Abstract
In transmissible spongiform encephalopathies (TSEs) and Alzheimer disease (AD) both misfolding and aggregation of specific proteins represent key features. Recently, it was observed that PrPC is a mediator of a synaptic dysfunction induced by Aβ oligomers. We tested a novel γ-secretase modulator (CHF5074) in a murine model of prion disease. Groups of female mice were intracerebrally or intraperitoneally infected with the mouse-adapted Rocky Mountain Laboratory prions. Two weeks prior infection, the animals were provided with a CHF5074-medicated diet (375 ppm) or a standard diet (vehicle) until they showed neurological signs and eventually died. In intracerebrally infected mice, oral administration of CHF5074 did not prolong survival of the animals. In intraperitoneally-infected mice, CHF5074-treated animals showed a median survival time of 21 d longer than vehicle-treated mice (p < 0.001). In these animals, immunohistochemistry analyses showed that deposition of PrPSc in the cerebellum, hippocampus and parietal cortex in CHF5074-treated mice was significantly lower than in vehicle-treated animals. Immunostaining of glial fibrillary acidic protein (GFAP) in parietal cortex revealed a significantly higher reactive gliosis in CHF5074-treated mice compared with the control group of infected animals. Although the mechanism underlying the beneficial effects of CHF5074 in this murine model of human prion disease is unclear, it could be hypothesized that the drug counteracts PrPSc toxicity through astrocyte-mediated neuroprotection. CHF5074 shows a pharmacological potential in murine models of both AD and TSEs thus suggesting a link between these degenerative pathologies.
Key words: TSE, prion, murine model, γ-secretase modulator, therapy
Introduction
Transmissible spongiform encephalopathies (TSEs), or prion diseases, are a group of rare and fatal neurodegenerative pathologies affecting both animals and humans. They may occur as sporadic form, which is the most common, and as inherited forms, associated with mutations within the prion protein gene (PRNP), or as acquired forms, due to transmission of an infectious agent.1–3 TSEs are characterized by progressive neuronal loss, often accompanied by a spongiform brain alteration and deposition of amyloid fibrils.2,8
According to the prion-only hypothesis, the pathology of TSEs is initiated by post-translational modification of a native glycoprotein, known as prion protein (PrPC) that develops the pathogenic isoform called prion (proteinaceous infectious only-PrPSc). Conversion of PrPC into PrPSc involves a conformational change. An α-helical conformation is prevalent in the protease-sensitive PrPC while a β-sheet conformation is dominant in the refolded and insoluble protease-resistant PrPSc.1–5 The localization of the prion protein in the membrane lipid rafts seems to play a role in sustaining the protein misfolding.6,7 PrPSc forms highly insoluble β-sheet aggregates within the brain where PrPC is abundantly expressed; it is also considered responsible for neurological damages occurring in prion diseases.
Protein misfolding and amyloid deposition in the central nervous system (CNS) are pathogenic characteristics of both TSEs and AD. AD is characterized by the formation of extracellular insoluble plaques mainly consisting of amyloid-β (Aβ), a 40–42 amino acid peptide. Aβ is formed by the cleavage of the amyloid precursor protein (APP).9 In human APP transgenic mice, prion infection promotes brain Aβ accumulation.10 The codon 129 polymorphism of the human PRNP gene was reported to increase Aβ deposition in aged brains and methionine homozygosis at codon 129 was suggested to be a risk factor for an early onset of AD and variant Creutzfeldt-Jakob Disease (vCJD).11–14 Both APP and PrPC are proteins located in the lipid rafts of the cell membrane. In physiological conditions, both proteins undergo a proteolytic cleavage mediated by α-secretases (ADAM 10 and ADAM 17) inside their potentially toxic core. In pathologic conditions, both APP and PrPC experience alternative cleavages that maintain their toxic domains intact (Aβ and PrPC 106–126 sequences, respectively). PrPSc seems to derive from a unique cleavage of PrPC sustained by an unknown activity.9
Aβ is produced from APP by sequential proteolytic cleavage of β- and γ-secretase. γ-Secretase is a complex of four different membrane proteins: presenilin, nicastrin, anterior pharynx defective-1 and presenilin enhancer 2. Presenilin is believed to contain the catalytic core of γ-secretase. γ-Secretase mediates the ultimate step in the release of Aβ, cleaving APP within the membrane. It has no sequence specificity since it generates different Aβ peptides of different lengths but it is conformation specific since it selectively cleaves α-helical substrates. γ-Secretase complex cleaves many substrates other than APP. The most important of these substrates is Notch-1 that is cleaved to the Notch-1 intracellular domain (NICD), a transcription factor. Translocation of NICD to neuronal nuclei stimulates expression of important genes such those encoding for the HES (Hairy and Enhancer of Split) proteins involved in the development of dendrites and axons.15 Studies in scrapie-infected mice have shown an increase of brain levels of both NICD and PrPSc preceding dendritic atrophy and loss.16 A recent study in scrapie-infected mice found that a potent γ-secretase inhibitor (LY411575) suppressed NICD and reduced PrPSc levels in the cortex but was not able to prevent dendritic atrophy or prolong survival of prion-inoculated mice compared with controls.17 Whether other γ-secretase interfering drugs, like γ-secretase modulators, can be used in place of prototypical γ-secretase inhibitors to prolong survival of prion-infected animals remains to be determined. Recently, it has been shown that PrPC mediates the impairment of synaptic plasticity induced by Aβ oligomers18 and that memory impairment in transgenic mouse models of AD mice requires PrPC.19 These findings prompted us to verify if a γ-secretase modulator that was shown to be neuropathologically and behaviorally effective in different transgenic mouse models of AD (CHF5074) could be also effective in a mouse model of scrapie. Indeed, CHF5074 {1-[3′,4′-dichloro-2-fluoro(1,1′-biphenyl)-4-yl]-cyclopropanecarboxylic acid} has been shown to attenuate brain Aβ deposition and memory deficit in two different transgenic murine models of AD.20–22 In rodents, the drug has a good oral bioavailability (50%) and is slowly eliminated from blood (half-life ∼ 20 h).23 Prolonged treatments with CHF5074 are well tolerated with no signs of Notch-mediated toxicity.20
Results
Animal survival.
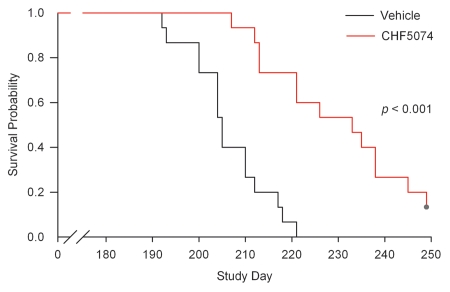
Uninfected treated and control animals did not show any clinical signs of disease. In infected vehicle-treated mice, early clinical signs of prion disease appeared at about Day 130 in the i.c.-inoculated animals and at Day 180 in i.p.-inoculated mice. Mice initially displayed ruffled coats, assumed kyphotic posture and a tendency to display a straight tail. These early signs of prion disease were followed by ragged or wobbly gait, ataxia and proprioceptive deficits, as evidenced by clasped feet when raised by tail. They then became extremely listless, lethargic and cachectic and appeared to adopt a frozen posture. All mice of the infected groups, except for two animals in the i.p.-infected CHF5074-treated group, reached the terminal disease phase. Sacrifice of the i.c.-infected mice in terminal phase started at Day 143 and ended at Days 177 and 178 in control and CHF5074-treated animals, respectively. Sacrifices of the i.p.-inoculated mice reaching clinical end-point took place between Day 192 and Day 221 for the control group and between Day 207 and Day 249 for the CHF5074-treated group. The two CHF5074-treated mice showing no abnormal clinical signs were steadily fed with the medicated diet until Day 318 and then put on standard diet (vehicle) for 44 additional days during which no clinical signs of disease were observed. At the end of this period, these two animals were sacrificed. As for i.c.-infected mice, no differences in median survival times were observed between CHF5074-treated (161 d) and control (161 d) mice. On the contrary, a significant difference emerged between the median survival times of i.p.-infected controls (205 d) and CHF5074-treated (226 d) animals (p < 0.001) (Fig. 1).
Figure 1.
Survival curve of intraperitoneally-infected mice. Log-Rank test indicates a significant difference (p < 0.001) between the median survival time of vehicle- and CHF5074-treated animals (205 vs. 226 d, respectively).
Western blot analysis.
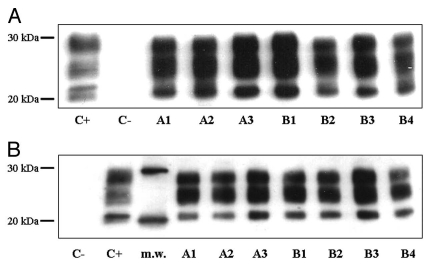
All mice sacrificed at clinical end stage of disease showed presence of PrPSc in their brains, characterized by three bands corresponding to di-, mono- and un-glycosylated forms with a molecular weight between 30 and 20 kDa. Representative western blot profiles of PrPSc extracted from the brains of i.c.- and i.p.-infected mice are shown in Figure 2, and the corresponding quantification of densitometric analysis is given in Figure 3. No significant differences appeared between vehicle- and CHF5074-treated groups in brain PrPSc levels both in i.c- and i.p.-inoculatedanimals. Western blot analysis performed on the brains of mice that were sacrificed without clinical signs of disease, including the two i.p.-infected mice treated with CHF5074, did not reveal any presence of PrPSc.
Figure 2.
Representative western blot profiles of PrPSc extracted from brains of intracerebrally-(A) and intraperitoneally-(B) infected mice. Lanes A1–A3 show three vehicle-treated mice, lanes B1–B4 deal with four CHF5074-treated mice, and lane C− considers a control non-infected mouse control. PrPSc signals of each sample were quantified comparing them with PrPSc signals of an ovine with classical scrapie (lane C+), used as reference. M.w. indicates molecular mass markers. Membrane was probed with monoclonal antibody SAF 70.
Figure 3.
Quantification of PrPSc signals obtained from western blot analyses of brains of intracerebrally-(A) and intraperitoneally-(B) infected mice treated with vehicle or CHF5074. Columns indicate the mean of optical densities. Error bars represent the standard error of the means.
Histological and immunohistochemical analysis.
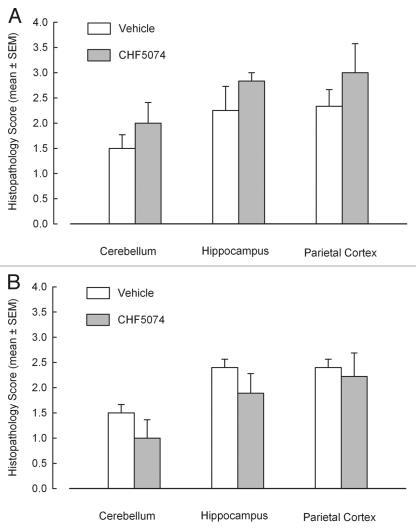
Hematoxylin-eosin staining allowed to detect spongiosis in nervous tissue and to create a lesion profile of the different encephalic areas. Figure 4 illustrates mean spongiosis severity score in different brain areas of i.c. and i.p. infected mice. No significant differences were found between vehicle- and CHF5074-treated groups.
Figure 4.
Severity of spongiosis in cerebellum, hippocampus and parietal cortex in hematoxilin-eosin stained samples of intracerebrally-(A) and intraperitoneally-(B) infected mice treated with vehicle or CHF5074. Columns indicate the mean of spongiosis severity for each group. Error bars represent the standard error of the means.
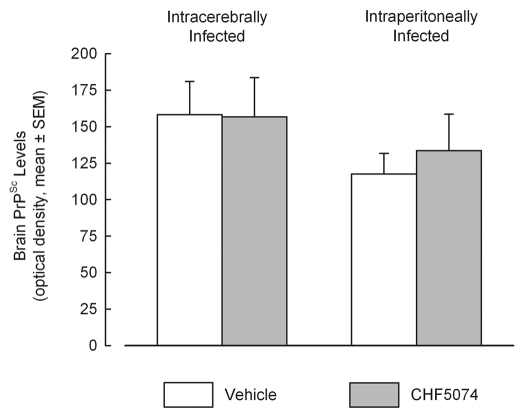
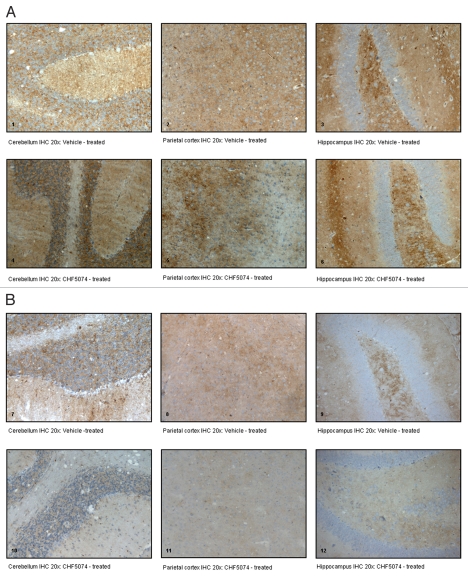
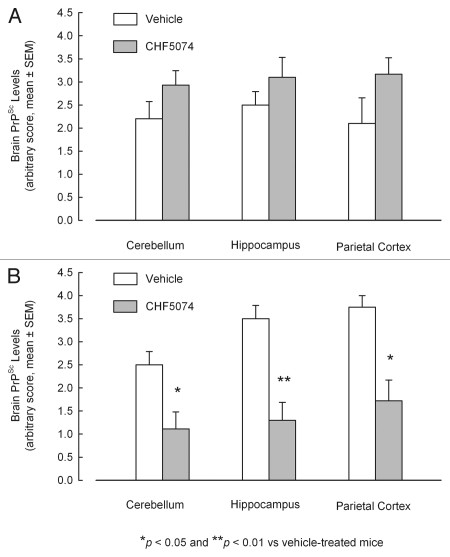
Immunohistochemical analysis confirmed presence of PrPSc in all samples from symptomatic animals. Representative images of i.c.- and i.p.-infected mice are presented in Figure 5. As for the i.c.-infected-mice, there was no difference in the quantification of PrPSc between vehicle- and CHF5074-treated mice (Fig. 6A). Conversely, within i.p. infected mice group, CHF5074-treated animals showed a significant lower amount of PrPSc in their cerebellum (p < 0.05), hippocampus (p < 0.01) and parietal cortex (p < 0.05) than vehicle-treated animals (Fig. 6B).
Figure 5.
Representative immunohistochemical staining with monoclonal antibody ICSM35. (A) coronal brain sections of intracerebrally-infected mice treated with vehicle (1. Cerebellum IHC 20x; 2. Parietal Cortex IHC 20x; 3. Hippocampus IHC 20x) or CHF5074 (4. Cerebellum IHC 20x; 5. Parietal Cortex IHC 20x; 6. Hippocampus IHC 20x); (B) coronal brain sections of intraperitoneally-infected mice treated with vehicle (7. Cerebellum IHC 20x; 8. Parietal Cortex IHC 20x; 9. Hippocampus IHC 20x) or CHF5074 (10. Cerebellum IHC 20x; 11. Parietal Cortex IHC 20x; 12. Hippocampus IHC 20x).
Figure 6.
Mean quantification scores of PrPSc deposition in cerebellum, hippocampus and parietal cortex of intracerebrally- (A) and intraperitoneally- (B) infected mice treated with vehicle or CHF5074. Columns indicate mean severity score of PrPSc staining by immunohistochemistry (ICSM 35 monoclonal antibodies). Error bars represent the standard error of the means.
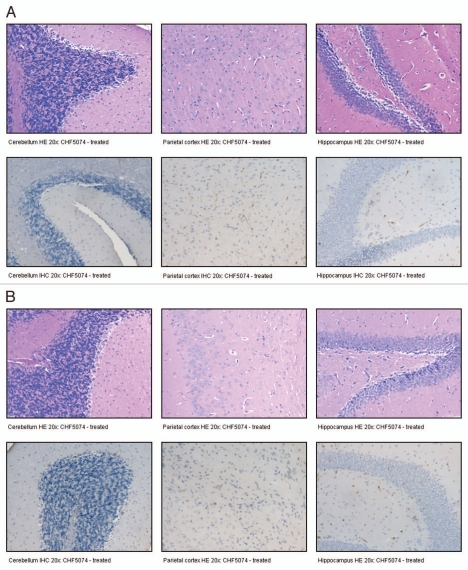
Histological and immunohistochemical analyses performed on the brains of the two i.p. infected animals treated with CHF5074 that did not show any neurological signs, disclosed neither presence of PrPSc nor of neuropathological lesions associated to prion diseases. Histopathology and immunohistochemistry images of these two animals are reported in Figure 7.
Figure 7.
Histopathology and immunohistochemistry images of two intraperitoneally-infected mice (A: animal H12; B: animal H13) treated with CHF5074 which did not develop neurological signs and survived.
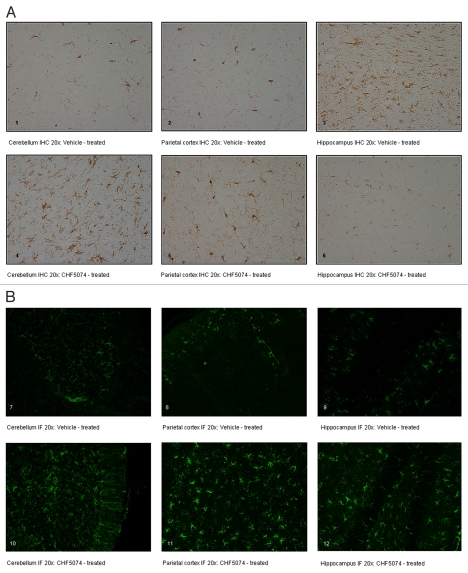
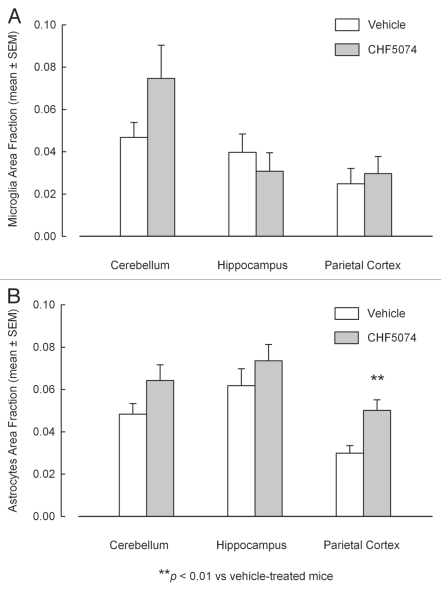
Iba-1 immunostaining in representative sections of cerebellum, hippocampus and parietal cortex of i.p. infected vehicle- and CHF5074-treated mice are shown in Figure 8A. Quantification of immunoreactivity did not reveal significant differences in brain area fraction occupied by immunoreactive microglia in vehicle- and CHF5074-treated mice (Fig. 9A).
Figure 8.
(A) Representative immunohistochemical staining with polyclonal antibody iba-1. Coronal brain sections of intraperitoneally-infected mice treated with vehicle (1. Cerebellum IHC 20x; 2. Parietal Cortex IHC 20x; 3. Hippocampus IHC 20x) or CHF5074 (4. Cerebellum IHC 20x; 5. Parietal Cortex IHC 20x; 6. Hippocampus IHC 20x). (B) Representative immunofluorescence staining with polyclonal antibody GFAP. Coronal brain sections of intraperitoneally infected mice treated with vehicle (7. Cerebellum IF 20x; 8. Parietal Cortex IF 20x; 9. Hippocampus IF 20x) or CHF5074 (10. Cerebellum IF 20x; 11. Parietal Cortex IF 20x; 12. Hippocampus IF 20x).
Figure 9.
Quantification of immunostaining of activated microglia (A) and reactive astrocytes (B) in cerebellum, hypothalamus and parietal cortex of intraperitoneally-infected mice treated with vehicle or CHF5074. Columns indicate the mean brain area fraction occupied by Iba-1 immunoreactive microglia (A) and GFAP-immunoreactive astrocytes (B). Error bars represent the standard error of the means.
GFAP immunostaining in representative sections of brain regions of i.p. infected vehicle- and CHF5074-treated mice are shown in Figure 8B. Quantification of immunoreactivity expressed as area fraction occupied by immunoreactive astrocytic filaments is shown in Figure 9B. Compared with vehicle-treated infected mice, the three brain regions analyzed in CHF5074-treated mice had higher area fraction of GFAP immunoreactivity, being the difference in parietal cortex statistically significant (p = 0.008).
Discussion
Chronic oral administration of CHF5074 with the diet (about 60 mg/kg x day) for up to 8 mo was well tolerated by both scrapie-infected and non-infected CD1 mice. Body weight and clinical signs of CHF5074-treated animals were similar to those of control mice. Also prolonged oral administration of CHF5074 in other mice strains was previously proved to have good tolerability.22
Two intraperitoneally-infected mice treated with a CHF5074-medicated diet did not develop any sign of prion disease. It is our belief that their survival cannot be due to a wrong scrapie inoculation. Indeed, 2 inefficient inoculations out of 60 are much unlikely to occur only by chance in the same group, i.e., CHF5074-treated, intraperitoneally-infected animals (p = 0.059, Fisher's exact test). These animals remained asymptomatic even after CHF5074 treatment was over, and showed no neuropathological or immunohistochemical abnormalities (Fig. 7). However, we cannot exclude that the administered dose of prions (50 µL of 1% mouse RML-infected brain homogenate) was too low to these two animals. Indeed, low doses of prions seem to correlate with fewer animals with PrPSc pathology, although this does not correlate with the onset of neurological symptoms.28
The time of onset and the pattern of clinical signs observed our study are compatible with those described in mice infected by RML scrapie strain.24,25 Histology showed spongiosis, a typical neuropathological feature of TSEs, while immunohistochemical and western blot analyses confirmed presence of PrPSc in the brain of these animals without significant regional differences.
The significant difference of survival time displayed by CHF5074-treated and control mice in i.p. infected group was not observed in i.c. infected animals. This suggests that CHF5074 concentrations at brain level were not high enough to counteract the high infection load achieved after intracerebral scrapie inoculation. Plasma CHF5074 concentrations measured in two i.p.-infected mice were around 270 µM (263 e 282 µM, respectively). Brain levels in these animals were not measured but, according to previous studies, they were expected to be about 2–3% of the corresponding plasma levels (i.e., 5–8 µM).20,21 This means that the delayed disease onset due to CHF5074 treatment should occur during prion propagation from periphery to brain.
Histology and immunohistochemistry analyses of the brains of i.c. infected mice did not show significant differences between vehicle- and CHF5074-treated animals. Conversely, immunohistochemical analysis of i.p. infected mice indicated noticeably lower PrPSc deposits in different brain areas (cerebellum, hippocampus and parietal cortex) of CHF5074-treated animals compared with controls. However, western blot analysis of a limited brain area did not confirm meaningful differences in PrPSc accumulation between treatment groups. This apparent discrepancy may be due to the fact that immunohistochemistry of paraffin-embedded tissues can highlight PrPSc deposits at cellular level and their distribution in different brain areas, whereas western blot assay detects PrPSc at molecular level but cannot localize the protein because sample preparation requires a homogenization step. On the other hand, it should be noted that PrPSc deposits in brain sections were quantified using an arbitrary evaluation instead of the more rigorous densitometric analysis of photomicrographs. Because of this limitation and of the apparently inconsistent effects observed by western blot in brain sections, the ability of CHF5074 in reducing PrPSc brain deposits needs to be confirmed through further experiments using quantitative immunohistochemistry and western blot recognizing the same PrP residues.
Histological analysis of the brains of i.p. infected mice did not encounter significant differences between treatment groups, maybe because spongiosis is not directly proportional to PrPSc deposition in the final stages of disease.29
The protective effects of CHF5074 in this murine model of human prion disease need to be further investigated. Scrapie-infected mice show marked reactive astrogliosis around neuropathological lesions.30 The appearance of astrogliosis has been postulated to provide neurotrophic factors to prevent or reduce neuronal cell loss in the pathogenesis of prion diseases.31 Previous studies have shown that CHF5074 may alter astrocyte reactivity in vitro.32 In addition, CHF5074 has been demonstrated to stimulate astrocytes migration toward large Aβ plaques in the Tg2576 mouse model of AD.22 Therefore, it could be hypothesized that CHF5074-induced beneficial effects in scrapie-infected mice may be due to its modulatory effects on reactive astrocytes with a migration of glial cells toward PrPSc aggregates.
Alternative processes can also be considered. Expression of PrPC is required for the neurotoxic effect of PrP 106–12633 and mice devoid of PrPC are resistant to scrapie.34 Similarly, PrPC is required for impairment of synaptic plasticity induced by Aβ oligomers in hippocampal slices,18 and familial AD transgenic mice knocked-out for PrPC showed brain Aβ accumulation without developing memory deficit.19 It could be hypothesized that CHF5074 may improve memory in AD transgenic mice by binding and inactivating PrPC on the surface of neuronal membrane.35 Similarly, CHF5074 may delay prion disease onset acting as antagonist of cell-surface receptors for PrPSc on neurons of scrapie-infected mice.
Therapeutic activity of CHF5074 in the present murine model of prion diseases might also be explained considering γ-secretase as the unknown protease involved in the pathologic cleavage of PrPC. It is an almost unlikely hypothesis, however, since γ-secretase exerts its proteolytic activity within the plasma membrane, whereas the pathologic breakdown of the prion protein is located at 90–91 N-terminal site.36,37 N-terminal portion is a copper and zinc binding octapeptide repeat region and it is usually reputed not to interact with a membrane, being PrPC a cell surface glycosyl-phosphatidylinositol (GPI)-anchored protein. Nevertheless, several in vitro studies indicated that GPI-anchor is not obligatory for the PrP raft association, which can be mediated by a determinant within N-terminal region of the ectodomain (residues 23–90).38–40 Furthermore, in vivo studies showed that N-terminal region strongly influenced both susceptibility to and incubation time of prion disease.3,41 Interaction between γ-secretase and N-terminal region may then be possible and plausible. This is an unexpected but not isolated event, being the involvement of disintegrin (ADAM) in the physiological catabolism of prion protein similarly unusual; in fact, substrates of disintegrins are generally transmembrane proteins, such as APP.9
In conclusion, this study has shown encouraging beneficial effects of CHF5074 in a murine animal model of prion disease but these preliminary findings need to be confirmed in further studies and the underlying mechanism of these potential beneficial effects need to be elucidated.
Materials and Methods
Animals and treatments.
Ninety-one CD1 female mice aged 3–4 weeks and initially weighing 10–12 g were under survey. Outbred CD1 mouse strain was used to mimic the conditions of a previous study with a γ-secretase inhibitor.17 Mice were maintained in a controlled environment (22 ± 1°C, 55 ± 5% relative humidity, 12 h light/dark cycles) and fed ad libitum. Animals were randomly divided into two groups according to the route of infection (intracerebrally, i.c., or intraperitoneally, i.p.) with the mouse-adapted Rocky Mountains Laboratories (RML) prions. A 10% (weight/volume) homogenate of RML-infected CD1 brain was diluted in sterile saline to a final concentration of 1%, and 25 µL or 50 µL of the suspension was injected i.c. or i.p., respectively, as described by Spilman et al.19 The day of scrapie inoculation was considered as Day 1 of the study. Animals of the two infected groups (i.c. or i.p.) were then split into two subgroups, each consisting of 15 treated or control mice (i.c. infected-treated, i.c. infected-controls; i.p. infected-treated and i.p. infected-controls). Similarly, uninfected animals were divided into two subgroups consisting of 8 animals each. These were then treated and i.c. or i.p. inoculated with the same volume of a 1% brain homogenate from uninfected CD1 mice (i.c. uninfected-treated and i.p. uninfected-treated). Furthermore, a negative control group was created (Table 1). Treated animals received a CHF5074 medicated oral diet (in the dosage of 375 ppm, corresponding to approximately 60 mg/kg × day) while control animals were given a standard diet (vehicle). CHF5074 was manufactured by Minakem (Beuvry-la-Forêt, France) and CHF5074-medicated diet by Mucedola (Settimo Milanese, Italy). CHF5074-medicated and standard diets were started 14 d before scrapie strain inoculation. Mice were daily monitored for onset and development of neurological signs, such as kyphotic posture, ataxia, proprioceptive deficits, lethargy and frozen posture. All mice were sacrificed when severe symptoms appeared as previously described in references 24 and 25.
Table 1.
Study groups
| Study group | Route of scrapie infection | Treatment | Number of animals |
| negative controls | none | standard diet (vehicle) | 15 |
| i.c. uninfected-treated | intracerebral | CHF5074-medicated diet | 8 |
| i.p. uninfected-treated | intraperitoneal | CHF5074-medicated diet | 8 |
| i.c. infected-controls | intracerebral | standard diet (vehicle) | 15 |
| i.c. infected-treated | intracerebral | CHF5074-medicated diet | 15 |
| i.p. infected-controls | intraperitoneal | standard diet (vehicle) | 15 |
| i.p. infected-treated | intraperitoneal | CHF5074-medicated diet | 15 |
Mice were randomly divided into groups depending on the route of scrapie infection and on the type of treatment (vehicle or CHF5074). The concentration of CHF5074 in the medicated diet was 375 ppm.
At necropsy, cerebral hemispheres, brain stem and cerebellum were removed. Each brain was then divided longitudinally; one part was fixed in 10% formalin for histopathological and immunohistochemical analysis, and the other one was stored at −20°C for western blot analysis.
Western blot analysis.
Ten percent (w/v) homogenates of each frozen brain were prepared in lysis buffer (10% N-lauroylsarcosine diluted in Tris Buffer Saline pH 7.4). After incubation for 20–30 min at room temperature, they were clarified by centrifugation at 22,000x g for 20 min at 10°C (Ultracentrifuge Optima TLX, Rotor TLA 55, Beckman Coulter, Fullerton, CA). A rate of 1 mL was removed from each supernatant and digested with proteinase K (40 µg/mL; Product No FS-M-112; Fisher Molecular Biology, Trevose, PA) for 1 h at 37°C with continuous shaking. After digestion, 10 µL of the proteinase K inhibitor phenylmethyl sulfonyl fluoride (PMSF 100 mM; Product No A0999; Applichem, Germany) was added. The samples were then centrifuged at 215,000x g for 1 h at 10°C (Ultracentrifuge Optima TLX, Rotor TLA 110, Beckman Coulter, Fullerton, CA). The obtained pellets were dissolved in 50 µL of Laemmli buffer. After 5–10 min boiling f at 99°C, 10 µL of each extract (10 mg of tissue) was separated by sodium dodecyl-sulfate PAGE on a 12% handmade minigel (acrylamide to bisacrylamide ratio of 37.5:1) and then transferred onto PVDF membranes (Product No IPVH20200; Immobilion P, Millipore, Billerica, MA). Blots were blocked with TBS-BSA 5% and incubated at 4°C overnight with monoclonal antibody SAF 70 (0.5 µg/mL; Product No A023206; Spi Bio, Cayman Chemical, Ann Arbor, MI), revealing r human PrP residues 142–160. Immunodetection was performed with an alkaline phosphatase-conjugated goat anti-mouse IgG (Product No 12000-09; Prionics AG, Switzerland) revealed by a chemiluminescent substrate (Product No 170-5012; ImmunoStar, Bio-Rad Laboratories, Hercules, CA) and then visualized onto Hyperfilm ECL (Product No 28906837; GE-Healthcare, St. Giles, UK) or by a UVI Prochemi (Uvitec, Cambridge, UK) analysis system. PrPSc signals of each sample were quantified using UVI Bandmap software (Uvitec, Cambridge, UK), that compared them with PrPSc signals of a classical reference scrapie sample.
Histopathological, immunohistochemical and immunofluorescence analysis.
Following fixation, brains were coronally cut into five sections (medulla, pons and cerebellum, mid-brain, diencephalon, telencephalon).26 These samples were processed and embedded in paraffin wax according to standard histopathological procedures. The 3 µm-thick sections obtained from each hemisphere were placed on slides with positive electrostatic charge and left for 24 h at 37°C. An hematoxylin-eosin staining was performed for each brain section. Slides for PrPSc immunohistochemical analysis were dewaxed and rehydrated according to routine methods and then immersed in 98% formic acid for 15 min. After water washing, the sections were autoclaved for 30 min at 121°C in citrate buffer (pH 6.1) to unmask antigenic sites. Endogenous peroxidase activity was blocked in 3% hydrogen peroxide diluted in methanol for 20 min at room temperature and samples were left overnight in distilled water at 2–8°C. In order to block non-specific tissue antigens, the sections were incubated with 2% horse blocking serum (pH 7.4) for 20 min at room temperature and then incubated for 1 h at room temperature with the mouse monoclonal antibody ICSM 35 that had been diluted 1:1,000 (Product No. 0130-03501, D-Gen Ltd., London UK). ICSM 35 monoclonal antibodies have shown to efficiently immunoprecipitate native PrPSc and recognize epitopes between residues 93–102.27 After rinsing in Tris-Buffered Saline Tween-20 (TBST), a biotinylated goat anti-mouse secondary antibody (1:200 dilution, Vectastain ABC kit PK-4002, Vector Laboratories) was applied to the tissue sections for 30 min at room temperature, followed by avidin-biotin-peroxidase complex (Vectastain ABC kit PK-4002, Vector Laboratories), according to the manufacturer's protocol. After rinsing in TBST, PrPSc immunoreactivity was visualized using 3,3′-diaminobenzidine (Dako Liquid DAB+ Substrate Chromogen System Cod. K3468, Dako) as a chromogen, blocked with distilled water. The sections were then counterstained with Meyer's hematoxylin.
Spongiosis and PrPSc deposition in different encephalic areas (medulla, cerebellum, mid-brain, hypothalamus, thalamus, hippocampus, para terminal body, frontal cortex and parietal cortex) were subjectively evaluated with light microscopy by two independent pathologists, and an intensity grade was assigned to vacuolation and to the different detected patterns: absent (0), slight (1), moderate (2), marked (3), very marked (4).
Microglia immunohistochemistry was performed on three brain areas: parietal cortex, hippocampus and cerebellum using 1:500 diluted anti Iba-1 (019-19741, Wako Chemicals, Osaka, Japan) as primary antibody. Slides were subjected to regular microwave heating (15 min), immersed in citrate buffer (pH = 6.1) to expose the epitope. Vectastain ABC System (PK 4001, Vector Laboratories, Burlingame, CA) was used as revealing system. Peroxidase activity was detected by treatment with 3,3′-diaminobenzidine (K3468, Dako, Glostrup, Denmark). Their sections were then photographed by a digital Nikon DS microscope color camera, using a 20x objective to perform counts in the above mentioned regions. Digital images were analyzed by NIS-Elements software (Nikon, Tokyo, Japan) using the automated target detection mode. Images size was 1280 × 960 pixels and target area had a size of 17,300 µm2. The software determined the microglia area fraction (immunopositive area/total area used as scan object). Eighteen counts were performed for each of the three above-mentioned areas.
Astrocytes immunofluorescence was performed on parietal cortex, hippocampus and cerebellum using 1:200 diluted anti glial fibrillar acidic protein (GFAP) (Z0334, Dako, Glostrup, Denmark) as primary antibody. Slides were subjected to regular microwave heating (15 min), immersed in citrate buffer (pH = 6.1). Anti-rabbit biotinylated (PK 4001, Vector Laboratories, Burlingame, CA) was used as secondary antibody and staining was visualized by fluorescein avidin DCS (A-2011, Vector Laboratories, Burlingame, CA). For counts in the above mentioned regions, digital images were captured by ViCo Eclipse 80i (Nikon, Tokyo, Japan) with motorize z-stage control using 20x objective. Immunofluorescence staining was analyzed by NIS-Elements software using the automated target detection mode. The target area had a size of 161,365 µm2. The software determined the astrocytes area fraction (immunopositive area/total area used as scan object). Six counts were performed on the three above-mentioned levels.
Statistical analysis.
Survival analysis was performed using Log-Rank test. Histological, immunohistochemical, western blot and immunofluorescence data were analyzed by Student test for unpaired samples.
Acknowledgments
The present work was sponsored by Chiesi Farmaceutici, Parma, Italy. We are also grateful to Mr. Massimo Dagrada, Mr. Domenico Gilardoni, Mr. Antonio Longo and Dr. Luana Dell'Atti for their technical support.
References
- 1.Prusiner SB. Novel proteinaceous infectious particles cause scrapie. Science. 1982;216:136–144. doi: 10.1126/science.6801762. [DOI] [PubMed] [Google Scholar]
- 2.Prusiner SB. Prions. Proc Natl Acad Sci USA. 1998;95:13363–13383. doi: 10.1073/pnas.95.23.13363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Moore RA, Taubner LM, Priola SA. Prion protein misfolding and disease. Curr Opin Struct Biol. 2009;19:1–2. doi: 10.1016/j.sbi.2008.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Griffith JS. Self-replication and scrapie. Nature. 1967;215:1043–1044. doi: 10.1038/2151043a0. [DOI] [PubMed] [Google Scholar]
- 5.Pan KM, Baldwin M, Nguyen J, Gasset M, Serban A, Groth D, et al. Conversion of α-helices into β-sheets features in the formation of the scrapie prion proteins. Proc Natl Acad Sci USA. 1993;90:10962–10966. doi: 10.1073/pnas.90.23.10962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gilch S, Kehler C, Schätzl HM. The prion protein requires cholesterol for cell surface localization. Mol Cell Neurosci. 2006;31:346–353. doi: 10.1016/j.mcn.2005.10.008. [DOI] [PubMed] [Google Scholar]
- 7.Taylor DR, Hooper NM. The prion protein and lipid rafts. [review] Mol Membr Biol. 2006;23:89–99. doi: 10.1080/09687860500449994. [DOI] [PubMed] [Google Scholar]
- 8.Heikenwalder M, Julius C, Aguzzi A. Prions and peripheral nerves: a deadly rendezvous. J Neurosci Res. 2007;85:2714–2725. doi: 10.1002/jnr.21246. [DOI] [PubMed] [Google Scholar]
- 9.Checler F, Vincent B. Alzheimer's and prion diseases: distinct pathologies, common proteolytic denominators. Trends Neurosci. 2002;25:616–620. doi: 10.1016/S0166-2236(02)02263-4. [DOI] [PubMed] [Google Scholar]
- 10.Baier M, Apelt J, Riemer C, Gültner S, Schwarz A, Bamme T, et al. Prion infection of mice transgenic for human APPSwe: increased accumulation of cortical formic acid extractable Abeta(1–42) and rapid scrapie disease development. Int J Dev Neurosci. 2008;26:821–824. doi: 10.1016/j.ijdevneu.2008.07.001. [DOI] [PubMed] [Google Scholar]
- 11.Zimmermann K, Turecek PL, Schwarz HP. Genotyping of the prion protein gene at codon 129. Acta Neuropathol. 1999;97:355–358. doi: 10.1007/s004010050998. [DOI] [PubMed] [Google Scholar]
- 12.Ironside JW, Head MW. Neuropathology and molecular biology of variant Creutzfeldt-Jakob disease. Curr Top Microbiol Immunol. 2004;284:133–159. doi: 10.1007/978-3-662-08441-0_6. [DOI] [PubMed] [Google Scholar]
- 13.Riemenschneider M, Klopp N, Xiang W, Wagenpfeil S, Vollmert C, Müller U, et al. Prion protein codon 129 polymorphism and risk of Alzheimer disease. Neurology. 2004;63:364–366. doi: 10.1212/01.wnl.0000130198.72589.69. [DOI] [PubMed] [Google Scholar]
- 14.Del Bo R, Scarlato M, Ghezzi S, Martinelli-Boneschi F, Fenoglio C, Galimberti G, et al. Is M129V of PRNP gene associated with Alzheimer's disease? A case-control study and a meta-analysis. Neurobiol Aging. 2006;27:770. doi: 10.1016/j.neurobiolaging.2005.05.025. [DOI] [PubMed] [Google Scholar]
- 15.Kageyama R, Ohtsuka T. The Notch-Hes pathway in mammalian neural development. Cell Res. 1999;9:179–188. doi: 10.1038/sj.cr.7290016. [DOI] [PubMed] [Google Scholar]
- 16.Ishikura N, Clever JL, Bouzamondo-Bernstein E, Samayoa E, Prusiner SB, Huang EJ, et al. Notch-1 activation and dendritic atrophy in prion disease. Proc Natl Acad Sci USA. 2005;102:886–891. doi: 10.1073/pnas.0408612101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Spilman P, Lessard P, Sattavat M, Bush C, Tousseyn T, Huang EJ, et al. A γ-secretase inhibitor and quinacrine reduce prions and prevent dendritic degeneration in murine brains. Proc Natl Acad Sci USA. 2008;105:10595–10600. doi: 10.1073/pnas.0803671105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Laurén J, Gimbel DA, Nygaard HB, Gilbert JW, Strittmatter SM. Cellular prion protein mediates impairment of synaptic plasticity by amyloid-beta oligomers. Nature. 2009;457:1128–1132. doi: 10.1038/nature07761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gimbel DA, Nygaard HB, Coffey EE, Gunther EC, Laurén J, Gimbel ZA, et al. Memory impairment in transgenic Alzheimer mice requires cellular prion protein. J Neurosci. 2010;30:6367–6374. doi: 10.1523/JNEUROSCI.0395-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Imbimbo BP, Del Giudice E, Colavito D, D'Arrigo A, Dalle Carbonare M, Villetti G, et al. 1-(3′,4′-Dichloro-2-fluoro[1,1′-biphenyl]-4-yl)-cyclopropanecarboxylic acid (CHF5074), a novel γ-secretase modulator, reduces brain β-amyloid pathology in a transgenic mouse model of Alzheimer's disease without causing peripheral toxicity. J Pharmacol Exp Ther. 2007;323:822–830. doi: 10.1124/jpet.107.129007. [DOI] [PubMed] [Google Scholar]
- 21.Imbimbo BP, Hutter-Paier B, Villetti G, Facchinetti F, Cenacchi V, Volta R, et al. CHF5074, a novel γ-secretase modulator, attenuates brain β-amyloid pathology and learning deficit in a mouse model of Alzheimer's disease. Br J Pharmacol. 2009;156:982–993. doi: 10.1111/j.1476-5381.2008.00097.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Imbimbo BP, Giardino L, Sivilia S, Giuliani A, Gusciglio M, Pietrini V, et al. CHF5074, a novel γ-secretase modulator, restores hippocampal neurogenesis potential and reverses contextual memory deficit in a transgenic mouse model of Alzheimer's disease. J Alzheimers Dis. 2010;20:159–173. doi: 10.3233/JAD-2010-1366. [DOI] [PubMed] [Google Scholar]
- 23.Peretto I, Radaelli S, Parini C, Zandi M, Raveglia LF, Dondio G, et al. Synthesis and biological activity of flurbiprofen analogues as selective inhibitors of β-amyloid(1–42) secretion. J Med Chem. 2005;48:5705–5720. doi: 10.1021/jm0502541. [DOI] [PubMed] [Google Scholar]
- 24.Thackray AM, Klein MA, Aguzzi A, Bujdoso R. Chronic subclinical prion disease induced by low-dose inoculum. J Virol. 2002;76:2510–2517. doi: 10.1128/jvi.76.5.2510-7.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Meeker HC, Ye X, Carp RI. The mouse model for scrapie: inoculation, clinical scoring and histopathological techniques. Methods Mol Biol. 2005;299:309–323. doi: 10.1385/1-59259-874-9:309. [DOI] [PubMed] [Google Scholar]
- 26.Fraser H, Dickinson AG. The sequential development of the brain lesion of scrapie in three strains of mice. J Comp Pathol. 1968;78:301–311. doi: 10.1016/0021-9975(68)90006-6. [DOI] [PubMed] [Google Scholar]
- 27.Khalili-Shirazi A, Summers L, Linehan J, Mallinson G, Anstee D, Hawke S, et al. PrP glycoforms are associated in a strain-specific ratio in native PrPSc. J Gen Virol. 2005;86:2635–2644. doi: 10.1099/vir.0.80375-0. [DOI] [PubMed] [Google Scholar]
- 28.Doré G, Leclerc C, Lazarini F. Treatment by CpG or Flt3-ligand does not affect mouse susceptibility to BSE prions. J Neuroimmunol. 2008;197:74–80. doi: 10.1016/j.jneuroim.2008.03.016. [DOI] [PubMed] [Google Scholar]
- 29.Mallucci G, Dickinson A, Linehan J, Klöhn PC, Brandner S, Collinge J. Depleting neuronal PrP in prion infection prevents disease and reverses spongiosis. Science. 2003;302:871–874. doi: 10.1126/science.1090187. [DOI] [PubMed] [Google Scholar]
- 30.Meeker HC, Ye X, Carp RI. The mouse model for scrapie: inoculation, clinical scoring and histopathological techniques. Methods Mol Biol. 2005;299:309–323. doi: 10.1385/1-59259-874-9:309. [DOI] [PubMed] [Google Scholar]
- 31.Lee YJ, Jin JK, Jeong BH, Carp RI, Kim YS. Increased expression of glial cell line-derived neurotrophic factor (GDNF) in the brains of scrapie-infected mice. Neurosci Lett. 2006;410:178–182. doi: 10.1016/j.neulet.2006.09.090. [DOI] [PubMed] [Google Scholar]
- 32.Lichtenstein MP, Carriba P, Baltrons MA, Wojciak-Stothard B, Peterson JR, García A, et al. Secretase-independent and RhoGTPase/PAK/ERK-dependent regulation of cytoskeleton dynamics in astrocytes by NSAIDs and derivatives. J Alzheimers Dis. 2010;22:1135–1155. doi: 10.3233/JAD-2010-101332. [DOI] [PubMed] [Google Scholar]
- 33.Brown DR, Herms J, Kretzschmar HA. Mouse cortical cells lacking cellular PrP survive in culture with a neurotoxic PrP fragment. Neuroreport. 1994;5:2057–2060. doi: 10.1097/00001756-199410270-00017. [DOI] [PubMed] [Google Scholar]
- 34.Büeler H, Aguzzi A, Sailer A, Greiner RA, Autenried P, Aguet M, et al. Mice devoid of PrP are resistant to scrapie. Cell. 1993;73:1339–1347. doi: 10.1016/0092-8674(93)90360-3. [DOI] [PubMed] [Google Scholar]
- 35.Balducci C, Mehdawy B, Mare L, Giuliani A, Lorenzini L, Sivilia S, et al. The γ-secretase modulator CHF5074 restores memory and hippocampal synaptic plasticity in plaque-free Tg2576 mice. J Alzheimers Dis. 2011;24:799–816. doi: 10.3233/JAD-2011-101839. [DOI] [PubMed] [Google Scholar]
- 36.Evin G, Sernee MF, Masters CL. Inhibition of γ-secretase as a therapeutic intervention for Alzheimer's disease: prospects, limitations and strategies. CNS Drugs. 2006;20:351–372. doi: 10.2165/00023210-200620050-00002. [DOI] [PubMed] [Google Scholar]
- 37.Vincent B, Paitel E, Saftig P, Frobert Y, Hartmann D, De Strooper B, et al. The disintegrins ADAM10 and TACE contribute to the constitutive and phorbol ester-regulated normal cleavage of the cellular prion protein. J Biol Chem. 2001;276:37743–37746. doi: 10.1074/jbc.M105677200. [DOI] [PubMed] [Google Scholar]
- 38.Sanghera N, Pinheiro TJ. Binding of prion protein to lipid membranes and implications for prion conversion. J Mol Biol. 2002;315:1241–1256. doi: 10.1006/jmbi.2001.5322. [DOI] [PubMed] [Google Scholar]
- 39.Walmsley AR, Zeng F, Hooper NM. The N-terminal region of the prion protein ectodomain contains a lipid raft targeting determinant. J Biol Chem. 2003;278:37241–37248. doi: 10.1074/jbc.M302036200. [DOI] [PubMed] [Google Scholar]
- 40.Taylor DR, Hooper NM. Role of lipid rafts in the processing of the pathogenic prion and Alzheimer's amyloid-β proteins. Semin Cell Dev Biol. 2007;18:638–648. doi: 10.1016/j.semcdb.2007.07.008. [DOI] [PubMed] [Google Scholar]
- 41.Fischer M, Rülicke T, Raeber A, Sailer A, Moser M, Oesch B, et al. Prion protein (PrP) with amino-proximal deletions restoring susceptibility of PrP knockout mice to scrapie. EMBO J. 1996;15:1255–1264. [PMC free article] [PubMed] [Google Scholar]