Abstract
The disulfide bond structures established decades ago for immunoglobulins have been challenged by findings from extensive characterization of recombinant and human monoclonal IgG antibodies. Non-classical disulfide bond structure was first identified in IgG4 and later in IgG2 antibodies. Although, cysteine residues should be in the disulfide bonded states, free sulfhydryls have been detected in all subclasses of IgG antibodies. In addition, disulfide bonds are susceptible to chemical modifications, which can further generate structural variants such as IgG antibodies with trisulfide bond or thioether linkages. Trisulfide bond formation has also been observed for IgG of all subclasses. Degradation of disulfide bond through β-elimination generates free sulfhydryls disulfide and dehydroalanine. Further reaction between free sulfhydryl and dehydroalanine leads to the formation of a non-reducible cross-linked species. Hydrolysis of the dehydroalanine residue contributes substantially to antibody hinge region fragmentation. The effect of these disulfide bond variations on antibody structure, stability and biological function are discussed in this review.
Key words: recombinant monoclonal antibody, disulfide bond, trisulfide bond, free sulfhydryl, dehydroalanine, thioether, aggregation
Introduction
The recombinant monoclonal IgG antibodies comprise a rapidly growing group of protein therapeutics. The disulfide bond structure of IgG is highly conserved through evolution and was once considered a uniform and homogeneous structural feature. However, detailed characterization of a large number of IgG molecules has revealed several new structural features in both recombinant and natural human IgG antibodies. These new findings and their effects on IgG structure, stability and biological function are reviewed here.
Classical Disulfide Bond Structures
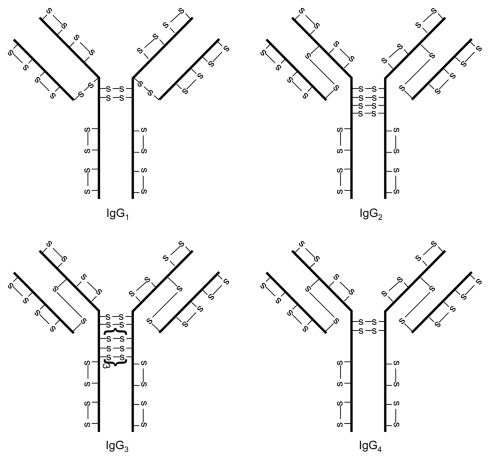
Disulfide bond structures of the four subclasses of IgG were established in the 1960s.1–8 These disulfide bond structures are referred to as the classical disulfide bond structures because they are widely accepted. As shown in Figure 1, there are many similarities and some differences with regard to the disulfide bond structures in the four subclasses of IgG antibodies, IgG1, IgG2, IgG3 and IgG4. Each IgG contains a total of 12 intra-chain disulfide bonds; each disulfide bond is associated with an individual IgG domain. The two heavy chains are connected in the hinge region by a variable number of disulfide bonds: 2 for IgG1 and IgG4, 4 for IgG2 and 11 for IgG3. The light chain of the IgG1 is connected to the heavy chain by a disulfide bond between the last cysteine residue of the light chain and the fifth cysteine residue of the heavy chain. However, for IgG2, IgG3 and IgG4, the light chain is linked to the heavy chain by a disulfide bond between the last cysteine residue of the light chain and the third cysteine residue of the heavy chain.
Figure 1.
Classical IgG disulfide bond structures.
The level of solvent exposure is different between intra-chain and inter-chain disulfide bonds. Cysteine residues that form inter-chain disulfide bonds are located in the hinge region with the exception of the third cysteine residue of the heavy chain in IgG2, IgG3 and IgG4, which is located between the interface of VH and CH1 domains.9 Therefore, inter-chain disulfide bonds are highly solvent exposed.9–12 On the other hand, intra-chain disulfide bonds are buried between the two layers of anti-parallel β-sheet structures within each domain and are not solvent exposed.9–12 The solvent exposure difference has important implications because exposed cysteine residues are considered more reactive than non-exposed cysteine residues.
Non-Classical Linkage
Disulfide bond structures other than the classical structures shown in Figure 1 have been observed mainly for IgG2 and IgG4, but not for IgG1 and IgG3. Only a trace amount of a disulfide bond variant with the two inter heavy chain disulfide bonds in the intra-chain form for IgG1 has been observed.13 IgG3 has repeated amino acid sequence in the hinge region and a total of 11 disulfide bonds in close proximity, which does not allow much flexibility for formation of disulfide bond variants.
Non-classical disulfide bond structures of IgG2 were first identified in recombinant monoclonal antibodies (mAbs) and then confirmed in human IgG2 molecules.14–16 In these publications, the classical disulfide bond structure was referred to as IgG2A, while the two major non-classical structures were referred to as IgG2B and IgG2-A/B, the latter being considered a structural intermediate between IgG2A and IgG2B (Fig. 2). Distribution of different disulfide bond isoforms is dependent on the type of light chain, IgG2A is the major form in molecules with λ light chain; IgG2B is the major form in molecules with κ light chain.15 A conversion from the IgG2A form to IgG2B was observed during cell culture, in vitro incubation with serum and in patient serum.17 Molecular dynamic simulation study revealed that the sulfur atoms of inter-chain disulfide bonds are highly mobile and can be in close proximity.18 Therefore, it is not a surprise to observe the coexistence of multiple disulfide bond isoforms for IgG2 antibodies. In addition to isoforms from different intra-molecule disulfide bond linkages, disulfide bond linked IgG2 dimer was also found in recombinant IgG2 from cell culture and in human serum.19
Figure 2.
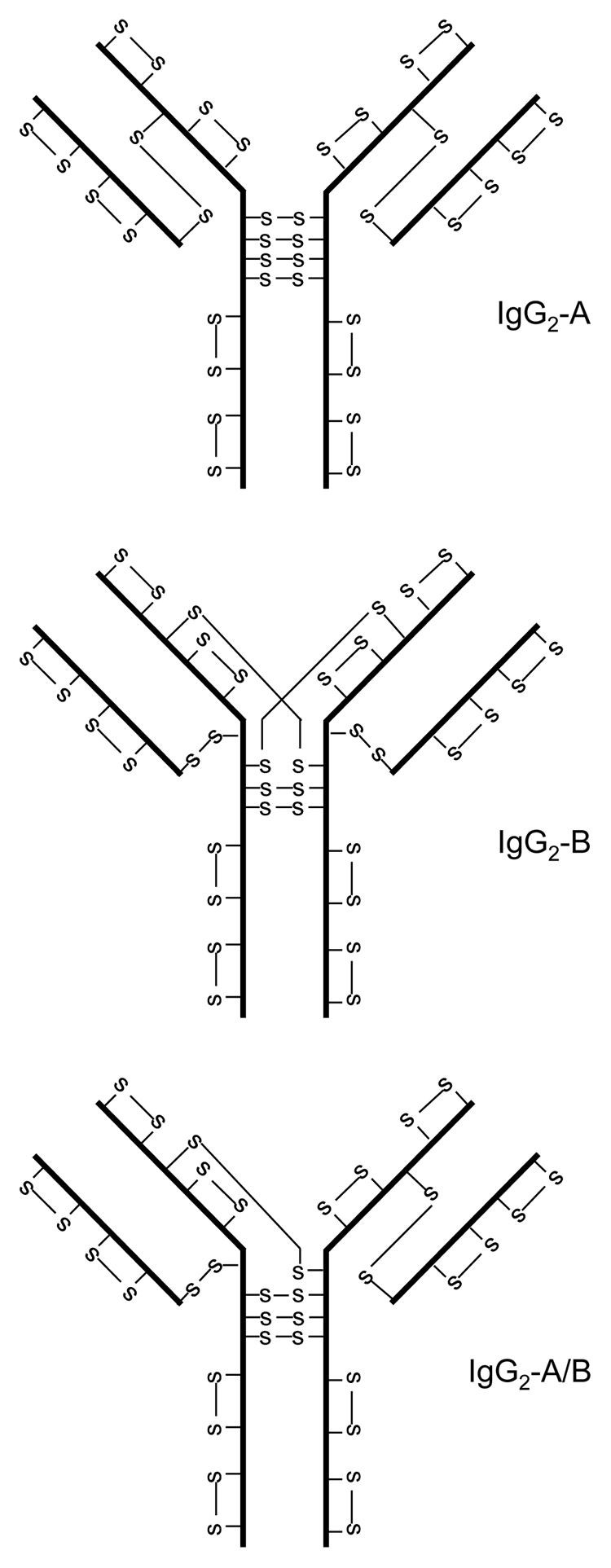
IgG2 disulfide bond isoforms.
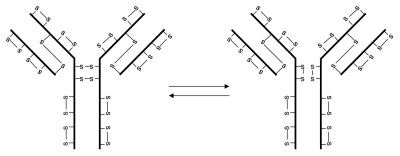
By far, IgG4 is the best known subclass of IgG molecule having non-classical disulfide bond structures (Fig. 3). Several interesting observations led to the ultimate finding of the non-classical disulfide bond structures. First, significant amounts of IgG4 were observed as half-molecules when analyzed by non-reducing sodium dodecyl sulfate-poly acrylamide gel electrophoresis (SDS-PAGE),20–24 but not by size-exclusion chromatography (SEC) run under native conditions,23 indicating that the two half-molecules are associated by non-covalent interactions rather than by covalent linkage. Second, polyclonal IgG4 is unable to cross-link antigen and behaves like a monovalent antibody,25 while monoclonal IgG4 can cross-link antigens.26 Third, IgG4 as a covalent-linked monomer demonstrates bispecificity in plasma.26 These observations were explained by the fact that the two inter heavy chain disulfide bonds of IgG4 are in equilibrium with intra-chain disulfide bond forms.13,23,24 IgG1 and IgG4 differ by one amino acid in the middle hinge region, i.e., there are two proline residues in IgG1 and a serine and a proline residue in IgG4. Stable inter heavy chain disulfide bonds of IgG4 were obtained by replacing the serine residue with a proline residue.13,23,24 Because of the instability of the inter heavy chain disulfide bonds, bispecific antibody can be formed in vitro in the presence of reducing reagents and in vivo by injection of equal amounts of two recombinant IgG4 antibodies specific for two different antigens into immunodeficient mice.27
Figure 3.
IgG4 disulfide bond isoforms.
Free Sulfhydryls
Presumably, all cysteine residues in IgG are in the disulfide bonded state. However, free sulfhydryls has been routinely detected in IgG molecules, including IgG from serum and recombinant mAbs (Table 1).28–35 It is worthwhile to discuss two important observations in Table 1. First, higher level of free sulfhydryls was detected under denaturing conditions compared with native conditions. This indicates that free sulfhydryl is associated with cysteine residues involved in both inter and intra chain disulfide bonds. Second, there is a large variation in the levels of free sulfhydryl under denaturing conditions among different studies. This large variation is likely due to a combination of multiple factors, including different IgG types (human or recombinant), different IgG subclasses and experimental variations, e.g., denaturing reagents and denaturing times, which may vary for different IgGs to be fully denatured. Degradation of disulfide bonds that produces free sulfhydryls may occur, as will be discussed later.
Table 1.
Level of free sulfhydryl in IgG
| Mole of free SH/Mole of IgG | |||
| Type of IgG | Reference | ||
| Native | Denatured | ||
| Human IgG2 | 0.24 | ND* | 28, 29 |
| Human IgG1 | 0.1–1.1 | 0.6–4.0 | 30 |
| Human IgG1, IgG4 and recombinant IgG4 | ND* | 0.9–2.2 | 31 |
| Recombinant IgG1, IgG2 and IgG4 | 0.02–0.08 | 0.08–0.09 | 32 |
| Recombinant IgG1 | ND* | 0.64 | 33 |
| Recombinant IgG2 | 0.06 | 0.59 | 34 |
| Recombinant IgG2 | 0.158 | 0.379 | 35 |
ND*, not determined.
While IgG molecules may have different levels of free sulfhydryl, studies suggested a similar distribution of free sulfhydryl among the domain structures, at least for recombinant IgG1.36,37 The variable domain has a higher level of free sulfhydryls than that in the constant domain in the light chain. The CH3 domain has the highest level of free sulfhydryls followed by CH1, CH2 and the variable domain in the heavy chain. The lowest level of free sulfhydryls is associated with inter-chain disulfide bonds, suggesting that low level of free sulfhydryls is most likely due to incomplete formation of disulfide bonds. Because inter-chain disulfide bonds with higher solvent exposure level are more prone to degradation than intra-chain disulfide bonds, higher level of free sulfhydryls associated with inter-chain disulfide bonds is expected if free sulfhydryl is generated due to disulfide bond degradation. Distribution other than described above may indicate special cases where particular disulfide bonds are not efficiently formed.
Two special cases have been reported in the literature so far. In both cases, the intra-chain disulfide bond in the heavy chain variable domain is not completely formed at such a level that antibodies with this incomplete disulfide bond were detected by hydrophobic interaction or weak cation exchange chromatography.38,39 Complete formation of this particular disulfide bond can be achieved by the addition of copper sulfate to cell culture,40 suggesting that cell culture conditions can affect disulfide bond formation. Antibodies after in vitro incubation in serum or recovered from rat serum after administration showed significant reduction in incomplete disulfide bond formation.39
β-Elimination
Under basic conditions, disulfide bonds can decompose through the β-elimination mechanism with the formation of dehydroalanine and persulfide, which can further revert back to a cysteine residue.41–43 Degradation of the inter light chain and heavy chain disulfide bond of IgG through the β-elimination mechanism followed by cross-linking of the resulting cysteine and dehydroalanine has led to the formation of a non-reducible thioether linkage,44 which was found at ∼0.4% for a recombinant monoclonal IgG1 stored at 4°C and up to 13.6% for a heat-stressed sample. Subsequent hydrolysis of the dehydroalanine is another important mechanism in addition to peptide bond hydrolysis that leads to antibody fragmentation in the hinge region.45
Trisulfide Bond Formation
Trisulfide bonding formation is a rare post-translational modification of proteins. The presence of trisulfide bonding was first reported for a recombinant monoclonal IgG2, where one or two of the four inter heavy chain disulfide bonds may exist as a trisulfide bond.46 Trisulfide bonds were later detected in all subclasses of recombinant IgG antibodies, as well as in human IgG from patients with myeloma.47 In all cases, higher levels of trisulfide bonds were observed between the cysteine residues that normally form the inter light chain and heavy chain disulfide bonds.47 Trisulfide bonds in recombinant mAbs are believed to be formed during fermentation as a result of the reaction of an intact disulfide bond with dissolved hydrogen sulfide (H2S).46,47 This conclusion is supported by the observation that incubation of IgG with H2S resulted in higher levels of trisulfide bonding.47 Substantial conversion of trisulfide bonds to disulfide bonds was observed when a recombinant IgG2 antibody was incubated with various reducing reagents at pH 7.5.46 In another study, it was found that trisulfide bonds were stable in buffers at pH 6.5 and in rat serum in vitro. However, complete conversion of the trisulfide bond to a disulfide bond was achieved when a recombinant IgG1 was recovered from rat serum 24 h after intraperitoneal injection.47 It is hypothesized that trisulfide bond is formed through several reaction intermediates produced by the initial nucleophilic attack of disulfide bond by HS- under an appropriate redox condition.48
Structure, Stability and Functions
Information about the structure, stability and functional impacts of non-classical linkage and trisulfide bond is limited. The non-classical structure of the IgG2B is more compact than that of IgG2A, as determined by SEC and analytical ultracentrifugation.15 IgG2A was shown to have either similar or higher binding affinity and biological activity than IgG2B.15,16 Studies determined that the presence of trisulfide bonds does not appear to affect the thermal stability or antigen binding.46,47 Limited information on IgG4 isoforms mainly comes from mutagenesis studies. Two mutants, one replacing the first middle hinge cysteine with a serine and the other replacing the serine in the middle hinge with a proline, resulted in more stable IgG4 molecules without affecting antigen binding activity.24 In a separate study, replacing the middle hinge serine with proline resulted in a more stable inter-chain disulfide bond and increased half-life, again without affecting antigen binding activity.23
The effect of free sulfhydryl on the structure, stability and biological functions of IgG has been studied using individual domains, as well as intact IgG molecules. Individual domains of CL domain,12 CH3 domain49,50 and single-chain variable fragment51 without the complete intra-chain disulfide bond showed lower stability, but no substantial structural changes. It is expected that the lack of intra-chain disulfide bond in other domains will have similar destabilizing effect because all IgG domain share similar folding.52 Incomplete formation of the disulfide bond in the heavy chain variable domain of a recombinant monoclonal antibody resulted in a significant decrease in potency.38,39 A natural antibody derived from the ABPC48 mouse plasmacytoma, in which the second cysteine residue in the heavy chain variable domain was replaced by a tyrosine residue, is capable of binding antigen,53 suggesting further that a complete disulfide bond is not a prerequisite for antigen binding. Higher amounts of free sulfhydryl resulted in lower thermal stability of both recombinant and human IgG antibodies.31 In addition, the higher aggregation propensity of IgG2 compared with IgG1 is also attributed to higher level of free sulfhydryl of IgG2.35
Partial reduction has been one of the commonly used methods to study the effect of inter-chain disulfide bond on the structure, stability and biological functions of IgG. Although a global conformational change was not observed,12,52,54–59 partial reduction increased the flexibility of the hinge region, probably as a result of reduction of inter-chain disulfide bonds, resulting in further separation of the two CH2 domains.52,55,56,60 An apparent increase in the hydrodynamic sizes of human IgG1, IgG2 and IgG4, but decreased size for IgG3 were also observed upon partial reduction and alkylation, which is again attributed to the structural change in the hinge region and CH2 domain.61 Highly dependent on the experimental conditions,62 partial reduction either has no impact59,63 or reduces complement activation efficiency.64–66 The effect of partial reduction on binding to Fc receptors and, consequently, antibody-dependent cell-mediated cytotoxicity (ADCC) is also not consistent, e.g., no effect67,68 and significantly reduced activity59,69,70 were observed for different antibodies. One of the critical issues is the degree of reduction of the intra-chain disulfide bonds in different studies. It has been reported that only inter-chain disulfide bonds of human IgG1 are susceptible to reduction under native conditions.71 However, reduction of intra-chain disulfide bonds of rabbit IgG under native conditions may be possible.62,64
Although levels of free sulfhydryls appear to be low, their presence poses some challenges for recombinant monoclonal antibody formulation. It was found that the majority of the IgG1 dimer is formed due to formation of intermolecular disulfide bonds,72 which could result from free sulfhydryls. Dimerization through disulfide bond formation is the major aggregation pathway for a recombinant monoclonal IgG2 antibody at pH 6.0 after heat stress.73 A substantial amount of covalently linked aggregates formed via disulfide bonds of an IgG2 was also found in the aggregates caused by agitation.34 It is possible that antibodies with incomplete disulfide bonds are more susceptible to unfolding under various stress conditions and, therefore, have a higher propensity for covalent aggregation through disulfide bond formation. IgG antibodies with higher levels of free sulfhydryls also have a greater tendency to expose hydrophobic regions, which can drive the formation of non-covalent aggregates through inter-molecule hydrophobic interactions.
Conclusion
Heterogeneity is a common feature of recombinant mAbs as a result of post-translational modifications and variation related specifically to disulfide bond structures is a potential major contributor to heterogeneity. It has been clearly demonstrated that intra-chain disulfide bonds are in general more stable after antibody assembly. Therefore, the low levels of free sulfhydryl associated with these intra-chain disulfide bonds are probably due to incomplete formation of disulfide bonds. On the other hand, inter-chain disulfide bonds are exposed and less stable, which explains why increased heterogeneity is associated with these bonds. It is thus reasonable to hypothesize that non-classical disulfide bond structures, trisulfide bonding and thioether linkages formation may occur after antibody assembly.
Close attention should be paid to these new disulfide bond-related structures during the development of recombinant mAbs because changes in structures and stability have been observed. Theoretically, administration of non-native disulfide bonded structures to humans has the potential to trigger immune response. Lowering stability can also ultimately lead to non-native structures because of the higher propensity to unfold and form aggregates. More experiments are thus warranted to improve understanding of the effects of disulfide bond related structural variants on the stability, structure and biological functions of IgG molecules.
Acknowledgments
We thank Larry Dick, Huijuan Li and Yi Du for their critical review of this manuscript.
References
- 1.Milstein C. The disulphide bridges of immunoglobulin kappa-chains. Biochem J. 1966;101:338–351. doi: 10.1042/bj1010338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Pink JR, Milstein C. Inter heavy-light chain disulphide bridge in immune globulins. Nature. 1967;214:92–94. doi: 10.1038/214092a0. [DOI] [PubMed] [Google Scholar]
- 3.Frangione B, Milstein C. Disulphide bridges of immunoglobin G-1 heavy chains. Nature. 1967;216:939–941. doi: 10.1038/216939b0. [DOI] [PubMed] [Google Scholar]
- 4.Pink JR, Milstein C. Disulphide bridges of a human immunoglobulin G protein. Nature. 1967;216:941–942. doi: 10.1038/216941a0. [DOI] [PubMed] [Google Scholar]
- 5.Frangione B, Milstein C, Franklin EC. Intrachain disulphide bridges in immunoglobulin G heavy chains. The Fc fragment. Biochem J. 1968;106:15–21. doi: 10.1042/bj1060015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Frangione B, Milstein C. Variations in the S-S bridges of immunoglobins G: interchain disulfide bridges of gammaG3 myeloma proteins. J Mol Biol. 1968;33:893–906. doi: 10.1016/0022-2836(68)90326-4. [DOI] [PubMed] [Google Scholar]
- 7.Edelman GM, Cunningham BA, Gall WE, Gottlieb PD, Rutishauser U, Waxdal MJ. The covalent structure of an entire gammaG immunoglobulin molecule. Proc Natl Acad Sci USA. 1969;63:78–85. doi: 10.1073/pnas.63.1.78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Frangione B, Milstein C, Pink JR. Structural studies of immunoglobulin G. Nature. 1969;221:145–148. doi: 10.1038/221145a0. [DOI] [PubMed] [Google Scholar]
- 9.Lefranc MP, Pommie C, Kaas Q, Duprat E, Bosc N, Guiraudou D, et al. IMGT unique numbering for immunoglobulin and T cell receptor constant domains and Ig superfamily C-like domains. Dev Comp Immunol. 2005;29:185–203. doi: 10.1016/j.dci.2004.07.003. [DOI] [PubMed] [Google Scholar]
- 10.Lefranc MP, Pommie C, Ruiz M, Giudicelli V, Foulquier E, Truong L, et al. IMGT unique numbering for immunoglobulin and T cell receptor variable domains and Ig superfamily V-like domains. Dev Comp Immunol. 2003;27:55–77. doi: 10.1016/S0145-305X(02)00039-3. [DOI] [PubMed] [Google Scholar]
- 11.Amzel LM, Poljak RJ. Three-dimensional structure of immunoglobulins. Annu Rev Biochem. 1979;48:961–997. doi: 10.1146/annurev.bi.48.070179.004525. [DOI] [PubMed] [Google Scholar]
- 12.Kikuchi H, Goto Y, Hamaguchi K. Reduction of the buried intrachain disulfide bond of the constant fragment of the immunoglobulin light chain: global unfolding under physiological conditions. Biochemistry. 1986;25:2009–2013. doi: 10.1021/bi00356a026. [DOI] [PubMed] [Google Scholar]
- 13.Bloom JW, Madanat MS, Marriott D, Wong T, Chan SY. Intrachain disulfide bond in the core hinge region of human IgG4. Protein Sci. 1997;6:407–415. doi: 10.1002/pro.5560060217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wypych J, Li M, Guo A, Zhang Z, Martinez T, Allen MJ, et al. Human IgG2 antibodies display disulfide-mediated structural isoforms. J Biol Chem. 2008;283:16194–16205. doi: 10.1074/jbc.M709987200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dillon TM, Ricci MS, Vezina C, Flynn GC, Liu YD, Rehder DS, et al. Structural and functional characterization of disulfide isoforms of the human IgG2 subclass. J Biol Chem. 2008;283:16206–16215. doi: 10.1074/jbc.M709988200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Martinez T, Guo A, Allen MJ, Han M, Pace D, Jones J, et al. Disulfide connectivity of human immunoglobulin G2 structural isoforms. Biochemistry. 2008;47:7496–7508. doi: 10.1021/bi800576c. [DOI] [PubMed] [Google Scholar]
- 17.Liu YD, Chen X, Enk JZ, Plant M, Dillon TM, Flynn GC. Human IgG2 antibody disulfide rearrangement in vivo. J Biol Chem. 2008;283:29266–29272. doi: 10.1074/jbc.M804787200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wang X, Kumar S, Singh S. Disulfide bond scrambling in IgG2 monoclonal antibodies: insights from molecular dynamics simulations. Pharm Res. 2011 doi: 10.1007/s11095-011-0503-9. [DOI] [PubMed] [Google Scholar]
- 19.Yoo EM, Wims LA, Chan LA, Morrison SL. Human IgG2 can form covalent dimers. J Immunol. 2003;170:3134–3138. doi: 10.4049/jimmunol.170.6.3134. [DOI] [PubMed] [Google Scholar]
- 20.Petersen JG, Dorrington KJ. An in vitro system for studying the kinetics of interchain disulfide bond formation in immunoglobulin G. J Biol Chem. 1974;249:5633–5641. [PubMed] [Google Scholar]
- 21.Colcher D, Milenic D, Roselli M, Raubitschek A, Yarranton G, King D, et al. Characterization and biodistribution of recombinant and recombinant/chimeric constructs of monoclonal antibody B72.3. Cancer Res. 1989;49:1738–1745. [PubMed] [Google Scholar]
- 22.King DJ, Adair JR, Angal S, Low DC, Proudfoot KA, Lloyd JC, et al. Expression, purification and characterization of a mouse-human chimeric antibody and chimeric Fab' fragment. Biochem J. 1992;281:317–323. doi: 10.1042/bj2810317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Angal S, King DJ, Bodmer MW, Turner A, Lawson AD, Roberts G, et al. A single amino acid substitution abolishes the heterogeneity of chimeric mouse/human (IgG4) antibody. Mol Immunol. 1993;30:105–108. doi: 10.1016/0161-5890(93)90432-B. [DOI] [PubMed] [Google Scholar]
- 24.Schuurman J, Perdok GJ, Gorter AD, Aalberse RC. The inter-heavy chain disulfide bonds of IgG4 are in equilibrium with intra-chain disulfide bonds. Mol Immunol. 2001;38:1–8. doi: 10.1016/S0161-5890(01)00050-5. [DOI] [PubMed] [Google Scholar]
- 25.van der Zee JS, van Swieten P, Aalberse RC. Serologic aspects of IgG4 antibodies. II. IgG4 antibodies form small, nonprecipitating immune complexes due to functional monovalency. J Immunol. 1986;137:3566–3571. [PubMed] [Google Scholar]
- 26.Schuurman J, Van Ree R, Perdok GJ, Van Doorn HR, Tan KY, Aalberse RC. Normal human immunoglobulin G4 is bispecific: it has two different antigen-combining sites. Immunology. 1999;97:693–698. doi: 10.1046/j.1365-2567.1999.00845.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.van der NeutKolfschoten M, Schuurman J, Losen M, Bleeker WK, Martinez-Martinez P, Vermeulen E, et al. Anti-inflammatory activity of human IgG4 antibodies by dynamic Fab arm exchange. Science. 2007;317:1554–1557. doi: 10.1126/science.1144603. [DOI] [PubMed] [Google Scholar]
- 28.Schauenstein E, Sorger S, Reiter M, Dachs F. Free thiol groups and labile disulfide bonds in the IgG fraction of human serum. J Immunol Methods. 1982;50:51–56. doi: 10.1016/0022-1759(82)90303-9. [DOI] [PubMed] [Google Scholar]
- 29.Schauenstein E, Dachs F, Reiter M, Gombotz H, List W. Labile disulfide bonds and free thiol groups in human IgG. I. Assignment to IgG1 and IgG2 subclasses. Int Arch Allergy Appl Immunol. 1986;80:174–179. doi: 10.1159/000234048. [DOI] [PubMed] [Google Scholar]
- 30.Gevondyan NM, Volynskaia AM, Gevondyan VS. Four free cysteine residues found in human IgG1 of healthy donors. Biochemistry (Mosc) 2006;71:279–284. doi: 10.1134/S0006297906030072. [DOI] [PubMed] [Google Scholar]
- 31.Lacy ER, Baker M, Brigham-Burke M. Free sulfhydryl measurement as an indicator of antibody stability. Anal Biochem. 2008;382:66–68. doi: 10.1016/j.ab.2008.07.016. [DOI] [PubMed] [Google Scholar]
- 32.Zhang W, Czupryn MJ. Free sulfhydryl in recombinant monoclonal antibodies. Biotechnol Prog. 2002;18:509–513. doi: 10.1021/bp025511z. [DOI] [PubMed] [Google Scholar]
- 33.Liu H, Gaza-Bulseco G, Chumsae C, Newby-Kew A. Characterization of lower molecular weight artifact bands of recombinant monoclonal IgG1 antibodies on non-reducing SDS-PAGE. Biotechnol Lett. 2007;29:1611–1622. doi: 10.1007/s10529-007-9449-8. [DOI] [PubMed] [Google Scholar]
- 34.Brych SR, Gokarn YR, Hultgen H, Stevenson RJ, Rajan R, Matsumura M. Characterization of antibody aggregation: role of buried, unpaired cysteines in particle formation. J Pharm Sci. 2010;99:764–781. doi: 10.1002/jps.21868. [DOI] [PubMed] [Google Scholar]
- 35.Franey H, Brych SR, Kolvenbach CG, Rajan RS. Increased aggregation propensity of IgG2 subclass over IgG1: role of conformational changes and covalent character in isolated aggregates. Protein Sci. 2010;19:1601–1615. doi: 10.1002/pro.434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chumsae C, Gaza-Bulseco G, Liu H. Identification and localization of unpaired cysteine residues in monoclonal antibodies by fluorescence labeling and mass spectrometry. Anal Chem. 2009;81:6449–6457. doi: 10.1021/ac900815z. [DOI] [PubMed] [Google Scholar]
- 37.Xiang T, Chumsae C, Liu H. Localization and quantitation of free sulfhydryl in recombinant monoclonal antibodies by differential labeling with 12C and 13C iodoacetic acid and LC-MS analysis. Anal Chem. 2009;81:8101–8108. doi: 10.1021/ac901311y. [DOI] [PubMed] [Google Scholar]
- 38.Harris RJ. Heterogeneity of recombinant antibodies: linking structure to function. Dev Biol (Basel) 2005;122:117–127. [PubMed] [Google Scholar]
- 39.Ouellette D, Alessandri L, Chin A, Grinnell C, Tarcsa E, Radziejewski C, et al. Studies in serum support rapid formation of disulfide bond between unpaired cysteine residues in the VH domain of an immunoglobulin G1 molecule. Anal Biochem. 2010;397:37–47. doi: 10.1016/j.ab.2009.09.027. [DOI] [PubMed] [Google Scholar]
- 40.Chaderjian WB, Chin ET, Harris RJ, Etcheverry TM. Effect of copper sulfate on performance of a serum-free CHO cell culture process and the level of free thiol in the recombinant antibody expressed. Biotechnol Prog. 2005;21:550–553. doi: 10.1021/bp0497029. [DOI] [PubMed] [Google Scholar]
- 41.Nashef AS, Osuga DT, Lee HS, Ahmed AI, Whitaker JR, Feeney RE. Effects of alkali on proteins. Disulfides and their products. J Agric Food Chem. 1977;25:245–251. doi: 10.1021/mjf60210a020. [DOI] [PubMed] [Google Scholar]
- 42.Florence TM. Degradation of protein disulphide bonds in dilute alkali. Biochem J. 1980;189:507–520. doi: 10.1042/bj1890507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Galande AK, Trent JO, Spatola AF. Understanding base-assisted desulfurization using a variety of disulfide-bridged peptides. Biopolymers. 2003;71:534–551. doi: 10.1002/bip.10532. [DOI] [PubMed] [Google Scholar]
- 44.Tous GI, Wei Z, Feng J, Bilbulian S, Bowen S, Smith J, et al. Characterization of a novel modification to monoclonal antibodies: thioether cross-link of heavy and light chains. Anal Chem. 2005;77:2675–2682. doi: 10.1021/ac0500582. [DOI] [PubMed] [Google Scholar]
- 45.Cohen SL, Price C, Vlasak J. Beta-elimination and peptide bond hydrolysis: two distinct mechanisms of human IgG1 hinge fragmentation upon storage. J Am Chem Soc. 2007;129:6976–6977. doi: 10.1021/ja0705994. [DOI] [PubMed] [Google Scholar]
- 46.Pristatsky P, Cohen SL, Krantz D, Acevedo J, Ionescu R, Vlasak J. Evidence for trisulfide bonds in a recombinant variant of a human IgG2 monoclonal antibody. Anal Chem. 2009;81:6148–6155. doi: 10.1021/ac9006254. [DOI] [PubMed] [Google Scholar]
- 47.Gu S, Wen D, Weinreb PH, Sun Y, Zhang L, Foley SF, et al. Characterization of trisulfide modification in antibodies. Anal Biochem. 2010;400:89–98. doi: 10.1016/j.ab.2010.01.019. [DOI] [PubMed] [Google Scholar]
- 48.Nielsen RW, Tachibana C, Hansen N, Winther J. Trisulfides in proteins. Antioxid Redox Signal. 2011;15:67–75. doi: 10.1089/ars.2010.3677. [DOI] [PubMed] [Google Scholar]
- 49.McAuley A, Jacob J, Kolvenbach CG, Westland K, Lee HJ, Brych SR, et al. Contributions of a disulfide bond to the structure, stability and dimerization of human IgG1 antibody CH3 domain. Protein Sci. 2008;17:95–106. doi: 10.1110/ps.073134408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Thies MJ, Mayer J, Augustine JG, Frederick CA, Lilie H, Buchner J. Folding and association of the antibody domain CH3: prolyl isomerization preceeds dimerization. J Mol Biol. 1999;293:67–79. doi: 10.1006/jmbi.1999.3128. [DOI] [PubMed] [Google Scholar]
- 51.Proba K, Honegger A, Pluckthun A. A natural antibody missing a cysteine in VH: consequences for thermodynamic stability and folding. J Mol Biol. 1997;265:161–172. doi: 10.1006/jmbi.1996.0726. [DOI] [PubMed] [Google Scholar]
- 52.Burton DR. Immunoglobulin G: functional sites. Mol Immunol. 1985;22:161–206. doi: 10.1016/0161-5890(85)90151-8. [DOI] [PubMed] [Google Scholar]
- 53.Rudikoff S, Pumphrey JG. Functional antibody lacking a variable-region disulfide bridge. Proc Natl Acad Sci USA. 1986;83:7875–7878. doi: 10.1073/pnas.83.20.7875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Goto Y, Hamaguchi K. The role of the intrachain disulfide bond in the conformation and stability of the constant fragment of the immunoglobulin light chain. J Biochem. 1979;86:1433–1441. doi: 10.1093/oxfordjournals.jbchem.a132661. [DOI] [PubMed] [Google Scholar]
- 55.Dorrington KJ, Smith BR. Conformational changes accompanying the dissociation and association of immunoglobulin-G subunits. Biochim Biophys Acta. 1972;263:70–81. doi: 10.1016/0005-2795(72)90160-2. [DOI] [PubMed] [Google Scholar]
- 56.Chan LM, Cathou RE. The role of the inter-heavy chain disulfide bond in modulating the flexibility of immunoglobulin G antibody. J Mol Biol. 1977;112:653–656. doi: 10.1016/S0022-2836(77)80170-8. [DOI] [PubMed] [Google Scholar]
- 57.Olins DE, Edelman GM. Reconstitution of 7S molecules from L and H polypeptide chains of antibody and gamma-globulins. J Exp Med. 1964;119:789–815. doi: 10.1084/jem.119.5.789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Björk I, Tanford C. Recovery of native conformation of rabbit immunoglobulin G upon recombination of separately renatured heavy and light chains at near-neutral pH. Biochemistry. 1971;10:1289–1295. doi: 10.1021/bi00784a003. [DOI] [PubMed] [Google Scholar]
- 59.Michaelsen TE, Naess LM, Aase A. Human IgG3 is decreased and IgG1, IgG2 and IgG4 are unchanged in molecular size by mild reduction and reoxidation without any major change in effector functions. Mol Immunol. 1993;30:35–45. doi: 10.1016/0161-5890(93)90424-A. [DOI] [PubMed] [Google Scholar]
- 60.Seegan GW, Smith CA, Schumaker VN. Changes in quaternary structure of IgG upon reduction of the interheavy-chain disulfide bond. Proc Natl Acad Sci USA. 1979;76:907–911. doi: 10.1073/pnas.76.2.907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Michaelsen TE. Alteration of the conformation of human IgG subclasses by reduction of the hinge S-S bonds. Mol Immunol. 1988;25:639–646. doi: 10.1016/0161-5890(88)90099-5. [DOI] [PubMed] [Google Scholar]
- 62.Johnson BA, Hoffmann LG. Effect of reduction and alkylation on structure and function of rabbit IgG antibody-I. Effect on ability to activate complement depends on conditions of reduction. Mol Immunol. 1981;18:181–188. doi: 10.1016/0161-5890(81)90084-5. [DOI] [PubMed] [Google Scholar]
- 63.Goers JW, Ziccardi RJ, Schumaker VN, Glovsky MM. The mechanism of activation of the first component of complement by a univalent hapten-IgG antibody complex. J Immunol. 1977;118:2182–2191. [PubMed] [Google Scholar]
- 64.Schur PH, Christian GD. The role of disulfide bonds in the complement-fixing and precipitating properties of 7S rabbit and sheep antibodies. J Exp Med. 1964;120:531–545. doi: 10.1084/jem.120.4.531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Press EM. Fixation of the first component of complement by immune complexes: effect of reduction and fragmentation of antibody. Biochem J. 1975;149:285–288. doi: 10.1042/bj1490285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Isenman DE, Dorrington KJ, Painter RH. The structure and function of immunoglobulin domains. II. The importance of interchain disulfide bonds and the possible role of molecular flexibility in the interaction between immunoglobulin G and complement. J Immunol. 1975;114:1726–1729. [PubMed] [Google Scholar]
- 67.Klein M, Neauport-Sautes C, Ellerson JR, Fridman WH. Binding site of human IgG subclasses and their domains for Fc receptors of activated murine T cells. J Immunol. 1977;119:1077–1083. [PubMed] [Google Scholar]
- 68.McNabb T, Koh TY, Dorrington KJ, Painter RH. Structure and function of immunoglobulin domains. V. Binding, University of immunoglobulin G and fragments to placental membrane preparations. J Immunol. 1976;117:882–888. [PubMed] [Google Scholar]
- 69.Michaelsen TE, Wisloff F, Natvig JB. Structural requirements in the Fc region of rabbit IgG antibodies necessary to induce cytotoxicity by human lymphocytes. Scand J Immunol. 1975;4:71–78. doi: 10.1111/j.1365-3083.1975.tb02601.x. [DOI] [PubMed] [Google Scholar]
- 70.Barnett Foster DE, Dorrington KJ, Painter RH. Structure and function of immunoglobulin domains. VII. Studies on the structural requirements of human immunoglobulin G for granulocyte binding. J Immunol. 1978;120:1952–1956. [PubMed] [Google Scholar]
- 71.Liu H, Chumsae C, Gaza-Bulseco G, Hurkmans K, Radziejewski CH. Ranking the susceptibility of disulfide bonds in human IgG1 antibodies by reduction, differential alkylation and LC-MS analysis. Anal Chem. 2010;82:5219–5226. doi: 10.1021/ac100575n. [DOI] [PubMed] [Google Scholar]
- 72.Remmele RL, Jr, Callahan WJ, Krishnan S, Zhou L, Bondarenko PV, Nichols AC, et al. Active dimer of Epratuzumab provides insight into the complex nature of an antibody aggregate. J Pharm Sci. 2006;95:126–145. doi: 10.1002/jps.20515. [DOI] [PubMed] [Google Scholar]
- 73.Van Buren N, Rehder D, Gadgil H, Matsumura M, Jacob J. Elucidation of two major aggregation pathways in an IgG2 antibody. J Pharm Sci. 2009;98:3013–3030. doi: 10.1002/jps.21514. [DOI] [PubMed] [Google Scholar]