group. Statistical analyses (within-group and between-group comparisons) of immunogenicity were performed at Months 7, 12, 18 and 24, as described previously in reference 21.
Memory B-cell and CD4+ T-cell analysis.
Memory B-cell and CD4+ T-cell responses for each stimulant at each timepoint were summarized for each group, HPV type, and age strata by the geometric mean (GM). In an exploratory analysis, the proportion of responders in each group was tested using Fisher's exact test.
The GMs of circulating antigen-specific memory B-cell responses were calculated only for subjects with no response at baseline and a response (≥1 cell/million cells) at the specific timepoint under analysis. The GMs were calculated only in responders as data for all subjects did not follow a normal distribution (samples with no detectable response were assigned a value of 1 for the purpose of GM calculation).
The GMs of circulating antigen-specific CD4+ T cells were assessed in all subjects (i.e., responders and non-responders). The GMR is the ratio between the GMs of vaccine groups. Both the ANOVA (analysis of variance) model and Kruskal-Wallis test were used to calculate p-values associated to GMRs; the ANOVA p-values are presented here.
Acknowledgments
We thank all study participants. This study was sponsored by GlaxoSmithKline Biologicals, Belgium. The team from GlaxoSmithKline Biologicals, US, included Kevin Carrick (Study Manager), J. Ann Jones (Field Monitors), and Joan Adler (Local Medical Monitor). Jesse Lepage (Cincinnati Children's Hospital) provided technical expertise in the preparation of samples for the evaluation of B-cell responses. B-cell assays were performed by the Human Cellular Immunity Team at GlaxoSmithKline Biologicals, Belgium (Olivier Jauniaux, Alexandre Smirnoff, Sarah Charpentier, Valerie Mohy, Dinis Fernandez-Ferreira, Samira Hadiy, Michael P. Mestre, Murielle Carton, Pierre Libert, Zineb Soussi and Luc Franssen). Pseudovirion-based neutralization assays and enzyme-linked immunosorbent assays were performed by the Global Vaccine Clinical Readout Development Unit (GVCR DU) team at GlaxoSmithKline Biologicals, Belgium (Rudy Crudenaire, Patrice Pierson, Stephanie Abderhamane, Rita Dereymaeker, France Dufranne, Benjamin Mathieu, Marie Gangarossa, Lieve Lauwers, Jeremy Leurquin, Mailys Pringels, Laurence Torset, Jessica Vanderhaegen for PBNA and Vinciane Lelivre, Michel Malevé, Mikael Lega for ELISA). Francis Dessy and Sylviane Poncelet (GlaxoSmithKline Biologicals, Belgium) led the PBNA and ELISA analysis, respectively. Jean-Louis Maroye (Modern Solutions for Business SPRI, Belgium) received the randomization list and performed the statistical analyses. Marie Lebacq (GlaxoSmithKline Biologicals, Belgium) supervised the data analyses (without access to the randomization list), and contributed to the statistical QC and writing of the statistical analysis report. The study report was prepared by Kim Nijs (Emtex, C/O GlaxoSmithKline Biologicals, Belgium) and Stéphanie Genevrois (GlaxoSmithKline Biologicals, Belgium). Medical writing assistance was provided by Meridian HealthComms Ltd., (Middlewich, UK) on behalf of GlaxoSmithKline Biologicals. Denis Sohy and Dirk Saerens (GlaxoSmithKline Biologicals, Belgium) provided manuscript coordination on behalf of GlaxoSmithKline.
Abbreviations
- AAHS
amorphous aluminum hydroxyphosphate sulphate
- ANOVA
analysis of variance
- AS04
adjuvant system 04
- ATP
according-to-protocol
- CI
confidence interval
- CIN
cervical intraepithelial neoplasia
- cLIA
competitive luminex immunoassay
- ED50
effective dose producing 50% response
- ELISA
enzyme-linked immunosorbent assay
- GM
geometric mean
- GMR
geometric mean (titer) ratio
- GMT
geometric mean titer
- GSK
GlaxoSmithKline
- HPV
human papillomavirus
- MPL
monophosphoryl lipid A
- PBNA
pseudovirion-based neutralization assay
- SD
standard deviation
- TLR-4
toll-like receptor 4
- TVC
total vaccinated cohort
- VLP
virus-like particle
Notes
Cervarix is a registered trade mark of the GlaxoSmithKline group of companies. Gardasil is a registered trade mark of Merck and Co., Inc.
Conflict of Interest Statement
All authors have completed the Unified Competing Interest form at www.icmje.org/coi_disclosure.pdf and declare: The submitted work got financial support from GlaxoSmithKline Biologicals.
M.E. has advised or participated in educational speaking activities, but does not receive payments from any companies. In specific cases, Montefiore Medical Center has received payment for M.E. time spent for these activities from GSK and Merck. Also, Montefiore has received grant funding for research related costs of clinical trials that M.E. has been the Montefiore PI from GSK and Merck. A.C., N.C., J.R. received support for travel to meetings for the study. A.C., M.J.L., N.C., J.R. received grants for their institutions. A.C. received financial support for board membership. M.J.L. received financial support for consultancy. A.C., B.F., N.C., received payment for lectures including service on speaker bureaus. A.S., D.D., M.L., S.D., P.M., S.G., RvdM are GSK employees. A.S., D.D., S.D., P.M., RvdM have stock options from GSK. S.S., B.F., M.B. declare no conflict of interest.
†HPV-010 Study Group
Principal and Co-Investigators: Keith Aqua (Visions Clinic Research, Boynton Beach, FL), Mira Baron (Rapid Medical Research, Cleveland, OH), Mark Blatter (Primary Physicians Research, Inc., Pittsburgh, PA), Archana Chatterjee (Creighton University, Omaha, NE), Christopher V. Chambers (Thomas Jefferson University, Philadelphia, PA), Nahida Chakhtoura (University of Miami, Miami, FL), Louis A. Civitarese (Preferred Primary Care Physicians, Inc., Carnegie, PA), Donna DeSantis (East Valley Family Physicians, Chandler, AZ), Rovena Reagan (Women's Health Care at Frost St., San Diego, CA), Mark H. Einstein (Montefiore Medical Center, Bronx, NY), Douglas K. Fenton (North Coast Women's Care, Vista, CA), Bradley Fox (Liberty Family Practice, Erie, PA), David L. Fried (Omega Medical Research, Warwick RI), Sidney A. Funk (Radiant Research, Atlanta, GA), Cheryl A. Hansen (Ridgeview Research, Chaska, MN), James A. Hedrick (Kentucky Pediatric and Adult Research, Bardstown, KY), Dan C. Henry (Foothill Family Clinic, Salt Lake City, UT), Bethany Hoffman (Aspen Medical Group, St. Paul, MN), Alan Johns (Texas Healthcare, Fort Worth, TX), Terry D. Klein (Heartland Research Assoc., Wichita, KS), Jacob Lalezari (Quest Clinical Research, San Francisco, CA), Myron J. Levin (University of Colorado Denver and Health Sciences Center, Aurora, CO), Michael J. Noss (Radiant Research, Cincinnati, OH), Kevin Pitts (Wenatchee Valley Medical Center, Wenatchee, WA), Alfred N. Poindexter III (Advances in Health, Inc., Houston, TX), Anthony Puopolo (Milford Emergency Associates, Inc., Milford, MA), Jeffrey Rosen (Clinical Research of South Florida, Coral Gables, FL), L. Sofia Scholar (Walla Walla Clinic, Walla Walla, WA), Michael A. Scutella (OB/GYN Associates of Erie, Erie, PA), James H. Silverblatt (Lake Medical Research, LLC, Willoughby Hills, OH), Dirk Smith (Heartland Research Associates, Arkansas City, KS), Rhoda S. Sperling (Mount Sinai School of Medicine, New York, NY), Karen G. Swenson (Professional Quality Research, Austin, TX), Mark Turner (Advanced Clinical Research, Boise, ID), Michael W. Warren (Research Across America, Lancaster, PA).
Study sponsor contributors: Dorothée Meric (GlaxoSmithKline Biologicals, Belgium) supervised the data analyses (without access to the randomization list); Francis Dessy (GlaxoSmithKline Biologicals, Belgium) led the pseudovirion-based neutralization assay; Sanjoy Datta and Dominique Descamps (GlaxoSmithKline Biologicals, Belgium), and Gary Dubin and Anne Schuind (GlaxoSmithKline Biologicals, United States) supervised study design and conduct, data collection, interpretation and reporting.
References
- 1.Ferlay J, Shin HR, Bray F, Forman D, Mathers C, Parkin DM. Estimates of worldwide burden of cancer in 2008: GLOBOCAN 2008. Int J Cancer. 2010;127:2893–2917. doi: 10.1002/ijc.25516. [DOI] [PubMed] [Google Scholar]
- 2.Muñoz N, Bosch FX, de Sanjosé S, Herrero R, Castellsagué X, Shah KV, et al. Epidemiologic classification of human papillomavirus types associated with cervical cancer. N Engl J Med. 2003;348:518–527. doi: 10.1056/NEJMoa021641. [DOI] [PubMed] [Google Scholar]
- 3.Smith JS, Lindsay L, Hoots B, Keys J, Franceschi S, Winer R, et al. Human papillomavirus type distribution in invasive cervical cancer and high-grade cervical lesions: A meta-analysis update. Int J Cancer. 2007;121:621–632. doi: 10.1002/ijc.22527. [DOI] [PubMed] [Google Scholar]
- 4.de Sanjose S, Quint WG, Alemany L, Geraets DT, Klaustermeier JE, Lloveras B, et al. Human papillomavirus genotype attribution in invasive cervical cancer: a retrospective cross-sectional worldwide study. Lancet Oncol. 2010;11:1048–1056. doi: 10.1016/S1470-2045(10)70230-8. [DOI] [PubMed] [Google Scholar]
- 5.Barr E, Tamms G. Quadrivalent human papillomavirus vaccine. Clin Infect Dis. 2007;45:609–617. doi: 10.1086/520654. [DOI] [PubMed] [Google Scholar]
- 6.Siddiqui MA, Perry CM. Human papillomavirus quadrivalent (types 6, 11, 16, 18) recombinant vaccine (Gardasil) Drugs. 2006;66:1263–1271. doi: 10.2165/00003495-200666090-00008. [DOI] [PubMed] [Google Scholar]
- 7.Vandepapeliere P, Barrasso R, Meijer CJ, Walboomers JM, Wettendorff M, Stanberry LR, et al. Randomized controlled trial of an adjuvanted human papillomavirus (HPV) type 6 L2E7 vaccine: infection of external anogenital warts with multiple HPV types and failure of therapeutic vaccination. J Infect Dis. 2005;192:2099–2107. doi: 10.1086/498164. [DOI] [PubMed] [Google Scholar]
- 8.Giannini SL, Hanon E, Moris P, Van Mechelen M, Morel S, Dessy F, et al. Enhanced humoral and memory B cellular immunity using HPV16/18 L1 VLP vaccine formulated with the MPL/aluminium salt combination (AS04) compared to aluminium salt only. Vaccine. 2006;24:5937–5949. doi: 10.1016/j.vaccine.2006.06.005. [DOI] [PubMed] [Google Scholar]
- 9.Keam SJ, Harper DM. Human papillomavirus types 16 and 18 vaccine (recombinant, AS04 adjuvanted, adsorbed) [Cervarix] Drugs. 2008;68:359–372. doi: 10.2165/00003495-200868030-00007. [DOI] [PubMed] [Google Scholar]
- 10.Caulfield MJ, Shi L, Wang S, Wang B, Tobery TW, Mach H, et al. Effect of alternative aluminum adjuvants on the absorption and immunogenicity of HPV16 L1 VLPs in mice. Hum Vaccin. 2007;3:139–145. doi: 10.4161/hv.3.4.4309. [DOI] [PubMed] [Google Scholar]
- 11.Tovar JM, Bazaldua OV. New quadrivalent HPV vaccine developments. Postgrad Med. 2008;120:14–16. doi: 10.3810/pgm.2008.11.1929. [DOI] [PubMed] [Google Scholar]
- 12.De Carvalho N, Teixeira J, Roteli-Martins CM, Naud P, De Borba P, Zahaf T, et al. Sustained efficacy and immunogenicity of the HPV-16/18 AS04-adjuvanted vaccine up to 7.3 years in young adult women. Vaccine. 2010;28:6247–6255. doi: 10.1016/j.vaccine.2010.07.007. [DOI] [PubMed] [Google Scholar]
- 13.FUTURE II Study Group, author. Quadrivalent vaccine against human papillomavirus to prevent high-grade cervical lesions. N Engl J Med. 2007;356:1915–1927. doi: 10.1056/NEJMoa061741. [DOI] [PubMed] [Google Scholar]
- 14.Harper DM, Franco EL, Wheeler C, Ferris DG, Jenkins D, Schuind A, et al. Efficacy of a bivalent L1 virus-like particle vaccine in prevention of infection with human papillomavirus types 16 and 18 in young women: a randomised controlled trial. Lancet. 2004;364:1757–1765. doi: 10.1016/S0140-6736(04)17398-4. [DOI] [PubMed] [Google Scholar]
- 15.Harper DM, Franco EL, Wheeler CM, Moscicki AB, Romanowski B, Roteli-Martins CM, et al. Sustained efficacy up to 4.5 years of a bivalent L1 virus-like particle vaccine against human papillomavirus types 16 and 18: follow-up from a randomised controlled trial. Lancet. 2006;367:1247–1255. doi: 10.1016/S0140-6736(06)68439-0. [DOI] [PubMed] [Google Scholar]
- 16.Olsson SE, Villa LL, Costa RL, Petta CA, Andrade RP, Malm C, et al. Induction of immune memory following administration of a prophylactic quadrivalent human papillomavirus (HPV) types 6/11/16/18 L1 virus-like particle (VLP) vaccine. Vaccine. 2007;25:4931–4939. doi: 10.1016/j.vaccine.2007.03.049. [DOI] [PubMed] [Google Scholar]
- 17.Paavonen J, Naud P, Salmeron J, Wheeler CM, Chow SN, Apter D, et al. Efficacy of human papillomavirus (HPV)-16/18 AS04-adjuvanted vaccine against cervical infection and precancer caused by oncogenic HPV types (PATRICIA): final analysis of a double-blind, randomised study in young women. Lancet. 2009;374:301–314. doi: 10.1016/S0140-6736(09)61248-4. [DOI] [PubMed] [Google Scholar]
- 18.Villa LL, Costa RL, Petta CA, Andrade RP, Ault KA, Giuliano AR, et al. Prophylactic quadrivalent human papillomavirus (types 6, 11, 16 and 18) L1 virus-like particle vaccine in young women: a randomised double-blind placebo-controlled multicentre phase II efficacy trial. Lancet Oncol. 2005;6:271–278. doi: 10.1016/S1470-2045(05)70101-7. [DOI] [PubMed] [Google Scholar]
- 19.Villa LL, Costa RL, Petta CA, Andrade RP, Paavonen J, Iversen OE, et al. High sustained efficacy of a prophylactic quadrivalent human papillomavirus types 6/11/16/18 L1 virus-like particle vaccine through 5 years of follow-up. Br J Cancer. 2006;95:1459–1466. doi: 10.1038/sj.bjc.6603469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Brown DR, Kjaer SK, Sigurdsson K, Iversen OE, Hernandez-Avila M, Wheeler CM, et al. The impact of quadrivalent human papillomavirus (HPV; types 6, 11, 16 and 18) L1 virus-like particle vaccine on infection and disease due to oncogenic nonvaccine HPV types in generally HPV-naive women aged 16–26 years. J Infect Dis. 2009;199:926–935. doi: 10.1086/597307. [DOI] [PubMed] [Google Scholar]
- 21.Einstein MH, Baron M, Levin MJ, Chatterjee A, Edwards RP, Zepp F, et al. Comparison of the immunogenicity and safety of Cervarix™ and Gardasil® human papillomavirus (HPV) cervical cancer vaccines in healthy women aged 18–45 years. Hum Vaccin. 2009;5:705–719. doi: 10.4161/hv.5.10.9518. [DOI] [PubMed] [Google Scholar]
- 22.Einstein MH, Baron M, Levin M, Chatterjee A, Fox B, Scholar S, et al. Comparative immunogenicity and safety of Cervarix® and Gardasil® human papillomavirus (HPV) vaccines: Follow-up from Month 12–24 in a Phase III study of healthy women ages 18–45 years. Hum Vaccin. 2011;7 doi: 10.4161/hv.7.12.18281. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Romanowski B. Efficacy of the HPV-16/18 AS04-adjuvanted vaccine against non-vaccine oncogenic HPV types: end-of-study results; 26th International Papillomavirus Conference; July 3–8, 2010; Montreal, Canada. [Google Scholar]
- 24.Paavonen J, Jenkins D, Bosch FX, Naud P, Salmeron J, Wheeler CM, et al. Efficacy of a prophylactic adjuvanted bivalent L1 virus-like-particle vaccine against infection with human papillomavirus types 16 and 18 in young women: an interim analysis of a phase III double-blind, randomised controlled trial. Lancet. 2007;369:2161–2170. doi: 10.1016/S0140-6736(07)60946-5. [DOI] [PubMed] [Google Scholar]
- 25.Bosch FX, Burchell AN, Schiffman M, Giuliano AR, de Sanjose S, Bruni L, et al. Epidemiology and natural history of human papillomavirus infections and type-specific implications in cervical neoplasia. Vaccine. 2008;26:1–16. doi: 10.1016/j.vaccine.2008.05.064. [DOI] [PubMed] [Google Scholar]
- 26.Tokoyoda K, Zehentmeier S, Chang H, Radbruch A. Organization and maintenance of immunological memory by stroma niches. Eur J Immunol. 2009;39:2095–2099. doi: 10.1002/eji.200939500. [DOI] [PubMed] [Google Scholar]
- 27.Smith JF, Brownlow M, Brown M, Kowalski R, Esser MT, Ruiz W, et al. Antibodies from women immunized with Gardasil cross-neutralize HPV 45 pseudovirions. Hum Vaccin. 2007;3:109–115. doi: 10.4161/hv.3.4.4058. [DOI] [PubMed] [Google Scholar]
- 28.Kemp TJ, Hildesheim A, Safaeian M, Dauner JG, Pan Y, Porras C, et al. HPV16/18 L1 VLP vaccine induces cross-neutralizing antibodies that may mediate cross-protection. Vaccine. 2011;29:2011–2014. doi: 10.1016/j.vaccine.2011.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Moscicki AB, Wheeler C, Romanowski B, Hedrick J, Gall S, Ferris D, et al. Anamnestic response elicited by a fourth dose of the HPV-16/18 AS04-adjuvanted vaccine in young women; EUROGIN; February 17–20, 2010; Monte Carlo, Monaco. [Google Scholar]
- 30.Moscicki B, Wheeler C, Romanowski B, Hedrick J, Gall S, Ferris D, et al. Anamnestic response to non-vaccine types elicited by a fourth dose of the HPV-16/18 AS04-adjuvanted vaccine; EUROGIN; February 17–20, 2010; Monte Carlo, Monaco. [DOI] [PubMed] [Google Scholar]
- 31.Krajden M, Cook D, Yu A, Chow R, Mei W, McNeil S, et al. Human Papillomavirus 16 (HPV 16) and HPV 18 Antibody Responses Measured by Pseudovirus Neutralization and Competitive Luminex Assays in a Two- versus Three-Dose HPV Vaccine Trial. Clin Vaccine Immunol. 2011;18:418–423. doi: 10.1128/CVI.00489-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Dessy F, Poncelet S, Xhenseval V, Méric S, Dubin G, et al. Comparative evaluation of the immunogenicity of two prophylactic HPV cervical cancer vaccines by Merck's competitive Luminex immunoassay (cLIA) and GSK's binding ELISA; EUROGIN; February 17–20, 2010; Monte Carlo, Monaco. [Google Scholar]
- 33.Langley JM, Frenette L, Ferguson L, Riff D, Sheldon E, Risi G, et al. Safety and cross-reactive immunogenicity of candidate AS03-adjuvanted prepandemic H5N1 influenza vaccines: a randomized controlled phase 1/2 trial in adults. J Infect Dis. 2010;201:1644–1653. doi: 10.1086/652701. [DOI] [PubMed] [Google Scholar]
- 34.Leroux-Roels I, Borkowski A, Vanwolleghem T, Drame M, Clement F, Hons E, et al. Antigen sparing and cross-reactive immunity with an adjuvanted rH5N1 prototype pandemic influenza vaccine: a randomised controlled trial. Lancet. 2007;370:580–589. doi: 10.1016/S0140-6736(07)61297-5. [DOI] [PubMed] [Google Scholar]
- 35.Moris P, van der Most R, Leroux-Roels I, Clement F, Drame M, Hanon E, et al. H5N1 influenza vaccine formulated with AS03 A induces strong cross-reactive and polyfunctional CD4 T-cell responses. J Clin Immunol. 2011;31:443–454. doi: 10.1007/s10875-010-9490-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Didierlaurent AM, Morel S, Lockman L, Giannini SL, Bisteau M, Carlsen H, et al. AS04, an aluminum salt- and TLR4 agonist-based adjuvant system, induces a transient localized innate immune response leading to enhanced adaptive immunity. J Immunol. 2009;183:6186–6197. doi: 10.4049/jimmunol.0901474. [DOI] [PubMed] [Google Scholar]
- 37.Baldridge JR, McGowan P, Evans JT, Cluff C, Mossman S, Johnson D, et al. Taking a Toll on human disease: Toll-like receptor 4 agonists as vaccine adjuvants and monotherapeutic agents. Expert Opin Biol Ther. 2004;4:1129–1138. doi: 10.1517/14712598.4.7.1129. [DOI] [PubMed] [Google Scholar]
- 38.Tiberio L, Fletcher L, Eldridge JH, Duncan DD. Host factors impacting the innate response in humans to the candidate adjuvants RC529 and monophosphoryl lipid A. Vaccine. 2004;22:1515–1523. doi: 10.1016/j.vaccine.2003.10.019. [DOI] [PubMed] [Google Scholar]
- 39.de Villiers EM, Fauquet C, Broker TR, Bernard HU, zur Hausen H. Classification of papillomaviruses. Virology. 2004;324:17–27. doi: 10.1016/j.virol.2004.03.033. [DOI] [PubMed] [Google Scholar]
- 40.Nakagawa S, Yoshikawa H, Yasugi T, Kimura M, Kawana K, Matsumoto K, et al. Ubiquitous presence of E6 and E7 transcripts in human papillomavirus-positive cervical carcinomas regardless of its type. J Med Virol. 2000;62:251–258. doi: 10.1002/1096-9071(200010)62:2<251::AIDJMV18>3.0.CO;2-V. [DOI] [PubMed] [Google Scholar]
- 41.Deschuyteneer M, Elouahabi A, Plainchamp D, Plisnier M, Soete D, Corazza Y, et al. Molecular and structural characterization of the L1 virus-like particles that are used as vaccine antigens in Cervarix™, the AS04-adjuvanted HPV-16 and -18 cervical cancer vaccine. Hum Vaccin. 2010;6:407–419. doi: 10.4161/hv.6.5.11023. [DOI] [PubMed] [Google Scholar]
- 42.Bishop B, Dasgupta J, Klein M, Garcea RL, Christensen ND, Zhao R, et al. Crystal structures of four types of human papillomavirus L1 capsid proteins: understanding the specificity of neutralizing monoclonal antibodies. J Biol Chem. 2007;282:31803–31811. doi: 10.1074/jbc.M706380200. [DOI] [PubMed] [Google Scholar]
- 43.Combita AL, Touze A, Bousarghin L, Christensen ND, Coursaget P. Identification of two cross-neutralizing linear epitopes within the L1 major capsid protein of human papillomaviruses. J Virol. 2002;76:6480–6486. doi: 10.1128/JVI.76.13.6480-6.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Day PM, Kines RC, Thompson CD, Jagu S, Roden RB, Lowy DR, et al. In vivo mechanisms of vaccine-induced protection against HPV infection. Cell Host Microbe. 2010;8:260–270. doi: 10.1016/j.chom.2010.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Brendle SA, Culp TD, Broutian TR, Christensen ND. Binding and neutralization characteristics of a panel of monoclonal antibodies to human papillomavirus 58. J Gen Virol. 2010;91:1834–1839. doi: 10.1099/vir.0.017228-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Giannini S, Lockman L, Christensen ND, Brendle SA, Balogh KK, Mossman S. HPV L1 VLP-based vaccines formulated with AS04 induce cross-protection against infection and disease in the rabbit HPV quasivirion challenge method; International. Papillomavirus Conference; Montreal, Canada. 2010. p. 193. [Google Scholar]
- 47.Schiller JT, Day PM, Kines RC. Current understanding of the mechanism of HPV infection. Gynecol Oncol. 2010;118:12–17. doi: 10.1016/j.ygyno.2010.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Fahey LM, Raff AB, Da Silva DM, Kast WM. A major role for the minor capsid protein of human papillomavirus type 16 in immune escape. J Immunol. 2009;183:6151–6156. doi: 10.4049/jimmunol.0902145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Fausch SC, Fahey LM, Da Silva DM, Kast WM. Human papillomavirus can escape immune recognition through Langerhans cell phosphoinositide-3-kinase activation. J Immunol. 2005;174:7172–7178. doi: 10.4049/jimmunol.174.11.7172. [DOI] [PubMed] [Google Scholar]
- 50.Horowitz A, Behrens RH, Okell L, Fooks AR, Riley EM. NK cells as effectors of acquired immune responses: effector CD4+ T cell-dependent activation of NK cells following vaccination. J Immunol. 2010;185:2808–2818. doi: 10.4049/jimmunol.1000844. [DOI] [PubMed] [Google Scholar]
- 51.Kenter GG, Welters MJ, Valentijn AR, Lowik MJ, Berends-van der Meer DM, Vloon AP, et al. Vaccination against HPV-16 oncoproteins for vulvar intraepithelial neoplasia. N Engl J Med. 2009;361:1838–1847. doi: 10.1056/NEJMoa0810097. [DOI] [PubMed] [Google Scholar]
- 52.Coleman N, Birley HD, Renton AM, Hanna NF, Ryait BK, Byrne M, et al. Immunological events in regressing genital warts. Am J Clin Pathol. 1994;102:768–774. doi: 10.1093/ajcp/102.6.768. [DOI] [PubMed] [Google Scholar]
- 53.Dessy FJ, Giannini SL, Bougelet CA, Kemp TJ, David MP, Poncelet SM, et al. Correlation between direct ELISA, single-epitope based inhibition ELISA and pseudovirion-based neutralization assay for measuring anti-HPV-16 and anti-HPV-18 antibody response after vaccination with the AS04-adjuvanted HPV-16/18 cervical cancer vaccine. Hum Vaccin. 2008;4:425–434. doi: 10.4161/hv.4.6.6912. [DOI] [PubMed] [Google Scholar]
- 54.Schiffman M, Wentzensen N. From human papillomavirus to cervical cancer. Obstet Gynecol. 2010;116:177–185. doi: 10.1097/AOG.0b013e3181e4629f. [DOI] [PubMed] [Google Scholar]
- 55.Schiffman M, Herrero R, DeSalle R, Hildesheim A, Wacholder S, Rodriguez AC, et al. The carcinogenicity of human papillomavirus types reflects viral evolution. Virology. 2005;337:76–84. doi: 10.1016/j.virol.2005.04.002. [DOI] [PubMed] [Google Scholar]