SUMMARY
BACKGROUND
Vitamin D increases cathelicidin production, and might alter mortality due to tuberculosis (TB) in human immunodeficiency virus (HIV) co-infection. However, due to abundant sun exposure, vitamin D levels might be excellent among Ugandans with HIV and TB.
METHODS
We measured 25(OH)D and calcium levels in 50 HIV-negative, 50 HIV-infected and 50 TB-HIV co-infected Ugandan adults.
RESULTS
Mean ± standard deviation 25(OH)D levels were 26 ± 7 ng/ml in HIV-negative, 28 ± 11 ng/ml in HIV-infected and 24 ± 11 ng/ml in TB-HIV co-infected adults (P > 0.05 all comparisons). Vitamin D deficiency (<12 ng/ml) was present in 10% of the HIV-infected subjects, 12% of the TB-HIV co-infected and none of the healthy controls (P = 0.03 for healthy vs. TB, P > 0.05 for other comparisons); 20% of the healthy controls, 22% of the HIV-positive and 38% of the TB-HIV co-infected subjects (P = 0.047 for healthy vs. TB, P > 0.05 for other comparisons) had suboptimal vitamin D levels (<20 ng/ml). No participant had hypercalcemia. Serum 25(OH)D levels correlated positively with body mass index (r = 0.22, P = 0.03) and serum calcium levels (r = 0.18, P = 0.03).
CONCLUSIONS
Ugandan HIV-infected adults with and without TB commonly had suboptimal vitamin D levels. Clinical trials are needed to evaluate the effect of vitamin D on health outcomes in HIV-infected patients with low vitamin D levels.
Keywords: HIV, tuberculosis, vitamin D, calcium
Human Immunodeficiency Virus (HIV) infected individuals have increased susceptibility to and greater morbidity and mortality due to tuberculosis (TB).1–5 These observations may in part be related to hypovitaminosis D, as low vitamin D levels are associated with decreased macrophage production of the peptide cathelicidin6 that exerts antimicrobial properties.
Cathelicidin, part of the innate immune system, plays a critical role in the fight against TB. Mycobacterium tuberculosis binds to toll-like receptors (TLR 2/1) on macrophages, leading to upregulation of 1α-hydroxylase gene expression to promote greater conversion of 25(OH)D to 1,25(OH)2D.6 Intracellular 1,25(OH)2D binds to the vitamin D receptor and induces production of the antimicrobial peptide cathelicidin.6 Cathelicidin localizes to monocytes infected with M. tuberculosis, where it has a direct antimicrobial effect.7 Low vitamin D levels could potentially blunt cathelicidin production, limiting the host's ability to fight TB.2
It was observed that serum containing lower 25(OH)D3 levels demonstrated lower production of c athelicidin mRNA than serum with higher 25(OH)D levels.6 When serum with low 25(OH)D3 was supplemented with 25(OH)D3, cathelicidin mRNA production increased. These findings suggest that vitamin D therapy among individuals with suboptimal levels might induce production of cathelicidin mRNA and improve the immune response to TB infection. Vitamin D could therefore be used as inexpensive adjunctive therapy among HIV patients, especially in settings where TB is highly prevalent, to reduce TB-related morbidity and mortality. In the present study, we describe the serum vitamin D, calcium and albumin levels in HIV-infected Ugandans with and without TB.
METHODS
Study design and setting
Study participants were consecutively enrolled into a prospective cross-sectional study at Mbarara Regional Referral Hospital, located in south-western Uganda, at a latitude of 0.6132 and longitude of 30.6582. Being at the equator, the area has year-round sunshine. Adults wear light clothing and typically spend between 8 and 10 h outside during daylight. The diet of the population is mainly plantain, green vegetables, milk and animal products.
Population
The study population included healthy HIV-negative adults (controls), individuals with HIV infection and individuals with TB-HIV co-infection. Individuals were enrolled as healthy controls if they had no medical ailment, were attending the hospital for HIV screening and were found to be HIV-negative. HIV-positive individuals were newly diagnosed with HIV and enrolling at the HIV clinic for the first time. These individuals were screened for TB using the Ministry of Health algorithm, and were confirmed not to have active TB disease. TB-HIV co-infected individuals had confirmed HIV infection, active TB infection and had not started TB treatment. We included only patients with sputum-positive TB, confirmed on the basis of a positive Ziehl-Neelsen (ZN) stain of a sputum sample. Study participants were excluded if they refused phlebotomy.
Data collection
All study participants were recruited during January and February 2009. In addition to drawing blood for measurement of serum 25(OH)D, albumin and calcium at enrollment, HIV-positive individuals with and without TB underwent baseline measurement of height, weight, complete blood counts and CD4 counts as part of routine HIV care. We also recorded whether participants were taking highly active antiretroviral therapy (HAART) at study entry. Results from routine tests were also recorded for study purposes.
The charts of the HIV-positive individuals were reviewed in November 2009, an average of 9 months after study entry, to record clinical outcomes. The 9-month interval was chosen because TB co-infected subjects would have completed therapy for TB by that time. Subject responses to TB therapy were assessed using the standard Ministry of Health tool, guided by the national TB treatment guidelines and the national HIV care guidelines.8,9 Among those with HIV only at study enrollment, clinical outcomes included the use of HAART, development of TB immune reconstitution inflammatory syndrome (IRIS) and death. Among those with HIV and TB at study entry, clinical outcomes included use of HAART, recovery from TB, the development of IRIS and death.
The study protocol was approved by the Institutional Review Boards of Mbarara University of Science and Technology and the University of Wisconsin–Madison. All participants provided written informed consent prior to phlebotomy. The consent form was written in English and in Runyankore, the most common local language in south-western Uganda.
Laboratory analysis
TB was confirmed on the basis of a positive ZN stain of a sputum sample. Individuals requesting an HIV test were initially screened using the Determine Rapid HIV-1/2 assay (Abbott Laboratories, Abbott Park, IL, USA). If the screening test was positive, the test was subsequently confirmed using the HIV-1/2 STAT-PAK Dipstick assay (Chembio Diagnostic Systems Inc, New York, NY, USA). If the Determine assay and the STAT-PACK assay provided discordant results, the sample was tested using the Uni-Gold rapid assay (Trinity Biotech, Wicklow, Ireland). A positive Uni-Gold assay confirmed a diagnosis of HIV infection.
We obtained blood from consenting subjects on one occasion for measurement of serum 25(OH)D, calcium and albumin. The blood was immediately centrifuged and serum and plasma were stored at –70°C. Frozen samples were shipped in April 2009 on dry ice to the University of Wisconsin (Centers for Disease Control and Prevention Permit #2010-03-040) for measurement of serum calcium, 25(OH)D and albumin. The shipment was sent overnight and the laboratory tests were performed immediately. Meriter Laboratory (Madison, WI, USA) measured serum calcium using o-cresolphthalein complexone and measured albumin using bromocresol green. We measured serum 25(OH)D in the University of Wisconsin Osteoporosis Research Laboratory using a semi-automated solid phase extraction reverse phase high performance liquid chromatography assay.10 Between-run precision coefficients of variance for the assay ranged from 2.6% to 4.9% for 25(OH)D3 and from 3.2% to 12.6% for 25(OH)D2. We corrected serum calcium levels for albumin using the following formula:
Corrected calcium = 0.8(normal albumin − patient's albumin) + serum calcium.
Statistical analysis
Power calculations were based on data from a prior study in which the mean 25(OH)D levels of 50 HIV-positive and 50 HIV-negative individuals were respectively 37 ± 9 ng/ml and 62 ± 8 ng/ml (P < 0.01). Assuming a standard deviation of 8 ng/ml for the difference in 25(OH)D levels between healthy controls and HIV-positive individuals,11 a sample size of 50 subjects with and without HIV would provide 90% power to detect a 5 ng/ml difference in 25(OH)D levels between groups. We therefore planned to recruit 50 healthy subjects, 50 subjects with HIV and 50 subjects with both HIV and TB.
The US Institute of Medicine recently published new definitions of vitamin D deficiency (<12 ng/ml), insufficiency (12–19 ng/ml), sufficiency (25(OH)D >20 ng/ml) and potentially toxicity (>50 ng/ml).12 In line with these definitions, we defined suboptimal vitamin D status as a serum 25(OH)D level < 20 ng/ml, and vitamin D deficiency as a serum 25(OH)D level < 12 ng/ml.12 We described serum 25(OH)D levels and other continuous data using mean ± standard deviation (SD). χ2 or Fisher's exact tests were used to compare proportions of subjects with vitamin D deficiency and insufficiency. We used analysis of variance and independent sample t-tests to compare continuous variables between the three groups of subjects. All analyses were completed using Analyze-It (Analyze-It Software Ltd, Leeds, UK).
RESULTS
We enrolled 150 individuals: 50 with HIV infection, 50 with TB-HIV co-infection and 50 healthy HIV-negative individuals (controls). Their demographic characteristics are summarized in Table 1. HIV-positive individuals with or without TB were older than healthy controls (P < 0.001). TB-HIV co-infected individuals were more likely to have a lower body mass index (BMI), hemoglobin, white cell count and CD4 cell counts than individuals with HIV only (Table 1).
Table 1.
Demographic and laboratory characteristics of participants
| Healthy controls (n = 50) mean ± SD or n (%) | HIV only (n = 50) mean ± SD or n (%) | HIV and TB (n = 50) mean ± SD or n (%) | P value* | |
|---|---|---|---|---|
| Demographic characteristics | ||||
| Age, years | 27 ± 7†‡ | 35 ± 10 | 37 ± 10§ | <0.001†‡ |
| 0.17§ | ||||
| Male sex | 25 (50)†‡ | 19 (38) | 29 (58)§ | 0.23† |
| 0.42‡ | ||||
| 0.05§ | ||||
| Weight, kg | — | 58 ± 11 | 52 ± 8 | <0.001 |
| Height, cm | — | 160 ± 9 | 162 ± 8 | 0.24 |
| Body mass index, kg/m2 | — | 22.8 ± 3.8 | 19.8 ± 3.8 | <0.001 |
| Laboratory characteristics | ||||
| Calcium, mg/dl | 9.5 ± 0.4†‡ | 8.8 ± 0.5 | 8.4 ± 1.3§ | <0.001†‡ |
| 0.03§ | ||||
| Albumin, g/dl | 4.4 ± 0.3†‡ | 3.7 ± 0.6 | 3.3 ± 0.7§ | <0.001†‡ |
| 0.008§ | ||||
| Corrected calcium, mg/dl | 9.2 ± 0.3 | 9.1 ± 0.3 | 8.9 ± 1.0 | 0.18 |
| 25(OH)D, ng/ml | 26 ± 7 | 28 ± 11 | 24 ± 11 | 0.14 |
| Hemoglobin, g/dl | — | 12.6 ± 2.4 | 11.6 ± 2.3 | 0.04 |
| White cell count, cells/mm3 | — | 5.0 ± 1.8 | 4.2 ± 1.5 | 0.01 |
| CD4 count, cells/μl | — | 372 ± 256 | 213 ± 151 | <0.001 |
| Medical history | ||||
| Prior tuberculosis | — | 3 (6) | 8 (16) | 0.006 |
| HAART | — | 14 (28) | 0.006 |
χ2 tests were used to compare dichotomous variables between groups.
One-way analysis of variance with Tukey correction was used to compare continuous data between the three groups, with subsequent pair-wise comparisons for significant P values, where
denotes the P value between healthy controls and subjects with HIV,
denotes the P value between healthy controls and subjects with TB and
denotes the P value between the HIV only and the HIV and TB groups
SD = standard deviation; HIV = human immunodeficiency virus; TB = tuberculosis, HAART = highly active antiretroviral therapy.
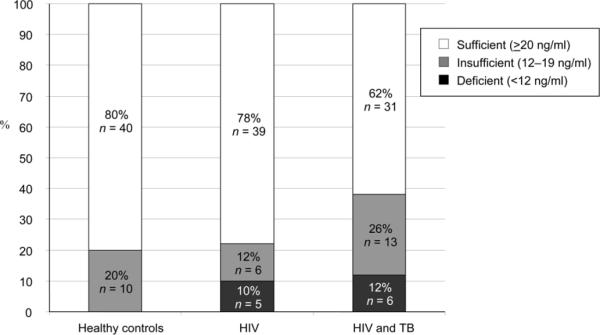
Among all 150 participants, the mean 25(OH)D level was 26 ± 10 ng/ml. Mean ± SD 25(OH)D levels were 26 ± 7 ng/ml in healthy controls, 28 ± 11 ng/ml in HIV-positives and 24 ± 11 ng/ml in TB-HIV co-infected adults (t-test P > 0.05, all comparisons). Vitamin D deficiency (<12 ng/ml) was present in none of the healthy controls, five (10%) of the HIV-positive subjects and six (12%) of the subjects with TB-HIV co-infection (P = 0.03 for healthy vs. TB, P > 0.05 for other comparisons). Suboptimal vitamin D levels (<20 ng/ml) were noted among 10 (20%) of the healthy controls, 11 (22%) of the HIV-positives and 19 (38%) of the TB-HIV co-infected subjects (P = 0.047 for healthy vs. TB, P > 0.05 for other comparisons; Figure). Two participants had potential vitamin D toxicity (>50 ng/ml), one with HIV infection (25(OH)D level, 51 ng/ml) and one with TB-HIV co-infection (25(OH)D level, 59 ng/ml). In linear regression models, weight was the only f actor significantly associated with 25(OH)D levels (P = 0.02).
Figure.
Vitamin D status in Ugandan adults with HIV and TB. Fisher's exact testing was used to analyze pair-wise comparisons. Analysis revealed P > 0.05 for all pair-wise comparisons, with the exception of two comparisons: compared to healthy controls, individuals with TB-HIV co-infection were more likely to have suboptimal vitamin D levels (<20 ng/ml, P = 0.047) and more likely to have vitamin D deficiency (P = 0.03).
Despite potential vitamin D toxicity in two individuals, no participant had hypercalcemia, defined as calcium level ≥10.4 mg/dl. Compared to healthy controls, calcium levels were significantly lower among subjects with HIV and among subjects with both HIV and TB (Table 1). However, when corrected for albumin levels, serum calcium levels were similar across the three groups.
Serum 25(OH)D levels correlated positively with body weight (r = 0.23, 95% confidence interval [CI] 0.04–0.41, P = 0.02), BMI (r = 0.22, 95%CI 0.02–0.40, P = 0.03) and serum calcium (r = 0.18, 95%CI 0.02–0.33, P = 0.03). We found no correlation between serum 25(OH)D levels and serum albumin, corrected serum calcium, white cell count, hemoglobin or CD4 count (Table 2).
Table 2.
Correlations between serum 25(OH)D levels and health parameters
| Parameter | Correlation coefficient (95%CI) | P value |
|---|---|---|
| Height (n = 100) | 0.01 (−0.19–0.20) | 0.96 |
| Weight (n = 100) | 0.23 (0.04–0.41) | 0.02 |
| Body mass index (n = 100) | 0.22 (0.02–0.40) | 0.03 |
| Serum calcium (n = 150) | 0.18(0.02–0.33) | 0.03 |
| Serum albumin (n = 150) | 0.13 (−0.03–0.29) | 0.11 |
| Corrected calcium (n = 150) | 0.08 (−0.08–0.24) | 0.31 |
| Hemoglobin (n = 97) | 0.12 (−0.08–0.31) | 0.23 |
| White cell count (n = 96) | −0.04(−0.23–0.17) | 0.73 |
| CD4 count (n = 97) | 0.07(−0.13–0.27) | 0.47 |
CI = confidence interval.
Among 100 subjects with HIV infection, those with TB were more likely to be taking HAART at enrollment (28% vs. 6%, P = 0.007). The mean duration of HAART for these patients was 1.7 months (interquartile range [IQR] 1.2–3.6). Prior history of TB disease was reported by three HIV-positive subjects, eight subjects with both HIV and TB infection and none of the HIV-negative individuals. Four (8%) patients in the HIV-only group were lost to follow-up. Among the 46 (92%) with follow-up data 9 months after enrollment, 22 (48%) were taking HAART and none had developed TB. Four (8%) of the subjects with TB-HIV co-infection were lost to follow-up. Among the 46 (92%) with outcome data at 9 months, 41 (89.1%) were cured of TB, three (6.6%) experienced treatment failure and two (4.3%) died. The two deaths occurred at home within a month of starting TB treatment and enrollment in the study. No post-mortem examinations were performed, and their cause of death was not determined. One had a 25(OH)D level of 16 ng/ml and the other a level of 26 ng/ml.
Fifteen subjects (30%) with TB-HIV developed IRIS.13 These individuals had higher serum albumin (3.7 ± 0.3 g/dl vs. 3.2 ± 0.8 g/dl, P = 0.03), higher CD4 counts (289 ± 124 cells/μl vs. 180 ± 151 cells/μl, P = 0.02) and higher hemoglobin levels (12.9 ± 1.6 g/dl vs. 11.1 ± 2.4 g/dl, P = 0.01) at study entry compared to the 35 subjects with TB who did not develop TB-IRIS. Vitamin D levels were not significantly different in subjects who developed TB-IRIS compared to those who did not (24 ± 12 vs. 26 ± 7 ng/ml, P = 0.46).
DISCUSSION
Our study found that despite year-round sun exposure, vitamin D insufficiency and deficiency were common among individuals with HIV, with or without co-existing TB. This was similar to observations in other studies evaluating 25(OH)D levels in HIV individuals.14–17 Our study findings are also similar to those observed in African settings such as Guinea-Bissau,18 Ethiopia,19 Morocco,20 and Nigeria,21 where TB patients were found to have low serum 25 (OH)D (Table 3). These studies, together with our own findings, indicate that suboptimal body vitamin D storage is common in African adults, whether healthy or TB-infected. Our study adds to the literature by comparing 25(OH)D levels among three groups of Ugandan adults: healthy controls, HIV-positive individuals and individuals with both HIV and TB infection.
Table 3.
Summary of studies evaluating vitamin D levels in African adults
| Author, year, reference | Patient population | Location, latitude | Time of recruitment | Vitamin D assay | 25(OH)D level, ng/ml mean ± SD |
|---|---|---|---|---|---|
| Felekem, 199919 | 30 adults aged 20–22 years; 31 pregnant women aged 22–28 years | Addis Ababa, Ethiopia 9°03′ North | September | HPLC | Healthy 9 Pregnant 10 (median values) |
| Wejse, 200718 | 362 TB patients; 494 healthy adults, aged 37 ± 13 years | Guinea-Bissau 12°00′ North | April 2005–February 2006 | HPLC-MS | TB 31 ± 9 Controls 43 ± 14 P < 0 001 for comparison between groups |
| Allali, 200920 | 415 healthy women aged 50 ± 9 years | Rabat, Morocco 34°02′ North | June–August | Chemiluminescence assay | 18 ± 8 |
| Glew, 201021 | 22 healthy Fulani men, aged 48 ± 8 years, 29 healthy Fulani women, aged 56 ± 14 years | Gombe, Nigeria 10°20′10″ North | Not stated | HPLC | Men 32 ± 2 Women 24 ± 1 |
| Current study | 50 healthy adults; 50 HIV-infected adults; 50 TB-HIV co-infected adults | Mbara, Uganda 0°37′ South | January–February | HPLC | Healthy 26 ± 7 HIV 28 ± 11 TB-HIV 24 ± 11 |
SD = standard deviation; HPLC = high performance liquid chromatography; HPLC-MS = HPLC-tandem mass spectrometry assay.
Our study also found that healthy Ugandans had vitamin D levels higher than those with TB. These results are congruent with the results of other studies and a recent meta-analysis.22 Collectively, these findings suggest that patients with TB might benefit from vitamin D supplementation to improve their innate immune response and mount an appropriate response against TB. Interestingly, we also found that the vitamin D status in healthy Ugandan adults closely resembled that of American adults of African origin.23,24
In the pre-antibiotic era, vitamin D was used to treat patients with TB, initially via sun exposure and later using supplements. More recently, three clinical studies reported the results of vitamin D therapy on outcomes related to TB infection. Previous clinical trials of vitamin D for individuals with TB were limited by a small sample size,25 the use of surrogate markers of illness,25 lack of serum 25(OH)D measurement during the trial,25,26 lack of randomization or blinding25 or excellent vitamin D status at study entry.27
In the largest randomized, double-blind clinical trial to date,27 365 West African adults starting treatment for TB were randomized to 1 year of placebo or 100 000 international units (IU) of cholecalciferol, administered orally at 0, 5 and 8 months. The primary outcome was the clinical severity score (TB score) at 12 months, and the secondary outcome was mortality at 12 months; 281 subjects (77%) returned for the 12-month study visit.
Researchers found no difference across treatment groups for either outcome measure (TB score or mortality). In a post-hoc analysis, researchers investigated whether vitamin D status at baseline influenced the effect of vitamin D therapy on study outcomes. Of note, only 10% of subjects in either treatment arm had 25(OH)D levels < 20 ng/ml, now considered to reflect suboptimal vitamin D stores.12 In the subgroup of 30 individuals with 25(OH)D levels < 20 ng/ml at baseline, subjects randomized to vitamin D therapy had a lower hazard ratio (HR) for death, but the P value was not significant (HR 0.7, 95%CI 0.1–6.4). In the subgroup of individuals with 25(OH)D levels < 30 ng/ml (previously considered to be vitamin D-insufficient), there was a non-significant trend toward greater mortality in subjects randomized to vitamin D (HR 1.4, 95%CI 0.5–3.7).
Given the limitations of previous clinical trials and the results of the current study, we, like others,28 call for carefully designed clinical trials in individuals with TB and low 25(OH)D levels, to clarify the role of vitamin D therapy for such individuals, including its safety. Clinical trials are also needed to clarify whether vitamin D reduces the rate of opportunistic infections among HIV-positive individuals or alters the risk of IRIS.
Our study has several strengths. We recruited subjects over a span of 2 months, and thus differences in 25(OH)D status between groups were not due to seasonal variation in sun exposure. We recruited subjects during the dry season in Uganda, when sun exposure is at a maximum. We measured 25(OH)D levels using a highly precise and reproducible assay10 considered one of two gold standard tests by the US National Institutes of Health. We measured both albumin and calcium levels, allowing us to adjust calcium levels for nutritional status.
Our study has some potential limitations. We did not score subjects' recent sun exposure nor measure their degree of skin pigmentation. However, the cultural habits of this population included regular daily sun exposure. We did not exclude subjects who may have been taking nutritional supplements. Although we routinely ask our HIV-positive individuals to take two multivitamins daily, providing 200 IU of vitamin D,29 most HIV-positive individuals were new to our clinic and therefore had not received this recommendation prior to participation in the study. We did not evaluate dietary habits that might influence vitamin D levels, and we did not assess the vitamin D content of commonly consumed foods. We did not systematically exclude subjects with significant proteinuria, which can cause vitamin D deficiency via urinary loss of vitamin D bound to vitamin D-binding protein.30 However, clinically, no patient presented with nephrotic syndrome.
CONCLUSIONS
Despite a geographic location permitting abundant year-round sun exposure along the equator, Ugandan HIV-positive individuals with and without TB commonly have vitamin D deficiency or insufficiency. Baseline hypercalcemia is unlikely to limit recruitment into a vitamin D clinical trial.27 We suggest the need for additional research studies in HIV-positive Ugandans, to evaluate the effect of vitamin D therapy on health outcomes, including the potential to prevent and treat TB.
Acknowledgements
The authors thank the subjects for taking part in the study and J Engelke for her laboratory expertise in measuring vitamin D levels. During this study, KEH received grant support from the National Institutes of Health (NIH; K23 AR050995 and R01 AG028739) and the American College of Rheumatology/Research Education Foundation and American Society for Specialty Physicians through the Hartford Foundation and Atlantic Philanthropies (Junior Career Development Award in Geriatric Medicine). FMG received grant support from the NIH (EY1252601) and DN received grant support from the NIH through the Fogarty International Center (International Clinical, Operational, and Health Services Research and Training Award-AIDS/TB, Award Number U2RTW006879). Sponsors had no role in any portion of the study, including its design, conduct, and data analysis and manuscript preparation.
References
- 1.Highleyman L. Mortality trends: towards a new definition of AIDS? Beta. 2005;17:18–28. [PubMed] [Google Scholar]
- 2.Stead WW, Senner JW, Reddick WT, Lofgren JP. Racial differences in susceptibility to infection by Mycobacterium tuberculosis. N Engl J Med. 1990;322:422–427. doi: 10.1056/NEJM199002153220702. [DOI] [PubMed] [Google Scholar]
- 3.Lochner JD, Schneider DJ. The relationship between tuberculosis, vitamin D, potassium and AIDS. A message for South Africa? S Afr Med J. 1994;84:79–82. [PubMed] [Google Scholar]
- 4.Garin B, Glaziou P, Kassa-Kelembho E, Yassibanda S, Mbelesso P, Morvan J. High mortality rates among patients with tuberculosis in Bangui, Central African Republic. Lancet. 1997;350:1298. doi: 10.1016/S0140-6736(05)62475-0. [DOI] [PubMed] [Google Scholar]
- 5.Van den Broek J, Mfinanga S, Moshiro C, O'Brien R, Mugomela A, Lefi M. Impact of human immunodeficiency virus infection on the outcome of treatment and survival of tuberculosis patients in Mwanza, Tanzania. Int J Tuberc Lung Dis. 1998;2:547–552. [PubMed] [Google Scholar]
- 6.Liu PT, Stenger S, Li H, et al. Toll-like receptor triggering of a vitamin D-mediated human antimicrobial response. Science. 2006;311:1770–1773. doi: 10.1126/science.1123933. [DOI] [PubMed] [Google Scholar]
- 7.Liu PT, Stenger S, Tang DH, Modlin RL. Cutting edge: vitamin D-mediated human antimicrobial activity against Mycobacterium tuberculosis is dependent on the induction of cathelicidin. J Immunol. 2007;179:2060–2063. doi: 10.4049/jimmunol.179.4.2060. [DOI] [PubMed] [Google Scholar]
- 8.Ministry of Health, Republic of Uganda . Manual of the National Tuberculosis and Leprosy Programme. 2nd ed MOH; Kampala, Uganda: 2010. [Google Scholar]
- 9.Ministry of Health Uganda . National antiretroviral treatment guidelines for adults, adolescents and children. 3rd ed Ministry of Health; Kampala, Uganda: 2009. [Google Scholar]
- 10.Lensmeyer GL, Wiebe DA, Binkley N, Drezner MK. HPLC method for 25-hydroxyvitamin D measurement: comparison with contemporary assays. Clin Chem. 2006;52:1120–1126. doi: 10.1373/clinchem.2005.064956. [DOI] [PubMed] [Google Scholar]
- 11.Teichmann J, Stephan E, Lange U, et al. Osteopenia in HIV-infected women prior to highly active antiretroviral therapy. J Infect. 2003;46:221–227. doi: 10.1053/jinf.2002.1109. [DOI] [PubMed] [Google Scholar]
- 12.Institute of Medicine . Dietary reference intakes for calcium and vitamin D. National Academies Press; Washington DC, USA: 2011. [PubMed] [Google Scholar]
- 13.Manosuthi W, Van Tieu H, Mankatitham W, et al. Clinical case definition and manifestations of paradoxical tuberculosis-associated immune reconstitution inflammatory syndrome. Aids. 2009;23:2467–2471. doi: 10.1097/QAD.0b013e32832f7b59. [DOI] [PubMed] [Google Scholar]
- 14.Van den Bout-Van den Beukel CJ, Fievez L, Michels M, et al. Vitamin D deficiency among HIV type 1-infected individuals in the Netherlands: effects of antiretroviral therapy. AIDS Res Hum Retroviruses. 2008;24:1375–1382. doi: 10.1089/aid.2008.0058. [DOI] [PubMed] [Google Scholar]
- 15.Rodriguez M, Daniels B, Gunawardene S, Robbins GK. High frequency of vitamin D deficiency in ambulatory HIV-positive patients. AIDS Res Hum Retroviruses. 2009;25:9–14. doi: 10.1089/aid.2008.0183. [DOI] [PubMed] [Google Scholar]
- 16.Coodley GO, Coodley MK, Nelson HD, Loveless MO. Micronutrient concentrations in the HIV wasting syndrome. AIDS. 1993;7:1595–1600. doi: 10.1097/00002030-199312000-00008. [DOI] [PubMed] [Google Scholar]
- 17.Kuehn EW, Anders HJ, Bogner JR, Obermaier J, Goebel FD, Schlondorff D. Hypocalcaemia in HIV infection and AIDS. J Intern Med. 1999;245:69–73. doi: 10.1046/j.1365-2796.1999.00407.x. [DOI] [PubMed] [Google Scholar]
- 18.Wejse C, Olesen R, Rabna P, et al. Serum 25-hydroxyvitamin D in a West African population of tuberculosis patients and unmatched healthy controls. Am J Clin Nutr. 2007;86:1376–1383. doi: 10.1093/ajcn/86.5.1376. [DOI] [PubMed] [Google Scholar]
- 19.Felekem Y, Abdulkadir J, Mshana R, et al. Low levels of serum calcidiol in an African population compared to a North European population. Eur J Endocrinol. 1999;141:358–360. doi: 10.1530/eje.0.1410358. [DOI] [PubMed] [Google Scholar]
- 20.Allali F, El Aichaoui S, Khazani H, et al. High prevalence of hypovitaminosis D in Morocco: relationship to lifestyle, physical performance, bone markers and bone mineral density. Semin Arthritis Rheum. 2009;38:444–451. doi: 10.1016/j.semarthrit.2008.01.009. [DOI] [PubMed] [Google Scholar]
- 21.Glew RH, Crossey MJ, Polanams J, Okolie HI, VanderJagt DJ. Vitamin D status of seminomadic Fulani men and women. J Natl Med Assoc. 2010;102:485–490. doi: 10.1016/s0027-9684(15)30556-3. [DOI] [PubMed] [Google Scholar]
- 22.Nnoaham KE, Clarke A. Low serum vitamin D levels and tuberculosis: a systematic review and meta-analysis. Int J E pidemiol. 2008;37:113–119. doi: 10.1093/ije/dym247. [DOI] [PubMed] [Google Scholar]
- 23.Looker AC, Pfeiffer CM, Lacher DA, Schleicher RL, Picciano MF, Yetley EA. Serum 25-hydroxyvitamin D status of the US population: 1988–1994 compared with 2000–2004. Am J Clin Nutr. 2008;88:1519–1527. doi: 10.3945/ajcn.2008.26182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zadshir A, Tareen N, Pan D, Norris K, Martins D. The prevalence of hypovitaminosis D among US adults: data from the NHANES III. Ethn Dis. 2005;15(Suppl 5):S97–S101. [PubMed] [Google Scholar]
- 25.Marcos MM, Gabr AA, Samuel S, et al. Vitamin D administration to tuberculous children and its value. Boll Chim Farm. 1998;137:157–164. [PubMed] [Google Scholar]
- 26.Nursyam EW, Amin Z, Rumende CM. The effect of vitamin D as supplementary treatment in patients with moderately advanced pulmonary tuberculous lesion. Acta Med Indones. 2006;38:3–5. [PubMed] [Google Scholar]
- 27.Wejse C, Gomes VF, Rabna P, et al. Vitamin D as supplementary treatment for tuberculosis: a double-blind, randomized, placebo-controlled trial. Am J Respir Crit Care Med. 2009;179:843–850. doi: 10.1164/rccm.200804-567OC. [DOI] [PubMed] [Google Scholar]
- 28.Yamshchikov AV, Desai NS, Blumberg HM, Ziegler TR, Tangpricha V. Vitamin D for treatment and prevention of infectious diseases: a systematic review of randomized controlled trials. Endocr Pract. 2009;15:438–449. doi: 10.4158/EP09101.ORR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fawzi WW, Msamanga GI, Spiegelman D, et al. A randomized trial of multivitamin supplements and HIV disease progression and mortality. N Engl J Med. 2004;351:23–32. doi: 10.1056/NEJMoa040541. [DOI] [PubMed] [Google Scholar]
- 30.Sato KA, Gray RW, Lemann J., Jr Urinary excretion of 25-h ydroxyvitamin D in health and the nephrotic syndrome. J Lab Clin Med. 1982;99:325–330. [PubMed] [Google Scholar]