Abstract
Most cases of hepatocellular carcinoma (HCC) are associated with cirrhosis related to chronic hepatitis B virus (HBV) or hepatitis C virus (HCV) infection. Changes in the time trends of HCC and most variations in its age-, sex-, and race-specific rates among different regions are likely to be related to differences in hepatitis viruses that are most prevalent in a population, the timing of their spread, and the ages of the individuals the viruses infect. Environmental, host genetic, and viral factors can affect the risk of HCC in individuals with HBV or HCV infection. This review summarizes the risk factors for HCC among HBV- or HCV-infected individuals, based on findings from epidemiological studies and meta-analyses, as well as determinants of patient outcome and the HCC disease burden, globally and in the US.
Keywords: Liver cancer, association, virology, genetics
Introduction
According to the International Agency for Research on Cancer, liver cancer is the fifth most common cancer in men worldwide (523,000 cases per year, 7.9% of all cancers) and the seventh in women (226,000 cases per year, 6.5% of all cancers). Liver cancer has high mortality; the geographic distribution of mortality is similar to that of incidence. Most of the burden of liver cancer is in developing countries, where almost 85% of the cases occur. Hepatocellular carcinoma (HCC) is the most common form of liver cancer; most cases of HCC (approximately 80%) are associated with chronic hepatitis B virus (HBV) or hepatitis C virus (HCV) infections. Variations in the age-, sex-, and race-specific rates of HCC rates in different geographic regions are likely to be related to differences in the prevalence of hepatitis viruses in the populations, as well as the timing of the spread of the viral infection and the age of individuals at the time of the infection.
Global Epidemiology of HCC
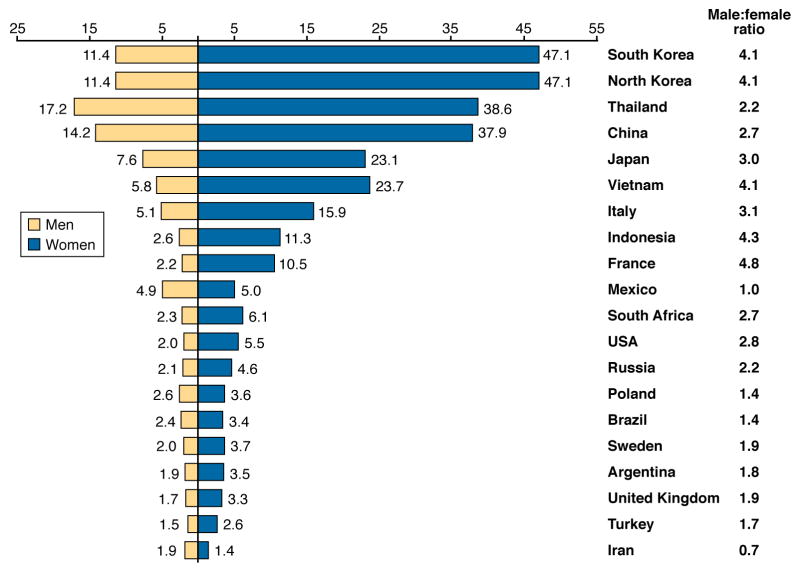
Most cases of HCC cases (>80%) occur in sub-Saharan Africa and in Eastern Asia, with typical incidence rates of > 20 per 100,000 individuals. Southern European countries, (such as Spain, Italy and Greece) tend have mid-incidence levels (10.0 to 20.0 per 100,000 individuals), whereas North America, South America, Northern Europe, and Oceania have a low incidence of HCC (<5.0 per 100,000 individuals) (Figure 1). Recent decreases in the incidence of HCC were reported among Chinese populations in Hong Kong, Shanghai, and Singapore; the incidence in Japan is also decreasing. However, cases of HCC are increasing in low-incidence areas such as the United States and Canada.
Figure 1.
Age-standardized incidence rates of liver cancer per 100,000 person-years, shown for different regions of the world and for men and women (GLOBOCAN 2002)
HCC is rarely seen during the first 4 decades of life, except in populations where HBV infection is hyperendemic. The mean ages of diagnosis with HCC were 55–59 years in China and 63–65 years in Europe and North America. In low-risk populations, the highest incidence of HCC is among individuals 75 or older. However, in Qidong, China, where HCC burden is among the world’s highest, the age-specific incidence rates among men increases until 45 years and then plateaus; among women, the incidence rate increases until 60 years and then plateaus. HCC is predominant among men, with the highest male:female ratios in areas of high incidence (Figure 1).
The Role of HBV and HCV in HCC
HBV and HCV promote cirrhosis, which is found in 80%–90% of patients with HCC. The 5-year cumulative risk of developing HCC for patients with cirrhosis ranges between 5% and 30%, depending on etiology (it is highest in individuals with HCV infection), region or ethnicity (it is highest in Asians), and stage of cirrhosis (it highest in individuals with decompensated disease)1.
Approximately 5% of the world population (350–400 million people) is chronically infected with HBV; 75% of infected people are Asian2, with a lower prevalence (0.3%–1.5%) in western countries. There is high ecological correlation between areas of HBV prevalence and HCC incidence and mortality worldwide (Figure 2). Chronic HBV infection accounts for approximately 50% of the total cases and virtually all of childhood HCC; it is the dominant risk factor in most areas of Asia and Sub-Saharan Africa that have high incidence of HCC, with the exception of Japan, where the major risk factor for HCC is chronic HCV infection. HB surface antigen (HBsAg) seroprevalence among persons with HCC varies widely: it is 3% in Sweden, 10% in the United States, 10%–15% in Japan, 19% in Italy, 55% in Greece, and 70% in South Korea.
Figure 2.
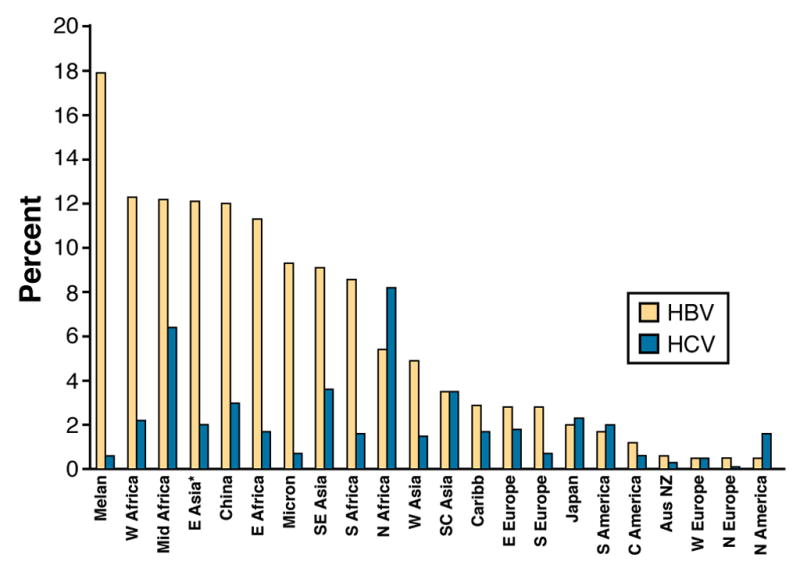
Prevalence of HBsAg carrier and chronic HCV status in different geographic regions (Custer et al., 2004)
Melanesia (includes the Amphlett Islands, Bismarck Archipelago, d'Entrecasteaux Islands, Fiji, Louisiade Archipelago, Maluku Islands, New Caledonia, New Guinea, Norfolk Island, Raja Ampat Islands, RotumaSchouten Islands, Santa Cruz Islands, and Solomon Islands) Micronesia (Banaba, Gilbert Islands, Mariana Islands, Marshall Islands, Caroline Islands, Nauru, and Wake Island)
The global prevalence of HCV is estimated to be 2% (approximately 180 million people worldwide) and varies considerably among different regions (Figure 2). Phylogenetic studies of HCV diversity described the chronology of the spread of HCV epidemics in Japan, Europe, and the US; these findings account for the geographical differences in the timing of the burden of HCV-related HCC 3. Based on these studies, HCV began to infect large numbers of young adults in Japan in the 1920s, in southern Europe in the 1940s, and in North America in the 1960s and 1970s [ref 4]. The HCV epidemic in the US originated from contaminated needles and/or injection drug use. The virus spread into national blood supplies and circulated until the late 1980s; the rate of new infections was greatly reduced thereafter. Although the seroprevalence of HCV is similar among the general populations of Japan, southern Europe, and North America, markers of HCV infection are highest among individuals with HCC in Japan (80%–90%), followed by Italy (44%–66%), and then the United States (30%–50%)5. The incidence of HCC is almost 3-fold higher in Japan than Italy and almost 6-fold higher than in the US. The burden of HCC in the US might therefore eventually equal that of Japan.
The age distribution of HCC in different regions is partly determined by type of virus and timing of infection. In areas that have high incidence of HCC in Asia, HBV infection is largely acquired by mother–child transmission, whereas transmission among siblings of young ages is more common in Africa. Therefore, individuals in these regions develop HCC at earlier ages than in low-incidence areas, where the main risk factors for HCV infection are encountered later in life. Differences in age-related prevalence of HCC might affect applicability and outcomes of therapies such as liver transplantation. The high male:female ratio of HCC might result, in part, from the higher prevalence of HBV and HCV infection among men than women.
It is estimated that >90% of countries routinely vaccinate newborns against HBV, and approximately 70% are now delivering 3 immunization doses. In 1984, Taiwan became the first country to vaccinate newborns against HBV, and give HB immunoglobulin (Ig) to infants of high-risk (HBsAg-positive) and HB e antigen (HBeAg)-positive mothers. Since then, the number of HBV carriers in the juvenile population has been greatly reduced, and incidence of HCC among children 6–14 years was reduced by 65%–75% 6. However, the HBV-related incidence of HCC is projected to increase for several decades, because of the high prevalence of chronic HBV infection and prolonged latency to HCC development.
Risk of HCC from HBV Infection
Prospective cohort studies showed a 5- to 100-fold increase in risk of developing HCC among persons chronically infected with HBV. Meta-analyses of case-control and cross-sectional studies indicated that the lifetime relative risk for HCC is 15–20 among HBsAg-positive individuals, compared with HBsAg-negative individuals. A systematic review of longitudinal (cohort) studies published until June 2007, by Fattovich et al., estimated the incidence rates of HCC in subjects with chronic HBV infection in East Asian countries to be 0.2 per 100 person-years in inactive carriers (HBsAg-positive but with normal levels of alanine aminotransferase, ALT), 0.6 person-years for those with chronic HBV infection without cirrhosis, and 3.7 person-years for those with compensated cirrhosis7. There have been few adequate studies in Europe or North America to determine the incidence of HCC in HBsAg-positive individuals—most studies included only small numbers of HBsAg-positive patients. Nevertheless, the summary HCC incidence rate was 0.02 per 100 person-years in inactive carriers, 0.3 in subjects with chronic HBV without cirrhosis, and 2.2 in subjects with compensated cirrhosis.
Most HBV-infected individuals who develop HCC have cirrhosis secondary to chronic necroinflammation. HBV can cause HCC in the absence of cirrhosis, although most cases of HBV-related HCC (70%–90%) occur in patients with cirrhosis8. Factors that have been reported to increase HCC risk among HBV carriers are demographic (male sex, older age, Asian or African ancestry, family history of HCC), viral (higher levels of HBV replication; HBV genotype; longer duration of infection; co-infection with HCV, HIV, or HDV), clinical (cirrhosis), and environmental (exposure to aflatoxin, heavy intake of alcohol or tobacco).
HBV Transmission and Replication
In many high-risk areas—particularly those in Asia—HBV is transmitted from mother to newborn (vertical transmission); as many as 90% of infected babies develop chronic infections. This pattern is different in areas that have low incidence of HCC—HBV infection is usually acquired in adulthood, through sexual and parenteral routes (horizontal transmission). More than 90% of these cases of acute HBV infection resolve spontaneously. The higher frequency and longer period of chronic HBV infections contribute to a greater risk for HCC in areas where HBV infection is common.
The risk of HCC is increased in patients with higher levels of HBV replication, determined by tests for HBeAg and levels of HBV DNA. One large study evaluated the effect of HBV replication on the risk of HCC among 11,893 Taiwanese men who were followed for a mean of 8.5 years. The incidence rate of HCC was 1169 per 100,000 person-years among men who were HBsAg-positive and HBeAg-positive, 324 per 100,000 person-years for those who were only HBsAg-positive, and 39 per 100,000 person-years for those who were HBsAg-negative. Similarly, the relative risks of HCC among men who were HBsAg-positive and HBeAg-positive were increased 60 fold. and 10 fold among those who were only HBsAg-positive 9. A community-based Taiwanese prospective study, the Risk Evaluation of Viral Load Elevation and Associated Liver Disease/Cancer–Hepatitis B Virus (REVEAL-HBV), reported that in a cohort of 3653 HBsAg positive participants, the incidence of cirrhosis and HCC increased in proportion to the serum level of HBV DNA, from <300 (undetectable) to ≥ 1,000,000 copies/mL (Figure 3). These associations remained significant after adjustment for age, sex, smoking, alcohol drinking, and HBeAg serostatus10. The increased incidence in HCC in relation to level of HBV DNA was also observed in a study that showed that inactive carriers of HBV (seronegative for HBeAg, serum levels of HBV DNA <10,000 copies/mL, and normal liver enzymes) had an almost 5-fold greater risk for HCC than controls (HBsAg-negative)11. It is not clear if the findings from REVEAL-HBV pertain to western populations, who acquire HBV as adults and have different risk factors for disease (obesity, diabetes, and alcohol use).
Figure 3.
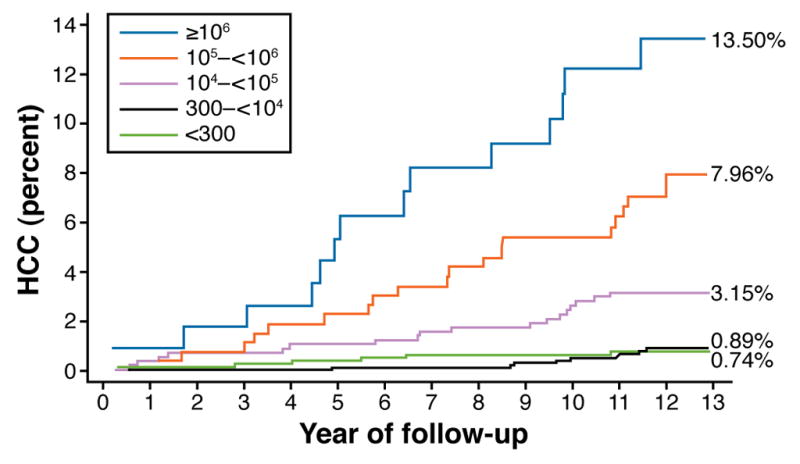
An association between baseline serum level of HBV DNA and future incidence of HCC. The cumulative incidence of HCC was also calculated for a subcohort of 2925 Taiwanese participants in the study REVEAL-HBV who were HBeAg-negative, had normal levels of ALT, and did not have cirrhosis when the study began. (modified from Chen CJ et al. JAMA. 2006;295:65–73)
Levels of viral replication are also affected by antiviral treatment. There is moderately strong evidence that effective antiviral therapy, which suppresses HBV infection in HBsAg-positive patients, substantially reduces but does not completely eliminate risk for HCC. In a large Asian study, patients with chronic HBV and cirrhosis or advanced fibrosis were given 100 mg per day of lamivudine or placebo for up to 5 years. A smaller percentage of patients given lamivudine developed HCC (3.9%), compared with those given placebo (7.4%), which mostly achieved a reduced level of HBV DNA12. Lower-quality evidence from non-randomized trials and observational studies indicate that therapy with interferon or lamivudine reduces the risk for HCC13.
HBV Genotypes
Several HBV genotypes (A–H) have been identified, based on differences of 8% or more in their whole-genome sequence. HBV genotypes have distinct geographical and ethnic distributions: genotypes A and D predominate in Africa, Europe, and India; genotypes B and C predominate in Asia; genotype E predominates in West Africa; and genotype F predominates in Central and South America. In the US, HBV genotypes A and D are more common in black and white persons, whereas HBV genotypes B and C are more common among persons of Asian ancestry.
HBV genotypes seem to affect clinical outcomes. In studies performed in Asia, there was a greater association between genotype C infection and severe liver disease, cirrhosis, and HCC than genotype B; in Western Europe and North America, individuals with genotype D had a greater incidence of severe liver disease or HCC than those with genotype A. However, some data associate genotype B HBV with the development of HCC in young carriers without cirrhosis. A study from Taiwan demonstrated that genotype B was significantly more common in patients with HCC under 50 years of age than in age-matched carriers with inactive infections (80% vs 52%)14. Most of these participants did not have cirrhosis. A Taiwanese, 15-year study of 460 carriers of HBV reported that genotype B was the most frequent genotype among 26 children with HBV-related HCC (found in 74%)15. Mutations in the region of the HBV genome that encode the basal core promoter, such as T1762 and A1764, 16 have been associated with increased incidence of HCC, whereas those in the precore region (G1896A) have been associated with decreased incidence of HCC17.
Aflatoxin B1 (AFB1)
AFB1 is a mycotoxin produced by fungi of the Aspergillus species (A. flavus and A. parasiticus) that grows readily on foods such as corn and peanuts stored in warm, damp conditions. In animals, AFB1 is a powerful hepatocarcinogen, leading the International Agency for Research on Cancer to classify it as a carcinogen 18.
Once ingested, AFB1 is metabolized to an active intermediate, AFB1-exo-8,9-epoxide, which can bind to and damage DNA. AFB1 causes a mutation at serine 249 in the tumor suppressor p53 19 that was detected in 30%–60% of HCC tumor samples collected from individuals in aflatoxin-endemic areas, most of whom had HBV infections 20, 21. Assays have been developed to measure aflatoxin metabolites in urine and AFB1–albumin adducts in serum, and to detect specific aflatoxin-associated DNA mutations in tissues.
Areas in which AFB1 exposure is an environmental problem also have a high prevalence of chronic HBV infection. Although AFB1 might contribute to hepatocarcinogenesis by other mechanisms, its role in pathogenesis of HCC is primarily mediated by its effects on chronic HBV infection. For example, prospective studies in Shanghai, China showed that urinary excretion of aflatoxin metabolites increased the risk of HCC up to 4-fold, and HBV infection increased the risk 7-fold. However, individuals who excreted AFB1 metabolites and were carriers of HBV had as much as a 60-fold increase in risk of HCC22. Importantly, prevention of HBV-related HCC would reduce the effects of aflatoxin on HCC risk.
Occult HBV Infection
Studies with sensitive amplification assays have shown that HBV DNA persists in serum or liver, as an occult HBV infection, among persons who have serological recovery from transient HBV infection (who are HBsAg-negative). In many instances, occult hepatitis B is associated with antibodies to hepatitis B core antigen (anti-HBc) and/or anti-HBs. A systematic review identified 16 studies of the association between occult HBV and HCC risk; 6 of these studies found no significant association. None of the studies included in this review was population-based—most had a small number of cases or controls, 11 were from Asia (only 1 was performed in the US), and they had varied and few adjustments for confounders. A pooled adjusted estimate could be calculated for only 4 longitudinal studies (3 from Japan) that indicated a modest association between occult HBV infection and HCC (a relative risk of 2.83)23. A recent small case-control study from Hong Kong showed a high prevalence of occult HBV in patients with cryptogenic HCC .[REFERENCE: Wong DK, Huang FY, Lai CL, Poon RT, Seto WK, Fung J, Hung IF, Yuen MF. Occult hepatitis B infection and HBV replicative activity in patients with cryptogenic cause of hepatocellular carcinoma. Hepatology. 2011 Sep 2;54(3):829–36. doi:10.1002/hep.24551. Epub 2011 Jul 21. PubMed PMID: 21809355.] However, there is no convincing evidence that occult HBV is an independent risk factor for HCC or a cofactor with HCV infection in most regions of the world.
Risk of HCC from HCV Infection
There is much evidence that HCV infection can cause HCC. Prospective studies have shown a significant increase in the incidence of HCC among HCV-infected cohorts, compared to HCV-negative cohorts 24. The rate of HCC among HCV-infected persons ranges from 1% to 3% over 30 years. Similarly, HCV infection is associated with a 15- to 20-fold increase in risk for HCC compared with HCV-negative subjects in cross-sectional and case-control studies.
HCV increases the risk for HCC by inducing fibrosis and, eventually, cirrhosis. Although HCC has been reported among individuals without or with low levels of fibrosis25–27, the risk of HCC increases with fibrosis stage; most cases of HCV-related HCC occur among patients with advanced fibrosis or cirrhosis, making it a condition listed for HCC surveillance in current recommendations. Once HCV-related cirrhosis is established, HCC develops at an annual rate of 1%–4%; though rates up to 8% have been reported in Japan. The incidence of cirrhosis (and consequently HCC) 25–30 years after HCV infection ranges from 15% to 35%,28 and is highest among recipients of HCV-contaminated blood-products and hemophiliacs and lowest among women who received a single dose of contaminated anti-D Ig. HCC risk might also vary based on the amount of virus in the contaminated product or repeated exposure. Other risk factors for HCC include the sex of the HCV-infected individual, comorbidities (co-infection with HBV or HIV, diabetes, obesity, steatosis), viral genotype (HCV 1b), level of alcohol consumption, and age. Among patients with HCV-related cirrhosis, low numbers of platelets or increased levels of α-fetoprotein are risk factors for HCC.
Viral Factors
HCV viremia of any level is a strong risk factor for HCC; conversely, treatment that eliminates the virus decreases risk for HCC. Evidence from randomized controlled studies and several non-randomized studies of HCV-infected patients with and without cirrhosis indicates a 57% to 75% reduction in risk of HCC in patients who received interferon-based therapy and achieved a sustained viral response. There are at least 6 HCV genotypes, which differ in 30%–35% of nucleotides in the complete genome. There are also several subtypes; HCV subtypes 1a and 1b are the most common in the US and Europe, whereas in Japan, 73% of HCV-infected individuals carry subtype 1b. Reports of the association between HCV genotype and HCC risk are inconsistent. However, a meta-analysis of 21 studies that calculated age-adjusted risk estimates reported that patients infected with HCV genotype 1b had an almost 2-fold greater risk of developing HCC than patients with other HCV genotypes (a pooled relative risk of 1.78). The pooled risk estimate remained significant but lower in an analysis limited to 8 studies conducted in patients with cirrhosis (1.60)29. There is no consistent evidence that other viral factors, such as HCV load or quasi-species, affect the risk of progression to cirrhosis or HCC. A study performed in Taiwan reported a correlation between level of HCV RNA and the risk of HCC 30, but studies from the US and Europe have not made this association.
HIV
Many studies examined the effect of HIV infection on the progression of HCV-related liver disease, measured by fibrosis, cirrhosis, HCC, decompensated liver disease, and liver-related death. These studies mostly used the retrospective cohort or cross-sectional study design. Despite the limitations of these studies and some inconsistent results, it was evident that persons co-infected with HIV have faster progression to cirrhosis and decompensated liver disease, especially during immunosuppression. However, the effect of anti-retroviral therapy on liver disease in patients co-infected with HCV and HIV is not clear. At least 4 studies included in a systematic review did not associate anti-retroviral therapy with risk for HCC, although it is difficult to make general conclusions because of the small number of cases of HCC in these studies. Anti-retroviral therapy might reduce the risk for HCC, given the association between HIV co-infection and accelerated liver disease31.
HBV infection persists in 25% of HIV-infected adults, compared with <5% of adults without HIV infection. Furthermore, individuals co-infected with HIV and HBV have an increased risk for liver-related mortality. However, there are few data on the effects of co-infection with HBV and HIV on risk for HCC.
Coffee
Population-based studies have associated high levels of coffee consumption (>2 cups/day) with reduced serum levels of ALT and γ-glutamyl transferase and reduced incidence of chronic liver disease. Consumption of high levels of caffeine was associated with milder fibrosis in patients with chronic HCV infection32. Coffee consumption was also inversely associated with progression of liver-disease among participants in the Hepatitis C Antiviral Long-Term Treatment against Cirrhosis (HALT-C) trial who had hepatitis C–related bridging fibrosis or cirrhosis and did not have a sustained virologic response to peginterferon and ribavirin treatment33. In the HALT-C study, consumption of caffeine from sources other than coffee or of decaffeinated coffee was not associated with reduced levels of liver enzymes or fibrosis.
Several studies suggested an inverse relation between coffee drinking and risk of HCC. One meta-analysis of studies published through 2007 included 10 studies (2260 cases of HCC; 6 case-control studies from southern Europe and Japan and 4 cohort studies from Japan). Given the geographic location of these studies, most patients with HCC were probably infected with HCV, or possibly HBV. All the studies observed an inverse relation between coffee consumption and risk of HCC, and in 6 studies the association was statistically significant. The summary relative risk for coffee drinkers vs non-coffee drinkers was 0.54 from case-control studies and 0.64 from cohort studies. It was estimated that, for an increase of 1 cup of coffee per day, the summary risk ratio was 0.77 from case-control studies, 0.75 from cohort studies. 34,35. The mechanisms by which coffee reduces the risk of liver disease, including HCC, are unclear, but could involve a reduced risk for cirrhosis. In addition to caffeine, coffee compounds such as cafestol and ditrepens are similar to enzymes involved in carcinogen detoxifixation. Coffee drinking might also protect against HCC by reducing levels of insulin and thereby the risk for type 2 diabetes 36—a risk factor for fatty liver disease, cirrhosis and HCC
Risk Factors for HCC Common to HBV and HCV
Sex
Men are at increased risk for HCC partly because they have a greater incidence of viral hepatitis and alcoholic cirrhosis. However, their risk is still increased after adjusting for these confounders. Men have an increased risk of cirrhosis and HCC from different diseases, such as HBV and HCV infection. High serum levels of testosterone have been associated with HCC risk in nested case-control studies of HBV carriers in Taiwan and Shanghai 37. Male carriers of HBV usually have higher viral loads. Studies of HBV infection in transgenic mice demonstrated that the androgen pathway can increase the transcription of HBV genes; androgens bind directly to sites in the viral genome, and conversely, the HBV protein HBx can increase the transcription of androgen receptors38, 39. Other studies have reported that estrogen protects against progression of HBV infection. There have been fewer studies of role of testosterone in HCV-related liver disease. There have been differing results from small, case-control studies of HCV-infected regarding total serum levels of testosterone and degree of HCV-related hepatic fibrosis. A cross-sectional study associated higher total serum levels of testosterone with risk of advanced hepatic fibrosis and inflammatory activity in male veterans with chronic HCV infections in the US. However, the association with HCC was not examined 40.
Co-Infection with HCV and HBV
There has been no single study large enough to adequately address the risk of HCC among patients with HBV and HCV co-infection. Two meta-analyses of studies from various countries (1998)41 and China (2005)42 reported the additive effects of HBV and HCV on risk for HCC. A meta-analysis of 32 case-control studies by Donato et al. 41 found that infection with HBV and HCV had an odds ratio for HCC of 165, compared with an odds ratio of 17 for HCV infection alone and an odds ratio of 23 for HBV infection alone. In a meta-analysis of Chinese studies that included 3201 cases and 4005 controls, the pooled odds ratio for HBsAg positivity was 14.1, for antibodies against HCV (anti-HCV) and HCV RNA the odds ratio was 4.6, and for HBsAg-positivity and anti-HCV and HCV RNA, the odds ratio was 35.742.
However, an updated meta-analysis that included 59 studies that assessed HBV and HCV co-infection reported a sub-additive effect on HCC risk, based on more recent studies (2000–2009), cohort studies, and studies conducted in areas in which HBV and HCV infection were not common; it reported an additive effect in older studies, case-control studies, and studies conducted areas where HCV infection was common43. A sub-additive effect of HBV and HCV co-infection is possible because infection with 1 virus can inhibit infection with the other.
Alcohol
There is evidence for a synergistic effect between heavy ingestion of alcohol and HCV infection and, to a lesser extent, HBV infection; these factors presumably operate together to promote cirrhosis. A meta-analysis of 20 studies published between 1995 and 2004 that involved more than 15,000 persons with chronic HCV infection reported that the pooled relative risk of cirrhosis associated with heavy alcohol intake was 2.33, compared with no or low-quantity alcohol intake 44. This synergistic effect has also been observed in development of HCC. Donato et al. 45 reported that, among alcohol drinkers, HCC risk increased in a linear fashion with daily intake >60 gm (6 cans of beer, glasses of wine, or shots of hard liquor). However, concomitant HCV infection increased this risk for HCC 2-fold (Figure 4).
Figure 4.
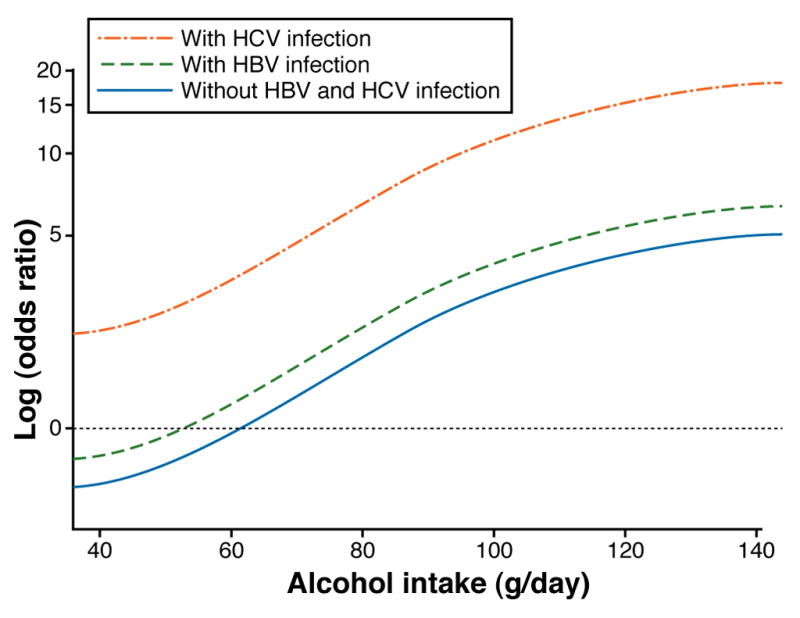
Odds ratios for hepatocellular carcinoma, according to alcohol intake and the presence of hepatitis B virus (HBV) or hepatitis C virus (HCV) infection. The plot was obtained by fitting spline regression models on data obtained in Brescia, Italy, 1995–2000. (modified from Donato F et al. Am. J. Epidemiol. 2002;155:323-331)
Few cohort studies have investigated the association between HBV infection and alcohol drinking or intake of different amounts of alcohol. A Japanese study of patients with compensated, HBV-related cirrhosis showed that heavy alcohol intake increased the risk for HCC 3-fold 46. A population-based cohort study performed in Korea found that among individuals with chronic HBV infection, the risk for HCC increased significantly among subjects with an alcohol intake of 50 g/day or more, with a relative risk of 1.2 for 50–99 g/day and of 1.5 for >100 g/day 47.
Tobacco Smoking
The relationship between cigarette smoking and HCC has been examined in more than 60 studies, in areas of high and low incidence of HCC; positive and no associations have been reported. Among studies reporting positive associations, several found that effects were limited to subgroups defined by HBV or HCV status. In a meta-analysis of 16 publications that evaluated the epidemiological interactions between HBV and HCV infection, cigarette smoking, and risk of HCC, there was a more-than additive interaction between HBV infection and cigarette smoking and a more-than multiplicative interaction between HCV infection and cigarette smoking48.
Metabolic Syndrome
Insulin resistance is associated with hepatic steatosis, advanced fibrosis, and HCC among patients with HCV infection. A meta-analysis of studies published through February 2005 reported that, of 13 case-control studies, diabetes was significantly associated with HCC in 9 studies (a pooled odds ratio of 2.5), and that of 13 cohort studies, diabetes was significantly associated with HCC in 7 studies (a pooled risk ratio of 2.5). The significant association between HCC and diabetes was independent of viral hepatitis or alcohol use in the 10 studies that examined these factors49. An updated review of studies published through February 2011 reported on a total of 17 case-control studies and 32 cohort studies. The pooled risk estimate of 17 case-control studies (an odds ratio of 2.40) was slightly higher than that from 25 cohort studies (a relative risk of 2.23)50. Cirrhosis causes glucose intolerance and type 2 diabetes, and also leads to HCC, making it difficult to interpret the association between HCC and diabetes. This bias is less likely to be present in longitudinal studies that exclude patients with liver disease at baseline.
The association between diabetes and HCC might depend on the type of viral infection. There is a significant (68%) increase in diabetes among HCV-infected individuals, compared with non-infected individuals, based on retrospective and prospective studies. Individuals with HCV infection also have a greater risk of diabetes than HBV-infected individuals 51. However, few studies found that HCV and diabetes synergize to increase the risk of HCC52, 53
The association between diabetes and HCC is less consistent in areas with a high incidence of HBV infection than in other regions. For example, although a large Korean cohort study reported a modest association between diabetes and risk for HCC54, several Taiwanese studies did not 55. Compared with HCV infection, there are less data on insulin or steatosis and risk of advanced liver disease, including HCC, among individuals with HBV infection. A prospective case-cohort study from Taiwan (of 124 HCC cases and 1084 controls, and measured baseline levels of insulin) reported that insulin resistance increased the risk of HCC among men with chronic HBV infection, with an hazard ratio of 2.36 for those in the highest tertile of insulin levels and a hazard ratio of 1.57 for those with the lowest levels of insulin, after adjusting for body mass index56.
Host Genetic Factors
Most individuals with HCV or HBV never develop cirrhosis or HCC. Family history of liver cancer has been associated with increased risk for HCC among HBV carriers (in cohort and case-control studies) and possibly among HCV-infected persons (in case control studies), irrespective of viral hepatitis57, 58. Host genetic factors might account for some of the variation in the risk of developing cirrhosis or HCC. Individual genetic association studies are frequently underpowered and often report small or variable effects. Meta-analysis has been recognized as an important tool to precisely define the effect of selected polymorphisms on risk of disease.
Tumor Necrosis Factor-α (TNFα) Variants
Two meta-analyses investigated the association of common polymorphisms in TNFα including –308 G >A, with risk for HCC. One meta-analysis analyzed 9 published studies that included 1362 cancer cases and 2426 controls and associated the TNFα –308 AA and AG variants (vs. GG) with a significantly increased risk of HCC in different genetic models, including a dominant inheritance model that produced an odds ratio of 1.59 59. The second meta-analysis summarized 10 case-control studies involving 1421 HCC cases and reported that patients with HCC had a significantly lower frequency of the TNFα polymorphism –308 GG than healthy controls, but not more than HBV-infected controls60.
A recent meta-analysis examined the relationship between polymorphisms in TNFα, IL-1B, and IL-10 and the risk for HCC in studies published through September 2010. Twenty studies were identified, involving 2763 patients with HCC and 4152 controls. This meta-analysis confirmed the significant association (odds ratio of 1.84) between a polymorphism at TNFα 308 and HCC in Asian subgroups. The polymorphisms TNFα 238 G/A, IL-1B 31 T/C and –511 C/T, and IL-10 1082 G/A were not associated with the risk for HCC61.
Glutathione S-Transferase (GST) Variants
Variants of GST genes are among the most extensively studied genetic risk factors for HCC. GSTs are a broadly expressed family of phase II isoenzymes that protect against endogenous oxidative stress. A meta-analysis evaluated the effect of polymorphisms that cause deletions in GSTM1 and GSTT1 62 in 14 studies (2514 cases and 4416 controls) indicated that forms of GSTM1 or GSTT1 that do not produce a functional product (null genotypes) slightly increased risk for HCC, though findings approached significance only for GSTT1 (odds ratio of 1.16). An updated meta-analysis of studies published through November 2009 that analyzed 24 individual case-control studies involving 3349 HCC cases and 5609 controls also showed a significant increase in risk for HCC among individuals with the null genotypes of GSTM1 (odds ratio of 1.2) and GSTT1 (odds ratio of 1.28). A subgroup analysis showed that the increase in risk for HCC was statistically significant in areas where HBV infection was common 63.
X-ray Repair Cross-Complementing Group 1 (XRCC1) Variants
A meta-analysis of 11 case-control studies, involving 2208 cases of HCC and 3265 controls, found no association between the XRCC1 polymorphism that encodes Arg399Gln and the risk of HCC64.
Using Epidemiologic Findings to Determine HCC Risk in the Clinic
Investigators from Taiwan examined the potential use of noninvasive clinical and laboratory measures, which have been demonstrated in epidemiological studies to be associated with HCC risk, to construct clinically usable nomograms to predict HCC risk in patients with chronic HBV infection65. A number of risk factors, including sex, age, family history of HCC, heavy alcohol consumption, serum levels of ALT, HBeAg sero-status, serum levels of HBV DNA, and HBV genotype were used to created predictive models based on data from 2435 subjects in the REVEAL-HBV study; these were validated in an analysis of 1218 subjects. The models have shown very good to excellent predictive and discriminant abilities. However, it is not clear whether these can be applied to the clinical setting and to non-Taiwanese populations. There is no such model for predicting HCC among HCV-infected patients.
HCC and Viral Hepatitis in the United States
In the United States, the age-adjusted incidence rates for HCC have tripled since the early 1980s. Incidence rates are 2–3-fold lower among Caucasians than African Americans, and 2–3-fold lower among African Americans than Asians, Pacific Islanders, or Native Americans. Asian men (Chinese, Korean, Filipino, and Japanese) have the highest age-adjusted incidence rates (as high as 23 per 100,000). However, the largest proportional increases have occurred among whites (Hispanic and non-Hispanic), whereas the lowest proportional increases have occurred among Asians. In addition, the age distribution of HCC patients has shifted to younger ages, with the greatest proportional increases among individuals 45–60 years old.
Secular Trends of HBV and HCV in HCC in the United States
Among patients with HCC in the US, 50%–60% are infected with HCV, 10%–15% are infected with HBV, less than 5% are infected with both viruses, and 30%–35% are infected with neither virus. HCV infection is the most frequently reported etiological factors in Hispanics and African Americans with HCC, whereas HBV infection is the most frequently reported factor in Asians with HCC.
Four studies have examined temporal variations in risk factors among patients with HCC in the US; data used in 2 studies came from large, single centers where viral risk factors were determined based on serological markers, and data used in the 2 others were collected from national administrative databases, in which risk factors were confirmed using International Classification of Disease codes in billing claims or discharge records. In all these studies, HCV-related HCC had the largest proportional increase, whereas the proportion of HCC associated with HBV infection remained stable in 3 studies and increased slightly in 1 study, among persons 65 years and older. The rate of HCC related to alcoholic liver disease was stable in all 4 studies. Similar national trends have been observed: increased HCV-related mortality and decreased or stabilized HBV-related mortality, based on data from liver transplant waitlist registration.
Future Burden of HBV- and HCV-Related HCC
In the US, the incidence of HBV-related HCC is likely to remain steady. Though vaccination against HBV could prevent HCC, it doesn’t prevent cancer in persons with chronic infections. The most recent (1999–2006) National Health and Nutrition Examination Survey (NHANES) estimated that only 0.27% of the US population, 6 years or older, had chronic HBV infection. [REFERENCE: Coleman PJ, McQuillan GM, Moyer LA, Lambert SB, Margolis HS. Incidence of hepatitis B virus infection in the United States, 1976–1994: estimates from the National Health and Nutrition Examination Surveys. J Infect Dis. 1998 Oct;178(4):954–9. PubMed PMID: 9806021.] However, screening studies of Native Americans (particularly Eskimos) and of Asian Americans and foreign-born persons that immigrated from the Middle East and Africa revealed a much higher prevalence of chronic HBV infection (10%–15%).
The incidence of HCV-related cirrhosis and HCC in the US has been progressively increasing (Figure 5) and will continue to do so for a few more decades. The NHANES also estimated that 1.3% of the non-institutionalized, civilian US population had chronic HCV infection; approximately 66% of those infected were born between 1945 and1964, and have therefore been living with the infection for several decades. This cohort also carries multiple risk factors for progression, such as alcohol drinking and obesity. It was estimated that approximately 50% of individuals with chronic HCV infections in the US are undiagnosed. Projections estimate that, without effective treatment, the annual number of patients with cirrhosis or HCC will roughly double by 2020 66.
Figure 5.
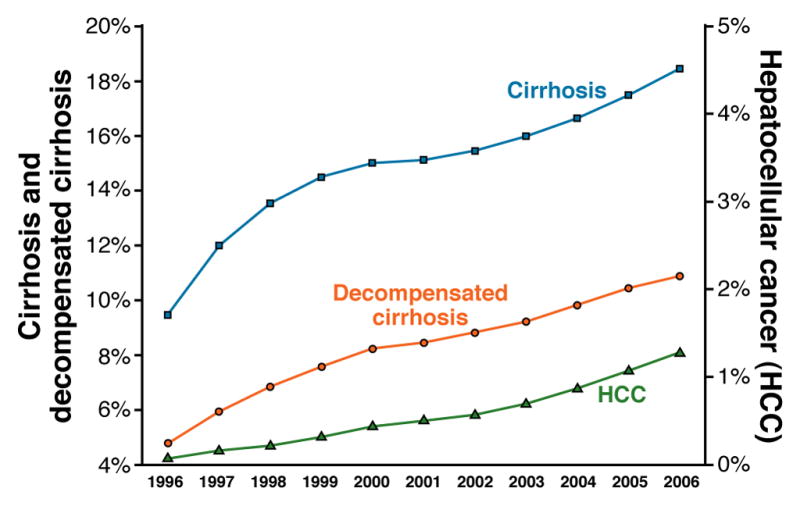
Secular trends in the prevalence of cirrhosis, decompensated cirrhosis (left axis), and HCC (right axis) between 1996 and 2006 among HCV-infected veterans. The annual prevalence rates of these conditions were calculated by dividing the number of HCV patients with either a new or prior diagnosis by the total number of HCV patients with > 1 visit to a veteran’s administration hospital during that particular year. Modified from Kanwal F et al Gastroenterology 2011
World Health Organization (WHO) data indicate a progressive increase in the total number of people diagnosed with primary liver cancer, mostly HCC, from 437,408 cases in 1990 to 714,600 in 200267. In general, HCC incidence and mortality (to be distinguished from number of cases) have been slowly decreasing in areas of high and intermediate incidence, including China and Japan, and increasing in low-incidence areas, including the US and Canada. The percentage of HCC cases associated with HBV has progressively decreased while the percentage associated with HCV has increased. However, each region or country can be its own case study. For example, WHO mortality data from several European countries indicated that between 1980 and 2004, the overall mortality from HCC among men increased in Austria, Germany, and Switzerland, while it decreased significantly in France and Italy68.
HBV and HCV will remain the main risk factors for HCC. It has been estimated that there will be a 2.5-fold increase in the HCV-related mortality worldwide between 2000 and 2020, which can be as high as 3.5-fold increase in Egypt, the country with the highest prevalence of HCV infection69. The 2010 Institute of Medicine report on Hepatitis and Liver Cancer highlighted the lack of awareness about HBV and HCV infections and insufficient understanding about the extent and seriousness of their public health impact. HBV- and HCV-related HCC might be prevented by increasing screening and detection of infected patients, approaches to reduce viral transmission, global vaccination of infants and susceptible adults against HBV, reducing aflatoxin exposure, treating patients with chronic HBV and HCV infections, reducing cofactors for progression (alcohol intake and metabolic syndrome), and identifying high-risk groups for surveillance, early detection, and treatment.
Acknowledgments
This material is based upon work supported in part by the Houston VA HSR&D Center of Excellence [HFP90-020].
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Reference List (FIRST 50 IN PRINT; REST IN SUPPLEMENTAL FILE)
- 1.Fattovich G, Stroffolini T, Zagni I, Donato F. Hepatocellular carcinoma in cirrhosis: incidence and risk factors. Gastroenterology. 2004 Nov;127(5 Suppl 1):S35–S50. doi: 10.1053/j.gastro.2004.09.014. [DOI] [PubMed] [Google Scholar]
- 2.McMahon BJ, Alberts SR, Wainwright RB, Bulkow L, Lanier AP. Hepatitis B-related sequelae. Prospective study in 1400 hepatitis B surface antigen-positive Alaska native carriers. Arch Intern Med. 1990 May;150(5):1051–4. doi: 10.1001/archinte.150.5.1051. [DOI] [PubMed] [Google Scholar]
- 3.Mizokami M, Orito E. Molecular evolution of hepatitis viruses. Intervirology. 1999 Sep;42(2–3):159–65. doi: 10.1159/000024975. [DOI] [PubMed] [Google Scholar]
- 4.Tanaka Y, Kurbanov F, Mano S, et al. Molecular tracing of the global hepatitis C virus epidemic predicts regional patterns of hepatocellular carcinoma mortality. Gastroenterology. 2006 Mar;130(3):703–14. doi: 10.1053/j.gastro.2006.01.032. [DOI] [PubMed] [Google Scholar]
- 5.El-Serag HB, Rudolph KL. Hepatocellular carcinoma: epidemiology and molecular carcinogenesis. Gastroenterology. 2007 Jun;132(7):2557–76. doi: 10.1053/j.gastro.2007.04.061. [DOI] [PubMed] [Google Scholar]
- 6.Chang MH. Hepatitis B virus and cancer prevention. Recent Results Cancer Res. 2011;188:75–84. doi: 10.1007/978-3-642-10858-7_6. [DOI] [PubMed] [Google Scholar]
- 7.Fattovich G, Bortolotti F, Donato F. Natural history of chronic hepatitis B: special emphasis on disease progression and prognostic factors. J Hepatol. 2008 Feb;48(2):335–52. doi: 10.1016/j.jhep.2007.11.011. [DOI] [PubMed] [Google Scholar]
- 8.Yang JD, Kim WR, Coelho R, et al. Cirrhosis is present in most patients with hepatitis B and hepatocellular carcinoma. Clin Gastroenterol Hepatol. 2011 Jan;9(1):64–70. doi: 10.1016/j.cgh.2010.08.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yang HI, Lu SN, Liaw YF, et al. Hepatitis B e antigen and the risk of hepatocellular carcinoma. N Engl J Med. 2002 Jul 18;347(3):168–74. doi: 10.1056/NEJMoa013215. [DOI] [PubMed] [Google Scholar]
- 10.Chen CJ, Yang HI, Iloeje UH. Hepatitis B virus DNA levels and outcomes in chronic hepatitis B. Hepatology. 2009 May;49(5 Suppl):S72–S84. doi: 10.1002/hep.22884. [DOI] [PubMed] [Google Scholar]
- 11.Chen JD, Yang HI, Iloeje UH, et al. Carriers of inactive hepatitis B virus are still at risk for hepatocellular carcinoma and liver-related death. Gastroenterology. 2010 May;138(5):1747–54. doi: 10.1053/j.gastro.2010.01.042. [DOI] [PubMed] [Google Scholar]
- 12.Liaw YF, Sung JJ, Chow WC, et al. Lamivudine for patients with chronic hepatitis B and advanced liver disease. N Engl J Med. 2004 Oct 7;351(15):1521–31. doi: 10.1056/NEJMoa033364. [DOI] [PubMed] [Google Scholar]
- 13.Sung JJ, Tsoi KK, Wong VW, Li KC, Chan HL. Meta-analysis: Treatment of hepatitis B infection reduces risk of hepatocellular carcinoma. Aliment Pharmacol Ther. 2008 Nov 1;28(9):1067–77. doi: 10.1111/j.1365-2036.2008.03816.x. [DOI] [PubMed] [Google Scholar]
- 14.Kao JH, Chen PJ, Lai MY, Chen DS. Basal core promoter mutations of hepatitis B virus increase the risk of hepatocellular carcinoma in hepatitis B carriers. Gastroenterology. 2003 Feb;124(2):327–34. doi: 10.1053/gast.2003.50053. [DOI] [PubMed] [Google Scholar]
- 15.Ni YH, Chang MH, Wang KJ, et al. Clinical relevance of hepatitis B virus genotype in children with chronic infection and hepatocellular carcinoma. Gastroenterology. 2004 Dec;127(6):1733–8. doi: 10.1053/j.gastro.2004.09.048. [DOI] [PubMed] [Google Scholar]
- 16.Liu CJ, Chen BF, Chen PJ, et al. Role of hepatitis B viral load and basal core promoter mutation in hepatocellular carcinoma in hepatitis B carriers. J Infect Dis. 2006 May 1;193(9):1258–65. doi: 10.1086/502978. [DOI] [PubMed] [Google Scholar]
- 17.Yang HI, Yeh SH, Chen PJ, et al. Associations between hepatitis B virus genotype and mutants and the risk of hepatocellular carcinoma. J Natl Cancer Inst. 2008 Aug 20;100(16):1134–43. doi: 10.1093/jnci/djn243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.IARC Monographs. Overall evaluations of carcinogenicity: An updating of IARC monographs. Suppl 7. 1–42. Lyon: IARCPress; 1987. pp. 83–7. [PubMed] [Google Scholar]
- 19.Garner RC, Miller EC, Miller JA. Liver microsomal metabolism of aflatoxin B 1 to a reactive derivative toxic to Salmonella typhimurium TA 1530. Cancer Res. 1972 Oct;32(10):2058–66. [PubMed] [Google Scholar]
- 20.Bressac B, Kew M, Wands J, Ozturk M. Selective G to T mutations of p53 gene in hepatocellular carcinoma from southern Africa. Nature. 1991 Apr 4;350(6317):429–31. doi: 10.1038/350429a0. [DOI] [PubMed] [Google Scholar]
- 21.Turner PC, Sylla A, Diallo MS, Castegnaro JJ, Hall AJ, Wild CP. The role of aflatoxins and hepatitis viruses in the etiopathogenesis of hepatocellular carcinoma: A basis for primary prevention in Guinea-Conakry, West Africa. J Gastroenterol Hepatol. 2002 Dec;17(Suppl):S441–S448. doi: 10.1046/j.1440-1746.17.s4.7.x. [DOI] [PubMed] [Google Scholar]
- 22.Qian GS, Ross RK, Yu MC, et al. A follow-up study of urinary markers of aflatoxin exposure and liver cancer risk in Shanghai, People's Republic of China. Cancer Epidemiol Biomarkers Prev. 1994 Jan;3(1):3–10. [PubMed] [Google Scholar]
- 23.Shi Y, Wu YH, Wei W, Zhang WJ, Yang J, Chen Z. Association between occult hepatitis B infection and the risk of hepatocellular carcinoma: a meta-analysis. Liver Int. 2011 Feb 23; doi: 10.1111/j.1478-3231.2011.02481.x. [DOI] [PubMed] [Google Scholar]
- 24.Goodgame B, Shaheen NJ, Galanko J, El Serag HB. The risk of end stage liver disease and hepatocellular carcinoma among persons infected with hepatitis C virus: publication bias? Am J Gastroenterol. 2003 Nov;98(11):2535–42. doi: 10.1111/j.1572-0241.2003.07678.x. [DOI] [PubMed] [Google Scholar]
- 25.De Mitri MS, Poussin K, Baccarini P, et al. HCV–associated liver cancer without cirrhosis. Lancet. 1995 Feb 18;345(8947):413–5. doi: 10.1016/s0140-6736(95)90400-x. [DOI] [PubMed] [Google Scholar]
- 26.Haydon GH, Jarvis LM, Simmonds P, Hayes PC. Association between chronic hepatitis C infection and hepatocellular carcinoma. Lancet. 1995 Apr 8;345(8954):928–9. doi: 10.1016/s0140-6736(95)90045-4. [DOI] [PubMed] [Google Scholar]
- 27.Tong MJ, Lai LP, Murakami-Mori K. Development of hepatocellular carcinoma after clearance of hepatitis C virus with interferon therapy. West J Med. 1997 Aug;167(2):103–5. [PMC free article] [PubMed] [Google Scholar]
- 28.Freeman AJ, Dore GJ, Law MG, et al. Estimating progression to cirrhosis in chronic hepatitis C virus infection. Hepatology. 2001 Oct;34(4 Pt 1):809–16. doi: 10.1053/jhep.2001.27831. [DOI] [PubMed] [Google Scholar]
- 29.Raimondi S, Bruno S, Mondelli MU, Maisonneuve P. Hepatitis C virus genotype 1b as a risk factor for hepatocellular carcinoma development: a meta-analysis. J Hepatol. 2009 Jun;50(6):1142–54. doi: 10.1016/j.jhep.2009.01.019. [DOI] [PubMed] [Google Scholar]
- 30.Lee MH, Yang HI, Lu SN, et al. Hepatitis C virus seromarkers and subsequent risk of hepatocellular carcinoma: long-term predictors from a community-based cohort study. J Clin Oncol. 2010 Oct 20;28(30):4587–93. doi: 10.1200/JCO.2010.29.1500. [DOI] [PubMed] [Google Scholar]
- 31.Kramer JR, Giordano TP, El-Serag HB. Effect of human immunodeficiency virus and antiretrovirals on outcomes of hepatitis C: a systematic review from an epidemiologic perspective. Clin Gastroenterol Hepatol. 2007 Nov;5(11):1321–8. doi: 10.1016/j.cgh.2007.08.006. [DOI] [PubMed] [Google Scholar]
- 32.Modi AA, Feld JJ, Park Y, et al. Increased caffeine consumption is associated with reduced hepatic fibrosis. Hepatology. 2010 Jan;51(1):201–9. doi: 10.1002/hep.23279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Freedman ND, Everhart JE, Lindsay KL, et al. Coffee intake is associated with lower rates of liver disease progression in chronic hepatitis C. Hepatology. 2009 Nov;50(5):1360–9. doi: 10.1002/hep.23162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bravi F, Bosetti C, Tavani A, et al. Coffee drinking and hepatocellular carcinoma risk: a meta-analysis. Hepatology. 2007 Aug;46(2):430–5. doi: 10.1002/hep.21708. [DOI] [PubMed] [Google Scholar]
- 35.Larsson SC, Wolk A. Coffee consumption and risk of liver cancer: a meta-analysis. Gastroenterology. 2007 May;132(5):1740–5. doi: 10.1053/j.gastro.2007.03.044. [DOI] [PubMed] [Google Scholar]
- 36.Huxley R, Lee CM, Barzi F, et al. Coffee, decaffeinated coffee, and tea consumption in relation to incident type 2 diabetes mellitus: a systematic review with meta-analysis. Arch Intern Med. 2009 Dec 14;169(22):2053–63. doi: 10.1001/archinternmed.2009.439. [DOI] [PubMed] [Google Scholar]
- 37.Yuan JM, Ross RK, Stanczyk FZ, et al. A cohort study of serum testosterone and hepatocellular carcinoma in Shanghai, China. Int J Cancer. 1995 Nov 15;63(4):491–3. doi: 10.1002/ijc.2910630405. [DOI] [PubMed] [Google Scholar]
- 38.Chiu CM, Yeh SH, Chen PJ, et al. Hepatitis B virus X protein enhances androgen receptor-responsive gene expression depending on androgen level. Proc Natl Acad Sci U S A. 2007 Feb 20;104(8):2571–8. doi: 10.1073/pnas.0609498104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Yeh SH, Chen PJ. Gender disparity of hepatocellular carcinoma: the roles of sex hormones. Oncology. 2010 Jul;78( Suppl 1):172–9. doi: 10.1159/000315247. [DOI] [PubMed] [Google Scholar]
- 40.White DL, Tavakoli-Tabasi S, Kuzniarek J, Pascua R, Ramsey DJ, El-Serag HB. Higher serum testosterone is associated with increased risk of advanced hepatitis C-related liver disease in males. Hepatology. 2011 Aug 19; doi: 10.1002/hep.24618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Donato F, Boffetta P, Puoti M. A meta-analysis of epidemiological studies on the combined effect of hepatitis B and C virus infections in causing hepatocellular carcinoma. Int J Cancer. 1998 Jan 30;75(3):347–54. doi: 10.1002/(sici)1097-0215(19980130)75:3<347::aid-ijc4>3.0.co;2-2. [DOI] [PubMed] [Google Scholar]
- 42.Shi J, Zhu L, Liu S, Xie WF. A meta-analysis of case-control studies on the combined effect of hepatitis B and C virus infections in causing hepatocellular carcinoma in China. Br J Cancer. 2005 Feb 14;92(3):607–12. doi: 10.1038/sj.bjc.6602333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Cho LY, Yang JJ, Ko KP, et al. Coinfection of hepatitis B and C viruses and risk of hepatocellular carcinoma: systematic review and meta-analysis. Int J Cancer. 2011 Jan 1;128(1):176–84. doi: 10.1002/ijc.25321. [DOI] [PubMed] [Google Scholar]
- 44.Hutchinson SJ, Bird SM, Goldberg DJ. Influence of alcohol on the progression of hepatitis C virus infection: a meta-analysis. Clin Gastroenterol Hepatol. 2005 Nov;3(11):1150–9. doi: 10.1016/s1542-3565(05)00407-6. [DOI] [PubMed] [Google Scholar]
- 45.Donato F, Tagger A, Gelatti U, et al. Alcohol and hepatocellular carcinoma: the effect of lifetime intake and hepatitis virus infections in men and women. Am J Epidemiol. 2002 Feb 15;155(4):323–31. doi: 10.1093/aje/155.4.323. [DOI] [PubMed] [Google Scholar]
- 46.Ikeda K, Saitoh S, Suzuki Y, et al. Disease progression and hepatocellular carcinogenesis in patients with chronic viral hepatitis: a prospective observation of 2215 patients. J Hepatol. 1998 Jun;28(6):930–8. doi: 10.1016/s0168-8278(98)80339-5. [DOI] [PubMed] [Google Scholar]
- 47.Jee SH, Ohrr H, Sull JW, Samet JM. Cigarette smoking, alcohol drinking, hepatitis B, and risk for hepatocellular carcinoma in Korea. J Natl Cancer Inst. 2004 Dec 15;96(24):1851–6. doi: 10.1093/jnci/djh334. [DOI] [PubMed] [Google Scholar]
- 48.Chuang SC, Lee YC, Hashibe M, Dai M, Zheng T, Boffetta P. Interaction between cigarette smoking and hepatitis B and C virus infection on the risk of liver cancer: a meta-analysis. Cancer Epidemiol Biomarkers Prev. 2010 May;19(5):1261–8. doi: 10.1158/1055-9965.EPI-09-1297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.El-Serag HB, Hampel H, Javadi F. The association between diabetes and hepatocellular carcinoma: a systematic review of epidemiologic evidence. Clin Gastroenterol Hepatol. 2006 Mar;4(3):369–80. doi: 10.1016/j.cgh.2005.12.007. [DOI] [PubMed] [Google Scholar]
- 50.Wang P, Kang D, Cao W, Wang Y, Liu Z. Diabetes mellitus and risk of hepatocellular carcinoma: a systematic review and meta-analysis. Diabetes Metab Res Rev. 2011 Sep 5; doi: 10.1002/dmrr.1291. [DOI] [PubMed] [Google Scholar]