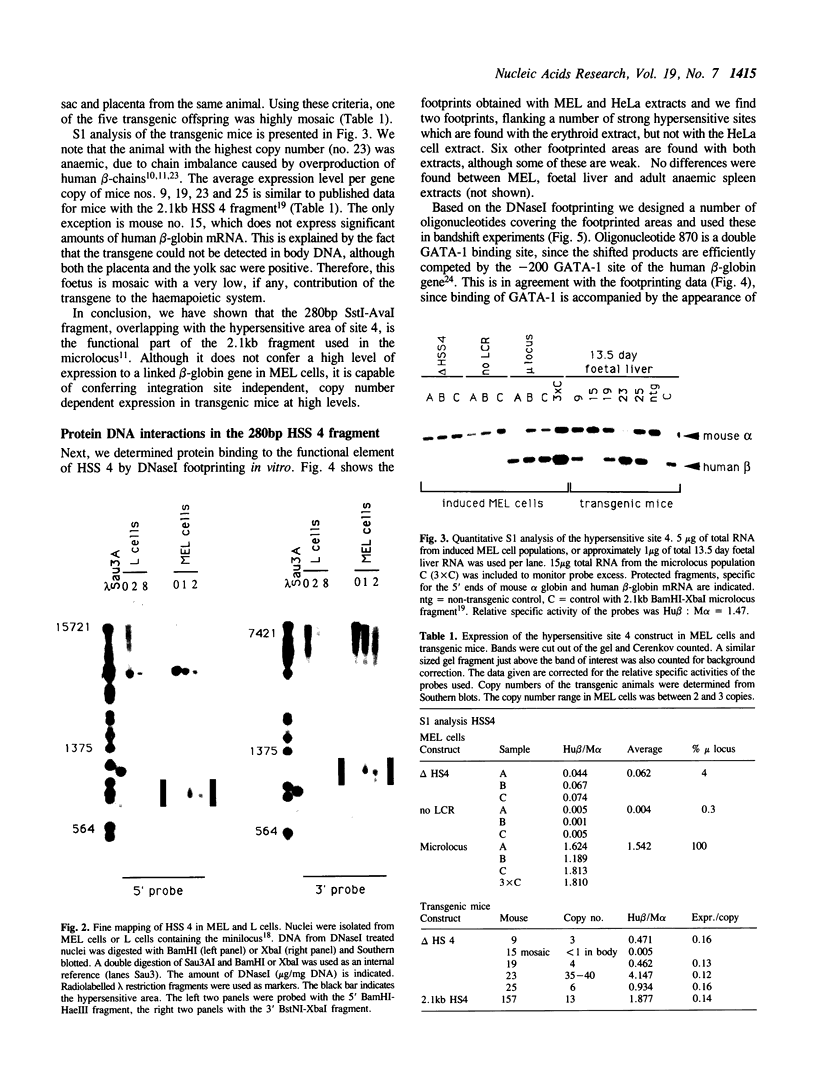
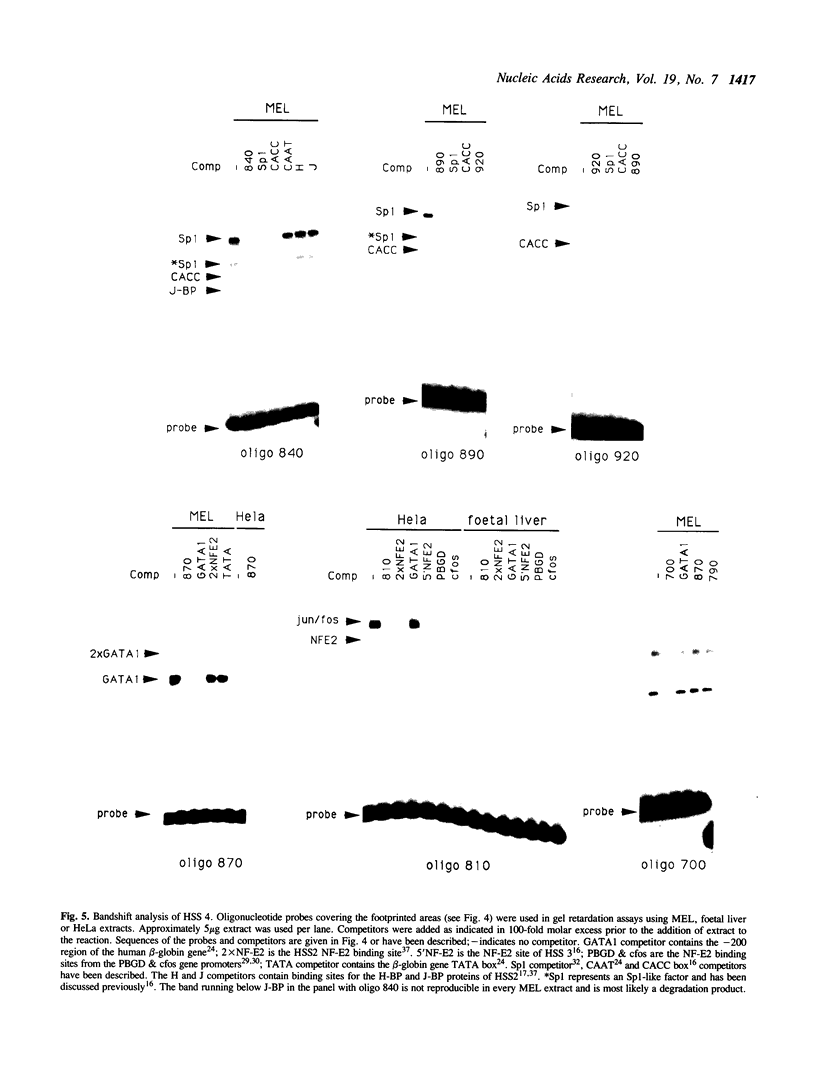
Abstract
The Locus Control Region (LCR) of the human beta globin gene domain is defined by four erythroid-specific DNasel hypersensitive sites (HSS) located upstream of this multigene cluster. The LCR confers copy number dependent high levels of erythroid specific expression to a linked transgene, independent of the site of integration. To assess the role of the individual hypersensitive sites of the LCR, we have localized HSS4 to a 280bp fragment that is functional both in murine erythroleukaemia (MEL) cells and in transgenic mice. This fragment coincides with the major area of hypersensitivity 'in vivo' and contains a number of DNasel footprints. Bandshift analysis shows that these footprints correspond to binding sites for the erythroid specific proteins GATA1 and NF-E2 and a number of ubiquitous proteins, including jun/fos, Sp1 and TEF2.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Blom van Assendelft G., Hanscombe O., Grosveld F., Greaves D. R. The beta-globin dominant control region activates homologous and heterologous promoters in a tissue-specific manner. Cell. 1989 Mar 24;56(6):969–977. doi: 10.1016/0092-8674(89)90630-2. [DOI] [PubMed] [Google Scholar]
- Collins F. S., Weissman S. M. The molecular genetics of human hemoglobin. Prog Nucleic Acid Res Mol Biol. 1984;31:315–462. doi: 10.1016/s0079-6603(08)60382-7. [DOI] [PubMed] [Google Scholar]
- Collis P., Antoniou M., Grosveld F. Definition of the minimal requirements within the human beta-globin gene and the dominant control region for high level expression. EMBO J. 1990 Jan;9(1):233–240. doi: 10.1002/j.1460-2075.1990.tb08100.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Driscoll M. C., Dobkin C. S., Alter B. P. Gamma delta beta-thalassemia due to a de novo mutation deleting the 5' beta-globin gene activation-region hypersensitive sites. Proc Natl Acad Sci U S A. 1989 Oct;86(19):7470–7474. doi: 10.1073/pnas.86.19.7470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emerson B. M., Nickol J. M., Jackson P. D., Felsenfeld G. Analysis of the tissue-specific enhancer at the 3' end of the chicken adult beta-globin gene. Proc Natl Acad Sci U S A. 1987 Jul;84(14):4786–4790. doi: 10.1073/pnas.84.14.4786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans T., Reitman M., Felsenfeld G. An erythrocyte-specific DNA-binding factor recognizes a regulatory sequence common to all chicken globin genes. Proc Natl Acad Sci U S A. 1988 Aug;85(16):5976–5980. doi: 10.1073/pnas.85.16.5976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forrester W. C., Novak U., Gelinas R., Groudine M. Molecular analysis of the human beta-globin locus activation region. Proc Natl Acad Sci U S A. 1989 Jul;86(14):5439–5443. doi: 10.1073/pnas.86.14.5439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fraser P., Hurst J., Collis P., Grosveld F. DNaseI hypersensitive sites 1, 2 and 3 of the human beta-globin dominant control region direct position-independent expression. Nucleic Acids Res. 1990 Jun 25;18(12):3503–3508. doi: 10.1093/nar/18.12.3503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gidoni D., Kadonaga J. T., Barrera-Saldaña H., Takahashi K., Chambon P., Tjian R. Bidirectional SV40 transcription mediated by tandem Sp1 binding interactions. Science. 1985 Nov 1;230(4725):511–517. doi: 10.1126/science.2996137. [DOI] [PubMed] [Google Scholar]
- Gorski K., Carneiro M., Schibler U. Tissue-specific in vitro transcription from the mouse albumin promoter. Cell. 1986 Dec 5;47(5):767–776. doi: 10.1016/0092-8674(86)90519-2. [DOI] [PubMed] [Google Scholar]
- Grosveld F., van Assendelft G. B., Greaves D. R., Kollias G. Position-independent, high-level expression of the human beta-globin gene in transgenic mice. Cell. 1987 Dec 24;51(6):975–985. doi: 10.1016/0092-8674(87)90584-8. [DOI] [PubMed] [Google Scholar]
- Hanscombe O., Vidal M., Kaeda J., Luzzatto L., Greaves D. R., Grosveld F. High-level, erythroid-specific expression of the human alpha-globin gene in transgenic mice and the production of human hemoglobin in murine erythrocytes. Genes Dev. 1989 Oct;3(10):1572–1581. doi: 10.1101/gad.3.10.1572. [DOI] [PubMed] [Google Scholar]
- Higgs D. R., Wood W. G., Jarman A. P., Sharpe J., Lida J., Pretorius I. M., Ayyub H. A major positive regulatory region located far upstream of the human alpha-globin gene locus. Genes Dev. 1990 Sep;4(9):1588–1601. doi: 10.1101/gad.4.9.1588. [DOI] [PubMed] [Google Scholar]
- Kioussis D., Vanin E., deLange T., Flavell R. A., Grosveld F. G. Beta-globin gene inactivation by DNA translocation in gamma beta-thalassaemia. Nature. 1983 Dec 15;306(5944):662–666. doi: 10.1038/306662a0. [DOI] [PubMed] [Google Scholar]
- Kollias G., Wrighton N., Hurst J., Grosveld F. Regulated expression of human A gamma-, beta-, and hybrid gamma beta-globin genes in transgenic mice: manipulation of the developmental expression patterns. Cell. 1986 Jul 4;46(1):89–94. doi: 10.1016/0092-8674(86)90862-7. [DOI] [PubMed] [Google Scholar]
- Martin D. I., Orkin S. H. Transcriptional activation and DNA binding by the erythroid factor GF-1/NF-E1/Eryf 1. Genes Dev. 1990 Nov;4(11):1886–1898. doi: 10.1101/gad.4.11.1886. [DOI] [PubMed] [Google Scholar]
- Mignotte V., Eleouet J. F., Raich N., Romeo P. H. Cis- and trans-acting elements involved in the regulation of the erythroid promoter of the human porphobilinogen deaminase gene. Proc Natl Acad Sci U S A. 1989 Sep;86(17):6548–6552. doi: 10.1073/pnas.86.17.6548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mignotte V., Wall L., deBoer E., Grosveld F., Romeo P. H. Two tissue-specific factors bind the erythroid promoter of the human porphobilinogen deaminase gene. Nucleic Acids Res. 1989 Jan 11;17(1):37–54. doi: 10.1093/nar/17.1.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ney P. A., Sorrentino B. P., Lowrey C. H., Nienhuis A. W. Inducibility of the HS II enhancer depends on binding of an erythroid specific nuclear protein. Nucleic Acids Res. 1990 Oct 25;18(20):6011–6017. doi: 10.1093/nar/18.20.6011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Philipsen S., Talbot D., Fraser P., Grosveld F. The beta-globin dominant control region: hypersensitive site 2. EMBO J. 1990 Jul;9(7):2159–2167. doi: 10.1002/j.1460-2075.1990.tb07385.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reitman M., Lee E., Westphal H., Felsenfeld G. Site-independent expression of the chicken beta A-globin gene in transgenic mice. Nature. 1990 Dec 20;348(6303):749–752. doi: 10.1038/348749a0. [DOI] [PubMed] [Google Scholar]
- Romeo P. H., Prandini M. H., Joulin V., Mignotte V., Prenant M., Vainchenker W., Marguerie G., Uzan G. Megakaryocytic and erythrocytic lineages share specific transcription factors. Nature. 1990 Mar 29;344(6265):447–449. doi: 10.1038/344447a0. [DOI] [PubMed] [Google Scholar]
- Talbot D., Collis P., Antoniou M., Vidal M., Grosveld F., Greaves D. R. A dominant control region from the human beta-globin locus conferring integration site-independent gene expression. Nature. 1989 Mar 23;338(6213):352–355. doi: 10.1038/338352a0. [DOI] [PubMed] [Google Scholar]
- Talbot D., Philipsen S., Fraser P., Grosveld F. Detailed analysis of the site 3 region of the human beta-globin dominant control region. EMBO J. 1990 Jul;9(7):2169–2177. doi: 10.1002/j.1460-2075.1990.tb07386.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taramelli R., Kioussis D., Vanin E., Bartram K., Groffen J., Hurst J., Grosveld F. G. Gamma delta beta-thalassaemias 1 and 2 are the result of a 100 kbp deletion in the human beta-globin cluster. Nucleic Acids Res. 1986 Sep 11;14(17):7017–7029. doi: 10.1093/nar/14.17.7017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Townes T. M., Lingrel J. B., Chen H. Y., Brinster R. L., Palmiter R. D. Erythroid-specific expression of human beta-globin genes in transgenic mice. EMBO J. 1985 Jul;4(7):1715–1723. doi: 10.1002/j.1460-2075.1985.tb03841.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tuan D. Y., Solomon W. B., London I. M., Lee D. P. An erythroid-specific, developmental-stage-independent enhancer far upstream of the human "beta-like globin" genes. Proc Natl Acad Sci U S A. 1989 Apr;86(8):2554–2558. doi: 10.1073/pnas.86.8.2554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tuan D., Solomon W., Li Q., London I. M. The "beta-like-globin" gene domain in human erythroid cells. Proc Natl Acad Sci U S A. 1985 Oct;82(19):6384–6388. doi: 10.1073/pnas.82.19.6384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van der Ploeg L. H., Konings A., Oort M., Roos D., Bernini L., Flavell R. A. gamma-beta-Thalassaemia studies showing that deletion of the gamma- and delta-genes influences beta-globin gene expression in man. Nature. 1980 Feb 14;283(5748):637–642. doi: 10.1038/283637a0. [DOI] [PubMed] [Google Scholar]
- Wall L., deBoer E., Grosveld F. The human beta-globin gene 3' enhancer contains multiple binding sites for an erythroid-specific protein. Genes Dev. 1988 Sep;2(9):1089–1100. doi: 10.1101/gad.2.9.1089. [DOI] [PubMed] [Google Scholar]
- Xiao J. H., Davidson I., Macchi M., Rosales R., Vigneron M., Staub A., Chambon P. In vitro binding of several cell-specific and ubiquitous nuclear proteins to the GT-I motif of the SV40 enhancer. Genes Dev. 1987 Oct;1(8):794–807. doi: 10.1101/gad.1.8.794. [DOI] [PubMed] [Google Scholar]
- Yamamoto M., Ko L. J., Leonard M. W., Beug H., Orkin S. H., Engel J. D. Activity and tissue-specific expression of the transcription factor NF-E1 multigene family. Genes Dev. 1990 Oct;4(10):1650–1662. doi: 10.1101/gad.4.10.1650. [DOI] [PubMed] [Google Scholar]
- deBoer E., Antoniou M., Mignotte V., Wall L., Grosveld F. The human beta-globin promoter; nuclear protein factors and erythroid specific induction of transcription. EMBO J. 1988 Dec 20;7(13):4203–4212. doi: 10.1002/j.1460-2075.1988.tb03317.x. [DOI] [PMC free article] [PubMed] [Google Scholar]






