Abstract
A point mutation in Prnp that converts tyrosine (Y) at position 145 into a stop codon leading to a truncated prion molecule as found in an inherited transmissible spongiform encephalopathy (TSE), Gertsmann-Sträussler-Scheincker syndrome, suggests that the N-terminus of the molecule (spanning amino acids 23–144) likely plays a critical role in prion misfolding as well as in protein-protein interactions. We hypothesized that Y145Stop molecule represents an unstable part of the prion protein that is prone to spontaneous misfolding. Utilizing protein misfolding cyclic amplification (PMCA) we show that the recombinant polypeptide corresponding to the Y145Stop of sheep and deer PRNP can be in vitro converted to PK-resistant PrPSc in presence or absence of preexisting prions. In contrast, recombinant protein full-length PrPC did not show a propensity for spontaneous conformational conversion to protease resistant isoforms. Further, we show that seeded or spontaneously misfolded Y145Stop molecules can efficiently convert purified mammalian PrPC into protease resistant isoforms. These results establish that the N-terminus of PrPC molecule corresponding to residues 23–144 plays a role in seeding and misfolding of mammalian prions.
Key words: prion protein, prions, recombinant prion protein, Y145Stop, protein misfolding cyclic amplification
Introduction
Transmissible spongiform encephalopathies (TSEs) are a group of prion diseases that include Creutzfeldt-Jakob disease (CJD), Gerstmann-Straussler Schiencker syndrome (GSS) and fatal familial insomnia (FFI) in humans, as well as scrapie in sheep, chronic wasting disease (CWD) in deer and bovine spongiform encephalopathy (BSE) in cattle.1–3 A critical aspect of the pathogenesis of TSEs involves conformational conversions of normal cellular prion protein (PrPC) to an abnormal isoform, termed PrPSc.4 Although it is currently unknown whether PrPSc from subclinical or preclinical animals of a variety of species can be present in conformations (or “strains”) that can cause human or animal TSEs, experimental studies have established that interspecies transmission of chronic wasting disease (CWD), bovine spongiform encephalopathy (BSE) and scrapie can occur under laboratory conditions. Further evidence supporting interspecies transmission is derived from the recent demonstration that the prion strain causing BSE is indistinguishable from the strain causing vCJD. Both share common incubation periods and lesion profiles in the brains of infected mice, macaques, and they have identical glycosylation profiles. Available data in the literature strongly suggest that conformations of PrPSc are critical for the interspecies transmission of TSE. Primary amino acid sequences of PRNP as well as the conformation of the infectious seeding prion appear to be important in defining strains and their potential transmissibility.
In addition to the acquired infections, over 20 mutations in human Prnp have been shown to be associated with prion phenotypes in humans.2 The mutations are hypothesized to destabilize the mutant PRNP, which then undergoes a spontaneous conformational change into the protease-resistant and pathogenic form. A point mutation in Prnp that converts tyrosine (Y) at position 145 into a stop codon leading to a truncated prion molecule, present in an autosomal dominant inherited genetic TSE, called Gertsmann-Sträussler-Scheincker syndrome, suggests that the N-terminus of the molecule (spanning amino acids 23–144) plays a critical role in prion misfolding as well as in protein-protein interactions. The N-terminus of the prion protein is largely unstructured and does not contain stable secondary structures5 and is highly conserved across different species. This disease-related mutation suggests that the N-terminus of the molecule (PrP23–144) likely plays a critical role in prion misfolding as well as in protein-protein interactions in multiple species. In the present study, the self-propagating conversion of the purified recombinant protein was demonstrated with peptides corresponding to N-terminal of PrPC-Y145Stop from sheep and deer. We postulate that this region of the molecule is critical in prion misfolding in other mammalian species. Furthermore, we compared the efficiency with which spontaneously converted PrP145Stop induced conversion of recombinant and mammalian PrPC.
Results
Cloning and expression of the recombinant PrP145Stop.
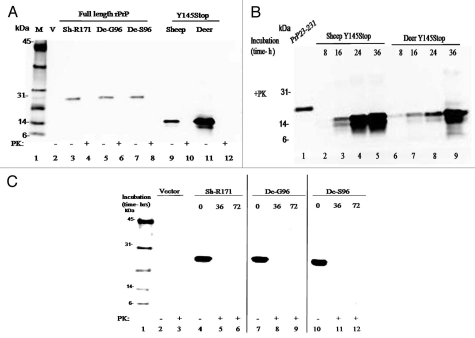
Open reading frame encoding Y145Stop of sheep and deer encompassing residues 23–144 of human prion protein (huPrP23–144) was cloned and expressed in E. coli and purified based on the affinity of the conserved octapeptide repeats for transition-metal cations. All expression plasmids were verified for sequence orientation and accuracy by DNA sequencing and the amino acid sequences of known deer and sheep sequences were compared by clustalW (Fig. 1). Purified Y145Stop from different species were seen on western blot using a prion specific monoclonal antibody (1E4; Fitzgerald Industries International, Concord, MA) targeting an epitope spanning amino acids 108–119 before and after PK digestion (Fig. 2A).
Figure 1.
Amino acid sequence alignment of Y145Stop from different species is shown. The alignment was obtained using ClustalW of huY145Stop with the corresponding sequences in cattle, deer and sheep genome.
Figure 2.
(A) Expression of full-length prion protein and Y145Stop of sheep and deer. Immunoblot of the purified proteins transfected with the expression vector alone or vectors containing constructs of the designated species (defined in the legend A) shows full degradation when incubated for 1 h in the presence of proteinase K. Positions of the molecular mass markers are designated in kDa. (B) Proteinase K digestion of sheep and deer Y145Stop molecules after PMCA at different time points. Human recombinant PrP 23–231 was used as a positive control. Spontaneously generated fibrillar proteins persisted after proteinase K digestion and were detected at 16 h with more conversion at 24 and 36 h after PMCA incubation. (C) Immunoblotting of PrP-sen of the purified recombinant full-length prion protein of sheep and deer strains; white-tailed deer (G96 and S96 variants) and sheep (171R) after PMCA and PK digestion at different time points. Proteins were fully degraded in the presence of proteinase K with no detectable conversion after 72 h of PMCA.
Spontaneous conversion of the recombinant PrP145Stop of sheep and deer.
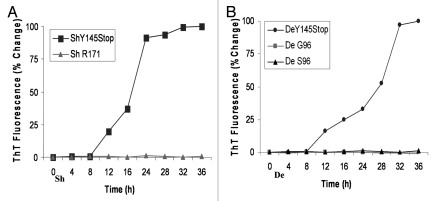
Freshly purified proteins were dialyzed against 10 mM sodium phosphate, pH 6.5 overnight. After prolonged periods of storage at room temperature, the proteins remained in a monomeric form with no sign of self-association as verified by the ThT assay. To test the conformational conversion of PrP145Stop, we performed two rounds of PMCA using the recombinant prion polypeptides of sheep and deer encompassing residues 23 to 144 (PrP23–144). In the first round, the reactions were incubated without addition of any seed. The subsequent round was seeded with a 1/10 volume of PMCA product from the previous round. Western blot analysis revealed that monomeric PrP145Stop without PMCA incubation was fully degraded when incubated for 1 h in the presence of 1 µg/ml of proteinase K (Fig. 2B). However, in the PMCA reactions, spontaneously generated fibrillar proteins persisted after proteinase K digestion. This conversion was consistent for the purified recombinant Y145Stop of both deer and sheep (Fig. 2B). We next examined the conformational conversion of the purified recombinant full-length prion protein of sheep and deer strains; white-tailed deer (G96 and S96 variants) and sheep (171R). In contrast to PrP145Stop, none of the purified recombinant protein corresponding to full-length protein showed a propensity for spontaneous conversion to protease resistant isoforms (Fig. 2C). To probe the progression of amyloid fibril formation, we used the fluorimetric ThT assay. A time course increase in ThT fluorescence was identified, suggesting that PrP145Stop from sheep and deer acquired the ability to bind ThT (Fig. 3). The kinetic curves for the fluorescent uptake were characterized by a lag phase ∼10 h (Fig. 3A and B). Overall, the above data indicate that the conformational conversion of sheep and deer PrP23–144 (Y145Stop) is a self-seeded reaction.
Figure 3.
Time-course analysis to document increase in Thioflavine T uptake and fluorescence by sheep and deer Y145Stop in the absence of seeds. (A) ShY145Stop (squares) and full-length Sheep PrP with R at codon 171 (triangles) incubated in the absence of seeds. (B) DeY145Stop (circles) and full length Deer PrP with G (squares) or S (triangles) at codon 96 incubated with no seeds. All samples were subjected to PMCA under identical conditions. ShY145Stop and DeY145Stop acquired the ability to bind ThT without addition of external seeds. In contrast, the full-length prion protein did not show detectable ThT binding. The kinetic curves were characterized by a lag phase (8–12 h).
Seeded conversion of the recombinant Y145Stop by PrPSc and PrPCWD.
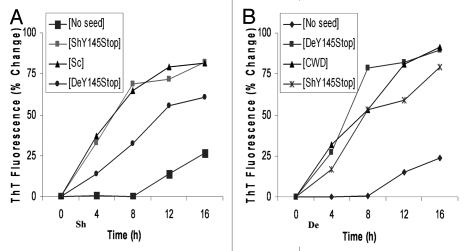
To test if in-vitro amplification of protease resistant PrP145Stop proceeds in a seeded fashion, we next applied the PMCA using PrPSc and PrPCWD, as seeds, from infected and confirmed brain tissues of sheep and deer, respectively. First, the minute amounts of brain-derived PrPSc (scrapie) and PrPCWD (chronic wasting disease; CWD) were PMCA amplified using PrPC from respective species. Purified recombinant Y145Stop of sheep and deer were also spiked with partially purified PrPSc from deer and sheep PMCA amplification in presence of small amounts (1% wt/wt) of the deer and sheep PrPSc resulted in elimination of the lag phase (Fig. 4A and B). Similar conversion rates were observed with the cross seeding between Y145Stop of the two species. Unlike PrP145Stop prion protein, the recombinant polypeptide corresponding to full-length prion protein (PrP 23–231) of sheep and deer did not show efficient amplification when the same molecules as above were used as seeding agents.
Figure 4.
The progress of amyloid fibril formation measured by fluorimetric Thioflavin T uptake assay in presence of seeds. One percent (w/w) performed fibrillar seeds (denoted in square brackets) were added to solutions containing monomers of sheep (A) or deer (B) Y145Stop. No seed control showed the characteristic lag phase, while seeded reactions with partially purified PrPSc from deer [CWD] or sheep [Sc] eliminated the lag phase. Similar conversion rates were observed with cross seeding by in-vitro converted Y145Stop of sheep [ShY145Stop] or deer [DeY145Stop].
Seeded conversion of mammalian PrPC by prion-seeded or spontaneously generated PrPRes of Y145Stop molecules.
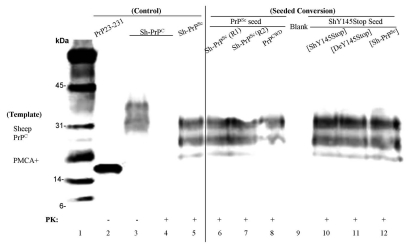
The efficiency of seeding between proteins derived from sheep and deer was examined next. A second passage experiment was performed with purified mammalian (deer and sheep) PrPC as templates. Unseeded control PMCA experiments showed no spontaneous conversion for any mammalian PrPC tested. Electrophoretic mobility patterns of seeded misfolded sheep PrPC was identical regardless of whether the seed was PrPSc, PrPCWD or any misfolded Y145Stop (Fig. 5). Identical results were obtained when PrPC from deer was used as a template for PMCA (data not shown). Further, the results suggest that in-vitro converted prions of deer and sheep carry similar protease cleavage sites and glycosylation profiles do not change during the process of PMCA.
Figure 5.
PMCA on purified PrPC from brain tissue of sheep and deer. Lane 1, molecular weight marker (biotinylated). Lane 2, human recombinant PrP 23–231 was used as a positive control. Lanes 3 and 4, samples containing purified PrPC from brain tissue of sheep before and after PK digestion. Lane 5, PrPSc extracted from sheep brain as a reference after PK digestion with 10 times the reaction equivalents of the seed protein concntration. Samples of purified PrPC from brain tissue of sheep were incubated with preformed fibrillar seed of PrPSc round one (lane 6), round two (lane 7), PrPCWD (lane 8), ShY145Stop seeded with performed fibrils of ShY145Stop (lane 10), DeY145Stop (lane 11), or PrPSc (lane 12) after PK digestion.
Discussion
The conversion of PrPC isoform into the PrPSc isoform may occur spontaneously or sporadically be genetic as in familial prion diseases or be acquired exogenously via consumption or iatrogenic routes as in infectious prion diseases. Conversion of the prion protein from the cellular to pathological isoform is central to the pathogenesis of prion disorders. The mechanism of this conformational conversion remains poorly understood and the critical part of the molecule involved in catalysis of misfolding is not known. To provide a better understanding of prion misfolding, we investigated the role of the N-terminus of PRNP in conversion. The Y145Stop mutation at PRNP is the basis of a naturally occurring familial spongiform encephalopathy; Gerstmann-Sträussler-Scheinker disease (GSS). Elucidation of how the Y145Stop molecule contributes to the mechanism of conformational conversion would provide insight into the underlying pathogenesis and, indeed, the prion concept itself. We propose that Y145Stop is sufficient for prion misfolding.
Y145Stop variant from sheep and deer can spontaneously polymerizes to protease resistant isoforms.
We focused on the N-terminal region of the prion protein as it is largely unstructured and lacks the C-terminal 146–231 amino acids including the glycophosphatidyl anchor, which links PrP to the cell surface and the two sites for N-glycosylation, which are known to stabilize the PrP molecule. Models of prion conversion have suggested that formation of PrPSc involves refolding of residues within the region between residues 90 and 140 into β-sheets.2,8 The region that contains the residues 113–128 is most highly conserved among all species studied.9 Our data demonstrate that the purified recombinant polypeptide corresponding to the Y145Stop variant of sheep and deer spontaneously polymerizes to β-sheets structures which bind Thioflavine T (ThT). The self-propagating conversion by PMCA not only occurs in HuPrPY145Stop,10–12 but also in the corresponding sheep and deer molecules. A similar conversion capacity among various species is likely due to the high percentage of amino acid sequence similarity in regions 113–128 and 138–141 in the N-terminal part of PrPC.10 The latter region has been reported to influence PrPSc formation13–15 and may be essential for nucleating intermolecular interactions.10 The conformational conversion of PrP145Stop is a nucleation-dependent reaction in which the growth of amyloid is preceded by a lengthy lag phase ranging from 8–10 h11 which is characteristic for nucleation-dependent polymerization.10–12 Many experimental evidences such as the infectious truncated HuPrP (PrP 27–30),16 the shorter version PrP106 acting as barrier for prion replication,17 and the Japanese patient carrying the mutation Y145Stop18 suggests a critical role for the segment MoPrP(89–143) in conversion. Tg196 mice expressing MoPrP(P101L) at low level, intracerebrally inoculated with the β-amyloid forms of MoPrP(89–143), exhibited neurological changes similar to those present in GSS patients19 and transmitted the disease to other Tg196 mice suggesting the de novo GSS prion formation.20 Similarly, Y145Stop of sheep and deer spontaneously misfold and the conserved sequence homology across species supports the idea that parts of the N-terminus of the prion molecule may be involved in prion conversion and may participate in crossing species barriers. More targeted studies are needed to confirm the role of Y145Stop in interspecies transmission. These studies on spontaneously misfolded Y145Stop from multiple species and their role in interspecies transmission are underway in our laboratory.
Conversion of the recombinant PrP145Stop is accelerated by addition of preexisting seeds.
Autocatalytic conversion from PrPC into PrPSc is a key feature of prion replication.21 We demonstrated that in vitro conversion of PrPY145Stop exhibits attributes of the autocatalytic mechanism, such as lag phase and seeding phenomena. Importantly, addition of a small quantity of spontaneously converted Y145Stop from sheep or deer to soluble protein monomer was able to seed the conversion and eliminate the lag phase. Therefore, sheep and deer proteins were found to be fully compatible, i.e., ShPrP23–144 fibrils readily seeded deer protein and DePrP23–144 fibrils acted as efficient seed for fibrilization of sheep PrP23–144. Furthermore, similar self-assembly curves were observed with cross-seeding by preformed aggregates from scrapie- or deer-infected brain. Kundu et al. found that the residues within the 138–141 region of PrP are important for amyloid formation. Interestingly, one of the mismatches between Hu and Syrian hamster PrP is located at position 139 (isoleucine in Hu PrP and methionine in sha PrP) which led to lack of cross seeding between the two species.11 This region shares 100% amino acid similarity in sheep and deer PrP145Stop. Whether this or other positions within the region 90–231 influences the species specificity of autocatalytic conversion remains to be determined. The present data show that there is little effect of species-specific sequence variation in PrPY145Stop sequence on the efficiency of cross-seeding between deer and sheep. These findings point to a role of the N-terminal unstructured segment for prion transmissibility between different species.
Full-length prion protein resists spontaneous conversion.
The full-length PrP obviously cannot mimic all aspects of conversion of the N-terminal region of the prion protein, in which the C-terminal domain might protect the full molecule from triggering the conversion of PrPC to PrPSc by shielding the critical amyloidogenic determinant(s) that is exposed in the unfolded N-terminal region of PrP.22,23 Similar studies with the recombinant full-length PrP were not possible due to practical difficulties in propagating efficient seeded conversion of full-length PrP, although certain aspects of seeded conversion of PrP23–231 or PrP90–231 could be reproduced using a reduction-oxidation process22 or under conditions requiring high concentrations of denaturants and very vigorous shaking.23 Recently, PMCA has been used to generate infectious prions from bacterially-expressed full-length murine24 and Syrian hamster25,26 recombinant PrP. However, additional cofactors were required for efficient PrPSc-seeded PMCA reaction. In one study,24 the proteinase K (PK)-resistance was detected after 17 rounds of PMCA which could be explained as a conformational adaptation allowing the newly emerged conformation (strain) to cross species barriers and serially propagate.
Spontaneously converted PrP145Stop is sufficient to induce in-vitro generation of PrPSc from mammalian PrPC.
Next we asked if the spontaneously misfolded Y145Stop molecules were sufficient to seed mammalian prions. Amplification of TSE infectivity was also accomplished by PMCA by employing purified PrPC as a substrate,24,27 exclusively in presence of polyanions. The efficient conversion of Y145Stop raises the question as to whether this molecule could also convert PrPC extracted from normal brain tissues. Our data suggest that protease resistant prions can indeed be generated de novo from mammalian PrP using spontaneously converted Y145Stop as a seed. The electrophoretic profiles of PrPSc from sheep protein inoculated with in vitro converted Y145Stop, as well as inoculated deer protein, were indistinguishable from those seeded with sheep (scrapie) and deer (chronic wasting disease; CWD), indicating similar PK cleavage site(s), as well as a similar ratio of different PrPSc glycoforms. The efficient conversion of mammalian prions of sheep and deer using spontaneously converted PrP145Stop indicates that PrP23–144 fibrils acted as an efficient seed for fibrillization of mammalian prions.
Several studies have shown that a variety of molecules, such as RNA,28 DNA,29 and heavy metals including copper30,31 appear to stimulate prion conversion. The N-terminal fragment of PrP has been shown to bind with copper,32 DNA,33,34 and RNA35 whereas the C-terminal fragment (122–231) does not demonstrate a similar binding affinity to these molecules. On the basis of the results presented here, we propose a two-step phenomenon in prion misfolding. We propose that in the first instance, any one of these cofactors maybe involved in binding to and misfolding of the N-terminal region of the molecule, which then likely catalyzes conversion of the full prion molecule. A detailed biochemical analyses and carefully designed studies that include PRNP structural variants would be needed to fully elucidate this model of prion conversion.
In summary, our findings demonstrate that the highly unstable part of the prion molecule, Y145Stop, can be converted in vitro to β-sheet rich oligomeric amyloid fibrils in presence or absence of preexisting seed in sheep and deer species. Moreover, spontaneously converted PrP145Stop is sufficient to induce de novo generation of PrPSc from mammalian prions. It is likely that the nucleotide sequence similarity and the spontaneous nature of misfolding in N-terminal segment of the prion protein could play a role in interspecies transmission.
Material and Methods
Plasmid construction.
DNA was extracted from normal brain extracts of sheep and deer using standard protocols (Qiagen DNA extraction Kits, Valencia, CA). Y145Stop segments of PrnP were amplified using the forward primer as 5′-GGA ATT CCA TAT GAA GAA GCG ACC AAA ACC TGG-3′ for both sheep and deer, while 5′-CCC AAG CTT GGG TTA GTC ATT GCC AAA ATG TAT AA-3′ and 5′-CCC AAG CTT GGG TTA GTC GTT GCC AAA ATG TAT AA-3′ as the reverse primers for sheep and deer respectively. Open reading frames were subsequently cloned into the NdeI and HindIII (NEB) restriction sites of the pET-41a vector (Novagen). The primer sets for Sh-R171 and De-G/S 96 were 5′-GGG ATC CAT CAT GAA GCG ACC AAA ACC TG-3′ as the forward primer and 5′-CGA ATT CTT ATG CCC CCC TTT GGT AAT AAG CC-3′ or 5′-CGA ATT CTT ATG CCC CTC TTT GGT AAT AAG-3′ as the reverse primers for Sh-R171 and De-G/S96 respectively. All constructs in the pET-41a were verified for accuracy of insert orientation by DNA sequencing and the amino acid sequences were compared by clustalW against human and cattle Y145Stop (Fig. 1).
Protein expression and purification.
Cloning and expression steps were combined by transforming the plasmid constructs into the Acella® chemically competent Cells (Edge BioSystems). IPTG (1 mM) induction was used to allow for larger scale expression of Y145Stop of each of the different species. Y145Stop purification method was based on the affinity of the conserved octapeptide repeats for transition-metal cations avoiding the use of a histidine tag. The cell pellets were lysed in 8 M Urea, 0.1 NaH2PO4, 0.01 M Tris.Cl; pH 8.0 for 1 h at 4°C followed by sonication. The prepared protein extracts were added onto HisPur Cobalt Resin (Pierce). The wash step with NaH2PO4/Tris.HCl buffer was used to eliminate the urea. Y145Stop was eluted by 300 mM imidazole solution and dialyzed against 10 mM sodium phosphate, pH 6.5 overnight. Under the same conditions, we expressed and purified recombinant full-length prion protein sheep and deer; white-tailed deer (G96 and S96) and sheep R at codon 171.
Purification of normal and pathological isoforms of prion protein from brain tissues.
PrPC and PrPSc were purified by sucrose density-gradient centrifugation and subjected to ultracentrifugation method. Brain samples of sheep or deer confirmed negative or positive for TSEs by immunohistochemistry at the Minnesota Veterinary Diagnostic Laboratory were used for extraction of prion proteins. Brain tissue was sliced and rinsed with cold PBS to remove the residual blood. Two syringes connected with a Three-Way Stopcock with Male Luer Slip Adaptor (Baxter Healthcare Corp., Deerfield, IL USA) were used to prepare 10% w/v brain suspension using cold 10% sarcosine (TEND) that is supplemented with 1 mM DTT and EDTA (EDTA)-free Protease Inhibitors cocktail (Roche, Mannheim, Germany) at each step where it was needed. Aliquots of this suspension were homogenized then transferred on ice for 1 h. The mixture was subjected to centrifugation at 3,000x g for 10 min to pellet of cellular debris. The supernatants were transferred to new Optiseal tubes (Beckman Coulter, Fullerton, CA) and centrifuged through 1 M sucrose cushion for 2 h 30 min at 4°C at 100,000x g. This pellet consists of a microsomal/synaptosomal membrane fraction containing PrPC. Then the pellets were rinsed using 10% NaCl, 1% SB 3–14 (TEND + PIs). DNAase I and RNAase treatment were used to remove nucleic acids from the aliquots. At this point, a portion of the material was digested with different concentrations (1, 5, 10 µg/ml) of proteinase K6 enzyme. The suspension was then subjected to centrifugation at 125,755x g for 2 h and pellets were rinsed using 0.5% SB 3–14/1x PBS and finally re-suspended in 1 ml 2% sarkosyl. The protein concentration was adjusted using bicinchonic acid assay (Pierce, Rockford, IL) to 0.5–1 mg/ml. protein purity was assessed by silver staining of gels (Fig. S1).
In vitro conversion of PrPC to PrPSc using PMCA assay.
Prions replicate in the body by inducing the misfolding of the normal prion protein. PMCA assay was developed to mimic this process. PMCA was performed as described,7 in 0.2 ml thin wall PCR tube strips as 80-µl solutions containing PBS with 0.05% (wt/vol) SDS and 0.05% Triton X-100. PK-resistant fragments seeded by PrPSc were generated with seed-to-substrate ratios of 1:100 (40 ng of PrPSc). The tube strips were placed in floating rack in the Sonicator (Misonix Model 4000, Farmingdale, New York) cup horn with water at 37°C and were subjected to repeated cycles of sonication. The samples were subjected to two rounds of PMCA reaction, each of them consisting of 24 cycles with 40 sec intermittent sonication at 100% power (16 min total sonication time), 59 min 20 sec incubations between each sonication. After the first round, an aliquot of the amplified samples was taken and diluted 10-fold into the conversion buffer containing the monomeric protein (0.5 mg/ml) as substrate. A second round of 24 cycles of sonication and incubation as described above was repeated. Buffer, vector control and unseeded sonication were used to test the tendency of the samples for spontaneous misfolding and/or contamination.
Proteinase K resistance assay.
Fifteen µl of the reaction sample (1 µg of rPrP) was diluted in PBS with 0.1% SDS and digested with PK. Typically; the PK concentration to a final volume of 3 µg/ml was used for one hour at 37°C. The reaction was stopped using Pefabloc (Roche) to final concentration of 4 mM. The samples were denaturized using 2x SDS-buffer containing 4 M urea then subjected the samples to 4–20% SDS-PAGE.
Immunoblotting.
Samples were separated in 4–20% SDS-PAGE and transferred to a polyvinylidene fluoride (PVDF) membrane. Monoclonal antibody (1E4) targeting an epitope spanning amino acids 108–119 was added with a 1:1,000 dilution. Secondary antibody conjugated with chemiluminescent compounds was added with a 1:10,000 dilution. All blots were developed using Supersignal West Pico Chemiluminescent Substrate (Pierce).
Thioflavine T (ThT) assay.
The progression of amyloid fibril formation was followed by a fluorimetric ThT assay. A stock solution of ThT dye (1 mg/ml; 3.14 mM) was prepared in distilled, deionized water. Small aliquots of each sample were withdrawn every 2 h up to 24 h and diluted to a final concentration of 4 µM in 50 mM Phosphate buffer, pH 6.5 containing 10 µM ThT. After 2 min incubation at room temperature, the fluorescence of ThT dye was measured using a Spectramax M2 plate reader (Molecular Devices, Sunnyvale, CA) set to at 482 nm by using the excitation wavelength of 450 nm. ThT fluorescence was normalized by (Fi-F0)/(Fmax-F0). Fi was the ThT intensity (fluorescence arbitrary unit) of samples, and F0 was the ThT background intensity. For the measurements of polymerization at different time points, Fmax was the maximum ThT intensity of samples at the end of PMCA cycle.
Acknowledgments
This study was funded by the US Department of Agriculture grant, CSREES 2007-35204-18359 (to S.S.), and NIAID-NIH, PO1 AI 77774-01 (to J.A.R.).
Abbreviations
- De
deer
- Hu
human
- PK
proteinase K
- PMCA
protein misfolding cyclic amplification
- PrPC
cellular form of prion protein
- PrPres
proteinase-resistant form of prion protein
- PrPSc
scrapie prion protein or prion
- Sh
sheep
- ThT
thioflavine T
Supplementary Material
References
- 1.Prusiner SB, DeArmond SJ. Prion diseases and neurodegeneration. Annu Rev Neurosci. 1994;17:311–339. doi: 10.1146/annurev.ne.17.030194.001523. [DOI] [PubMed] [Google Scholar]
- 2.Prusiner SB. Prions. Proc Natl Acad Sci USA. 1998;95:13363–13383. doi: 10.1073/pnas.95.23.13363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Collinge J. Prion diseases of humans and animals: their causes and molecular basis. Annu Rev Neurosci. 2001;24:519–550. doi: 10.1146/annurev.neuro.24.1.519. [DOI] [PubMed] [Google Scholar]
- 4.Prusiner SB. Novel proteinaceous infectious particles cause scrapie. Science. 1982;216:136–144. doi: 10.1126/science.6801762. [DOI] [PubMed] [Google Scholar]
- 5.Bruce M, Chree A, McConnell I, Foster J, Pearson G, Fraser H. Transmission of bovine spongiform encephalopathy and scrapie to mice: strain variation and the species barrier. Philos Trans R Soc Lond B Biol Sci. 1994;343:405–411. doi: 10.1098/rstb.1994.0036. [DOI] [PubMed] [Google Scholar]
- 6.Raymond GJ, Bossers A, Raymond LD, O'Rourke KI, McHolland LE, Bryant PK, 3rd, et al. Evidence of a molecular barrier limiting susceptibility of humans, cattle and sheep to chronic wasting disease. EMBO J. 2000;19:4425–4430. doi: 10.1093/emboj/19.17.4425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Atarashi RMR, Moore RA, Sim VL, Hughson AG, Dorward DW, Onwubiko HA, et al. Ultrasensitive detection of scrapie prion protein using seeded conversion of recombinant prion protein. Nat Methods. 2007;4:645–650. doi: 10.1038/nmeth1066. [DOI] [PubMed] [Google Scholar]
- 8.Muramoto T, Scott M, Cohen FE, Prusiner SB. Recombinant scrapie-like prion protein of 106 amino acids is soluble. Proc Natl Acad Sci USA. 1996;93:15457–15462. doi: 10.1073/pnas.93.26.15457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Prusiner SB. Prion diseases and the BSE crisis. Science. 1997;278:245–251. doi: 10.1126/science.278.5336.245. [DOI] [PubMed] [Google Scholar]
- 10.Kundu B, Maiti NR, Jones EM, Surewicz KA, Vanik DL, Surewicz WK. Nucleation-dependent conformational conversion of the Y145Stop variant of human prion protein: structural clues for prion propagation. Proc Natl Acad Sci USA. 2003;100:12069–12074. doi: 10.1073/pnas.2033281100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Vanik DL, Surewicz KA, Surewicz WK. Molecular basis of barriers for interspecies transmissibility of mammalian prions. Mol Cell. 2004;14:139–145. doi: 10.1016/S1097-2765(04)00155-8. [DOI] [PubMed] [Google Scholar]
- 12.Jones EM, Surewicz WK. Fibril conformation as the basis of species- and strain-dependent seeding specificity of mammalian prion amyloids. Cell. 2005;121:63–72. doi: 10.1016/j.cell.2005.01.034. [DOI] [PubMed] [Google Scholar]
- 13.Priola SA, Chesebro B. A single hamster PrP amino acid blocks conversion to protease-resistant PrP in scrapie-infected mouse neuroblastoma cells. J Virol. 1995;69:7754–7758. doi: 10.1128/jvi.69.12.7754-7758.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Goldmann W, Martin T, Foster J, Hughes S, Smith G, Hughes K, et al. Novel polymorphisms in the caprine PrP gene: a codon 142 mutation associated with scrapie incubation period. J Gen Virol. 1996;77:2885–2891. doi: 10.1099/0022-1317-77-11-2885. [DOI] [PubMed] [Google Scholar]
- 15.Zahn R, Liu A, Lührs T, Riek R, von Schroetter C, López García F, et al. NMR solution structure of the human prion protein. Proc Natl Acad Sci USA. 2000;97:145–150. doi: 10.1073/pnas.97.1.145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Prusiner SB, Groth DF, Bolton DC, Kent SB, Hood LE. Purification and structural studies of a major scrapie prion protein. Cell. 1984;38:127–134. doi: 10.1016/0092-8674(84)90533-6. [DOI] [PubMed] [Google Scholar]
- 17.Supattapone S, Bosque P, Muramoto T, Wille H, Aagaard C, Peretz D, et al. Prion protein of 106 residues creates an artifical transmission barrier for prion replication in transgenic mice. Cell. 1999;96:869–878. doi: 10.1016/S0092-8674(00)80596-6. [DOI] [PubMed] [Google Scholar]
- 18.Kitamoto T, Iizuka R, Tateishi J. An amber mutation of prion protein in Gerstmann-Sträussler syndrome with mutant PrP plaques. Biochem Biophys Res Commun. 1993;192:525–531. doi: 10.1006/bbrc.1993.1447. [DOI] [PubMed] [Google Scholar]
- 19.Kaneko K, Ball HL, Wille H, Zhang H, Groth D, Torchia M, et al. A synthetic peptide initiates Gerstmann-Sträussler-Scheinker (GSS) disease in transgenic mice. J Mol Biol. 2000;295:997–1007. doi: 10.1006/jmbi.1999.3386. [DOI] [PubMed] [Google Scholar]
- 20.Tremblay P, Ball HL, Kaneko K, Groth D, Hegde RS, Cohen FE, et al. Mutant PrPSc conformers induced by a synthetic peptide and several prion strains. J Virol. 2004;78:2088–2099. doi: 10.1128/JVI.78.4.2088-99.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gajdusek DC. Transmissible and non-transmissible amyloidoses: autocatalytic post-translational conversion of host precursor proteins to beta-pleated sheet configurations. J Neuroimmunol. 1988;20:95–110. doi: 10.1016/0165-5728(88)90140-3. [DOI] [PubMed] [Google Scholar]
- 22.Lee S, Eisenberg D. Seeded conversion of recombinant prion protein to a disulfide-bonded oligomer by a reduction-oxidation process. Nat Struct Biol. 2003;10:725–730. doi: 10.1038/nsb961. [DOI] [PubMed] [Google Scholar]
- 23.Baskakov IV. Autocatalytic conversion of recombinant prion proteins displays a species barrier. J Biol Chem. 2004;279:7671–7677. doi: 10.1074/jbc.M310594200. [DOI] [PubMed] [Google Scholar]
- 24.Wang F, Wang X, Yuan CG, Ma J. Generating a prion with bacterially expressed recombinant prion protein. Science. 2010;327:1132–1135. doi: 10.1126/science.1183748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Makarava N, Kovacs GG, Bocharova O, Savtchenko R, Alexeeva I, Budka H, et al. Recombinant prion protein induces a new transmissible prion disease in wild-type animals. Acta Neuropathol. 2010;119:177–187. doi: 10.1007/s00401-009-0633-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kim JI, Cali I, Surewicz K, Kong Q, Raymond GJ, Atarashi R, et al. Mammalian prions generated from bacterially expressed prion protein in the absence of any mammalian cofactors. J Biol Chem. 2010;285:14083–14087. doi: 10.1074/jbc.C110.113464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Deleault NR, Harris BT, Rees JR, Supattapone S. Formation of native prions from minimal components in vitro. Proc Natl Acad Sci USA. 2007;104:9741–9746. doi: 10.1073/pnas.0702662104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Deleault NR, Lucassen RW, Supattapone S. RNA molecules stimulate prion protein conversion. Nature. 2003;425:717–720. doi: 10.1038/nature01979. [DOI] [PubMed] [Google Scholar]
- 29.Cordeiro Y, Machado F, Juliano L, Juliano MA, Brentani RR, Foguel D, et al. DNA converts cellular prion protein into the beta-sheet conformation and inhibits prion peptide aggregation. J Biol Chem. 2001;276:49400–49409. doi: 10.1074/jbc.M106707200. [DOI] [PubMed] [Google Scholar]
- 30.Quaglio E, Chiesa R, Harris DA. Copper converts the cellular prion protein into a protease-resistant species that is distinct from the scrapie isoform. J Biol Chem. 2001;276:11432–11438. doi: 10.1074/jbc.M009666200. [DOI] [PubMed] [Google Scholar]
- 31.Leclerc E, Serban H, Prusiner SB, Burton DR, Williamson RA. Copper induces conformational changes in the N-terminal part of cell-surface PrPC. Arch Virol. 2006;151:2103–2109. doi: 10.1007/s00705-006-0804-1. [DOI] [PubMed] [Google Scholar]
- 32.Viles JH, Cohen FE, Prusiner SB, Goodin DB, Wright PE, Dyson HJ. Copper binding to the prion protein: structural implications of four identical cooperative binding sites. Proc Natl Acad Sci USA. 1999;96:2042–2047. doi: 10.1073/pnas.96.5.2042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nandi PK. Interaction of prion peptide HuPrP106-26 with nucleic acid. Arch Virol. 1997;142:2537–2545. doi: 10.1007/s007050050261. [DOI] [PubMed] [Google Scholar]
- 34.Takemura K, Wang P, Vorberg I, Surewicz W, Priola SA, Kanthasamy A, et al. DNA aptamers that bind to PrP(C) and not PrP(Sc) show sequence and structure specificity. Exp Biol Med (Maywood) 2006;231:204–214. doi: 10.1177/153537020623100211. [DOI] [PubMed] [Google Scholar]
- 35.Gabus C, Derrington E, Leblanc P, Chnaiderman J, Dormont D, Swietnicki W, et al. The prion protein has RNA binding and chaperoning properties characteristic of nucleocapsid protein NCP7 of HIV-1. J Biol Chem. 2001;276:19301–19309. doi: 10.1074/jbc.M009754200. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.