Abstract
The middle cingulate cortex (MCC) has been implicated in pain processing by studies of cingulotomy for chronic pain and imaging studies documenting increased MCC blood flow in response to acute pain. The only previous report of quantitative sensory testing following cingulotomy described increased intensity and unpleasantness ratings of painful hot and cold stimuli in a single patient with psychiatric disease. We now report a case in which perception of pain and temperature was assessed before and after cingulotomy for obsessive-compulsive disorder (OCD). Positron emission tomographic (PET) studies of the bloodflow response to acute pain were carried out using a single subject design which allowed for statistical evaluation of postoperative blood flow changes in this case.
Postoperatively, the patient demonstrated increased intensity and unpleasantness ratings of painful thermal waterbath stimuli. The PET studies demonstrated preoperative contact heat pain-evoked activation of the bilateral MCC/SMA (supplementary motor area) and the left (contralateral) fronto-parietal operculum. Postoperative pain-evoked activation was demonstrated in the right (ipsilateral) parasylvian cortex but not of the MCC/SMA. Prior studies of forebrain lesions, and of cortical synchrony during the application of painful stimuli suggest the presence of functional connectivity between components of the MCC/SMA and the fronto-parietal opercula. Therefore present results suggest that cingulate lesions disinhibit ipsilateral parasylvian cortex and so are independent evidence of functional connectivity between these cortical areas, the defining characteristic of components in a pain network.
Keywords: Cingulotomy, Pain Intensity, Pain Unpleasantness, Human Anterior Cingulate Cortex, Insula, S2
INTRODUCTION
The MCC is comprised of the cingulate cortical structures which are just anterior to the central sulcus, and which may mediate the unpleasantness of pain among other functions [Abdelaziz and Cosgrove 2002; Apkarian et al. 2005; Vogt 2005]. Cingulotomy is a rare surgical procedure for treatment of psychiatric disease or pain. This surgical procedure lesions both the MCC, and the cingulum bundle, which includes fibers coursing to and from and within the MCC. The only prior study of acute pain psychophysics following cingulotomy reported increased ratings of the intensity and unpleasantness of painful thermal stimuli in a single subject with psychiatric disease [Davis et al. 1994]. We now report results of psychophysical testing, and a single subject PET study of pain-evoked blood flow responses following cingulotomy in a patient with OCD. The postoperative results demonstrate increased pain ratings, and altered blood flow which was decreased in MCC/SMA and increased in ipsilateral parasylvian cortical structures. The present results suggest that cingulate lesions disinhibit ipsilateral parasylvian cortex as a result of functional connectivity between these areas, a defining characteristic of the pain network [Ohara et al. 2006].
SUBJECT AND METHODS
Subject description
The patient (male, 31 years old) was diagnosed with OCD which was refractory to medical therapy. In such patients, the cingulum bundle can be lesioned (cingulotomy), a rare procedure which is effective in approximately 60% of patients with OCD [Dougherty et al. 2002]. Throughout these studies he was treated with clonazepam 1 mg (a benzodiazepine), fluvoxamine (Serotonin Selective Reuptake Inhibitor – SSRI anti-depressant) 100mg twice per day, and Mirtazapine (SSRI) 75 mg each night. Preoperatively, he had a NIMH (National Institute of Mental Health, National Institute of Health, Bethesda, Maryland, USA: NIH-NINDR) Global Obsessive Compulsive Scale of 12 (upper limit of normal - 6, definite disability - 12), a Yale-Brown Obsessive-Compulsive Scale of 14 for obsessions and of 14 for compulsions summing to 28 (upper limit of normal - 8, definite disability - 18)[Blacker 1999]. At all times relevant to this study he was stable clinically and was on the medications listed above.
The patient was approached for participation in this protocol after he decided to undergo cingulotomy. Sensory testing and PET scan protocols were carried out approximately two weeks preoperatively and two weeks postoperatively. The sensory testing protocols were approved by the Johns Hopkins and the University of Maryland at Baltimore Institutional Review Boards (IRBs), and the PET imaging protocols were approved by the NIH-NIDR IRB. The patient gave written informed consent for both protocols.
Sensory Testing Protocol
Thermal thresholds
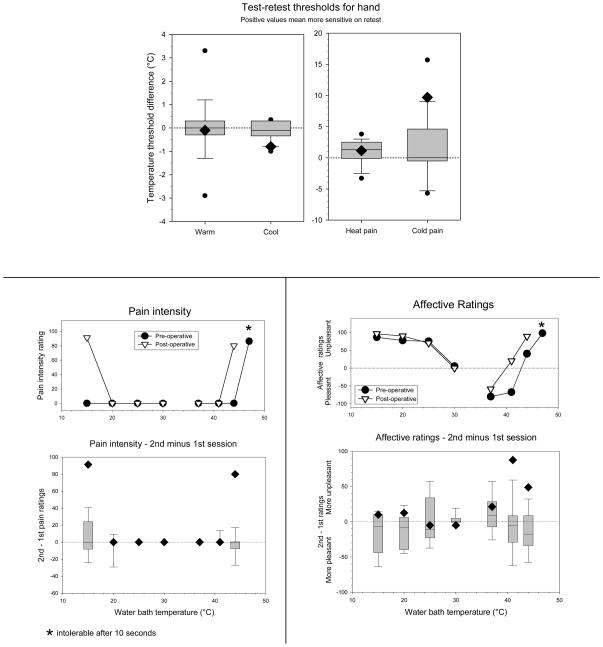
These tests determined the threshold temperature levels for evoking each of four sensations: warm, cool, heat pain, and cold pain. This test used a small (7cm2) computer-controlled Peltier thermode (Mark IX, Florida State Univ., Tallahassee, FL), which was applied to the dorsum of the hands. In this procedure, the thermode was gradually heated (or cooled; 1°C/sec) from the adapting temperature, (33°C for warm and cool thresholds, 30°C for cold pain threshold, and 35°C for heat pain threshold). The patient was instructed to press a button when he first felt the sensation specified in the instructions e.g. warmth (modified Marstock method [Fruhstorfer et al. 1976]). The temperature of the thermode at the time of the button push was recorded, and then the thermode was then returned to the baseline temperature. This was repeated 4 times on each hand for each sensation. Data were averaged across both hands, since no laterality differences were noted. For any given test site, thresholds were determined in the following sequence: warm, cool, heat pain, and then cold pain.
The threshold data from the patient were compared to a control database derived from 19 healthy men tested in a similar manner, and comparing their change in threshold between test and retest sessions separated by 2–3 weeks (M Backonja - personal communication). Patient data were considered significantly different from control if they fell outside the 5th or 95th percentile of values from this database (Table 1). Data falling outside the 10th and 90th percentiles, but within the 5th and 95th percentiles is also noted in Table 1.
Table 1.
Pre- and Post-operative Thermal Thresholds.
| Normative distibution | Warm | Cool | Heat Pain | Cold Pain | ||||
|---|---|---|---|---|---|---|---|---|
| 5th and 95th percentile | −2.2; 2.4 | −0.9; 0.3 | −2.9; 3.4 | −5.5; 12.7 | ||||
| 10th and 90th percentile | −1.2; 1.2 | −0.8; 0.3 | −2.2; 3.0 | −4.4; 8.2 | ||||
| Patient results | R | L | R | L | R | L | R | L |
| Difference | N/A | − 0.1 | −0.8 | −0.8 | 0.5 | 1.8 | 9.8* | 9.6* |
| Preoperative | 2.2°C | 2.1°C | −0.5°C | −0.6°C | 46.8°C | 45.2°C | 12.8°C | 13.9°C |
| Postoperative | N/A | 2.2°C | −1.3°C | −1.4°C | 46.3°C | 43.4°C | 22.6°C | 23.5°C |
Normative distribution shows percentiles of the difference in thresholds between two successive data collection sessions. These data were derived from 19 male subjects tested in a similar manner as the patient (courtesy of Miroslav M. Bakonja).
Patient results shows actual thresholds, as well as the preoperative-postoperative difference. Warm and cool thresholds (two ratings for each hand) are shown as changes from the adapting temperature (33°C). Heat pain and cold pain thresholds are shown as actual threshold temperatures. Positive difference values indicate that the patient was more sensitive on postoperative than preoperative testing.
Difference values are beyond the 90th percentile of normative test-retest differences.
N/A: Data unavailable.
2) Hot and cold thermal ratings
This test measured sensations evoked by placing a hand in a series of water baths maintained at temperatures ranging from 10–47°C, as in our previous protocol [Sarlani et al. 2003]. The patient rated three different aspects of perception: a) thermal intensity of non-painful sensations, b) the level of unpleasantness of the pain sensation, and c) the intensity of the pain. These data were compared to ratings collected from a group of ten healthy age-matched male subjects tested in the same way, from two sessions separated by two weeks.
Functional Brain Imaging
Brain activation was assessed by measuring relative changes in cerebral blood flow with H215O PET using a standard, single subject protocol [Chmielowska et al. 1998]. In brief, the subject was placed in the PET scanner (GE Advance, General Electric, Milwaukee, Wisconsin, USA) and transmission scans were performed for attenuation correction during image reconstruction. A sham scan (saline injection) was carried out to minimize anxiety associated with the PET scan. Thereafter, each PET scan was initiated upon intravenous bolus injection of 10mCi H215O, with data acquisition (3D mode with septa retracted) during the 60 sec following tracer arrival in the brain. The subject had 15 PET scans one day prior to surgery, and again ten days after surgery.
In both preoperative and post-operative sessions, the subject was scanned during 3 different stimulus conditions: 1) rest (no somatosensory stimulation), 2) 35°C stimulation, and 3) 48°C stimulation. A 1 cm diameter contact thermal stimulator was applied sequentially to 6 regions (2 X 3 grid, 2 cm between spots, 5 sec stimulus/spot: 0.5 sec between spots) on the ventral surface of the right forearm, to be consistent with previous studies [Chmielowska, Coghill, Maisog, Carson, Herscovitch, Honda, chen, and Hallett 1998; Coghill et al. 2001]. Stimulation was initiated 5 sec prior to tracer injection and was continued for ~90 sec until completion of data acquisition. At the end of every PET scan the subject rated pain using two separate VAS’s each with a range 0–10. One scale was the intensity VAS where 0 represented no pain sensation and 10 represented the most intense pain sensation imaginable. The other was the unpleasantness VAS where 0 represented not at all unpleasant, and 10 represented the most unpleasant sensation imaginable. Each scanning condition was repeated 5 times, and conditions were presented in pseudorandom order. The number of replications was limited by the institutional regulations regarding limits on annual allowed exposure to radioactivity.
Image Processing and Analysis
All image processing and analysis operations were executed with the FSL software library (Image Analysis Group FMRIB, Oxford, UK). T1 weighted magnetic resonance imaging (MRI) scans were obtained both preoperative and postoperatively, and were used for transformation of respective PET data sets into standard stereotaxic space [Jenkinson et al. 2002]. After spatial normalization, background correction, and movement correction, PET data were smoothed with a 10x10x10 mm Gaussian filter to minimize the spatial variability further [Chmielowska, Coghill, Maisog, Carson, Herscovitch, Honda, Chen, and Hallett 1998; Jenkinson, Bannister, Brady, and Smith 2002]. The smoothness of the image is determined by the spatial filter in part, and also determines how many multiple, statistically independent comparisons are performed. The lower the smoothness of the image, the greater the number of multiple comparisons. Therefore, a cluster of X volume in a dataset that was smoother as a result of being spatially smoothed with a large filter (i.e. 10x10x10mm) would yield a smaller (i.e. more significant) p value versus a cluster of the exact same number of voxels obtained from a dataset with a 5x5x5mm filter. Finally, to minimize variability produced by global CBF changes, each PET scan was normalized to grey matter values by dividing each voxel value by the average of whole brain CBF.
Standard general linear modeling techniques (GLM) were then used to identify activation that was significantly related to perceived pain intensity. Gaussian random field theory based statistics were used to correct for multiple comparisons in the calculation of the experiment-wise false positive rate according to [Friston et al. 1994]. Clusters of contiguous voxels exceeding a z-score of 3.1 and a p<0.01 were considered to be statistically reliable and were adopted for the Z statistic images. This criterion was very conservative because the 10x10x10mm spatial filtering (see above) minimized the numbers of multiple comparisons made in this analysis.
We specifically tested the hypothesis that the preoperative MCC/SMA locus would be activated post-operatively. Preoperative and postoperative changes in pain-induced activation of the MCC/SMA were assessed using a region of interest (ROI) analysis and evaluated using a paired t-test. This ROI was defined by the voxels within the MCC/SMA that exhibited a statistically reliable relationship with perceived pain intensity during the preoperative scans. The use of the preoperative scan to define the MCC/SMA ROI may introduce bias to this single subject analysis. However, it avoids the false negatives caused by inter-individual variability in the location of this ROI which may be located outside the MCC/SMA activation, if the ROI were to be determined on the basis of analysis of an arbitrary group of subjects [Vogt et al. 1996].
The cingulotomy was carried out stereotactically using the Leksell frame, as previously described [Abdelaziz and Cosgrove 2002]. Coronal images were taken through the frontal lobe and used to target the cingulum bundle in the coronal plane 2.5 cm posterior to the anterior pole of the lateral ventricles. Burrholes were fashioned 2 centimeters anterior to the coronal suture and 2.5 cm lateral to the midline, bilaterally. The radiofrequency electrode (exposed tip 1cm, diameter 1.8mm, Radionics, Boston MA) was placed at the target and heated to 75°C for one minute and then to 85°C for one minute. The electrode was then backed off by 1 cm and the electrode was heated to the same temperatures at this position.
RESULTS
Lesion Location
Figure 1 shows that the lesions (black arrowheads on right side of postoperative images) involved a cylinder of cingulate bundle and cortex (radius 6mm) which was centered 33 mm dorsal to the AC-PC line and 22 mm rostral to the AC [Talairach and Tournoux 1988]. The left and right lesions were centered 12 and 16 mm lateral to the midline, as determined by the MRI location of the cingulum bundle on either side. The location shown in this Figure corresponds to the lesion locations demonstrated in many previous reports of cingulotomy (reviewed by [Abdelaziz and Cosgrove 2002]).
Figure 1. Anatomic location and blood flow consequences of cingulotomy.
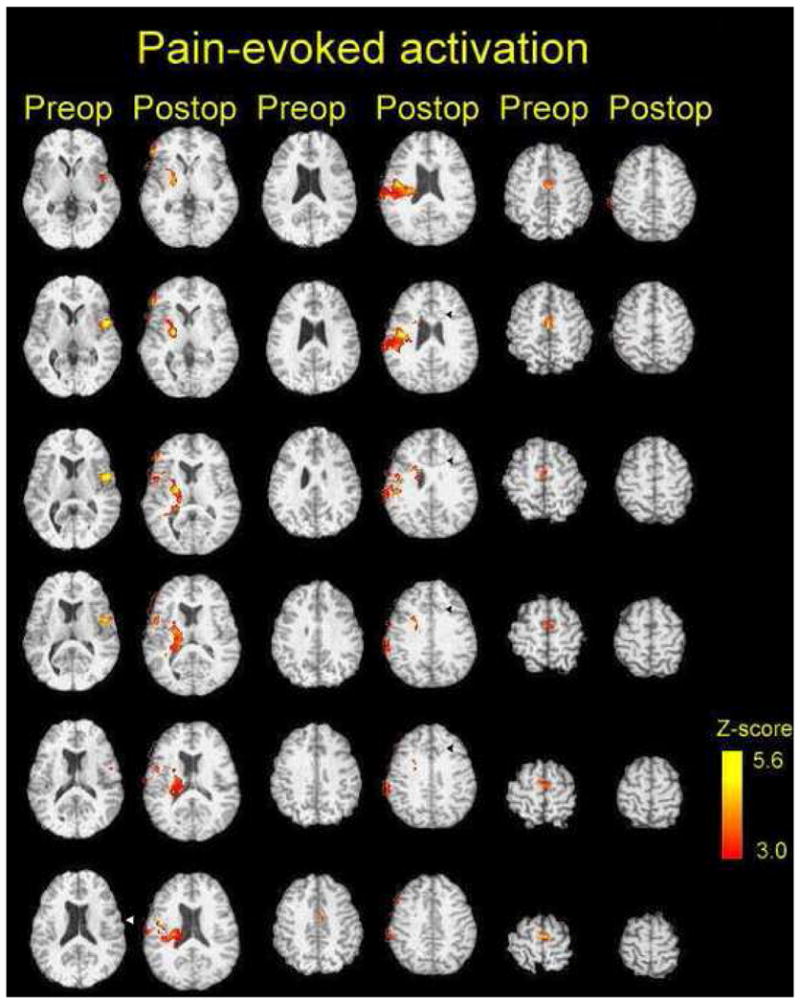
The cingulotomy lesions are seen on postoperative (T1 weighted axial) scans as circular structures (black arrowheads) bilaterally with a light center composed of blood products, and a surrounding, darker ring composed of edematous tissue. The color axis on these images indicates pain-related bloodflow changes pre- and post-cingulotomy with the Z-score as indicated in the calibration scale. See text. The left side of each image corresponds to the patient’s right side; the stimulus was applied to the subject’s right forearm, during all imaging studies.
Psychophysical results
The preoperative psychophysical test session revealed thresholds within the normal range, and with no indication of a laterality difference (Table 1). The changes in warm, cool, and heat pain thresholds were not significantly outside the normative data range for test-retest values. However, the post-operative increase of nearly 10°C in cold pain sensitivity fell outside the 90th percentile of the normative data.
The patient showed significant post-operative increases in the intensity ratings of heat pain and cold pain during water bath testing (Figure 2A). Most notably, temperatures that were not painful preoperatively (15°C and 44°C) were intensely painful postoperatively, such that the postoperative change in ratings was well outside the test-retest difference in ratings from the normative data set. The 47°C stimulus became intolerable postoperatively, such that the intensity and unpleasantness difference from the preoperative ratings could not be calculated. However, the patient’s unpleasantness ratings for heat pain stimuli were elevated (greater than the 90% percentile of the normative test-retest data set) at the other two hot water bath temperatures (41 and 44°C, Figure 2B), which indicates a significant overall increase in unpleasantness ratings for painful and non-painful heat stimuli (P=0.028, Binomial).
Figure 2. Psychophysical consequences of cingulotomy.
A, Ratings of pain intensity during exposure of the hand to water baths of various temperatures, based on the average of four ratings (two ratings for each hand). Top: Preoperative (filled circle) and postoperative (open triangle) ratings. Bottom: Change in ratings from preoperative to postoperative. The diamond symbol represents the patient’s data. The box and whisker plot represents the range of test-retest differences recorded for a group of 10 age-matched healthy male subjects. For these data the box identifies the range between the 25th and the 75th percentiles, while the whiskers define the 10th and 90th percentiles of the group data. Positive values indicate that the ratings were higher for the second testing session.
B, ratings of unpleasantness during exposure of the hand to water baths of various temperatures. Positive values represent unpleasantness ratings, and negative values represent pleasantness ratings. Conventions as in the legend for A.
Differences between intensity and unpleasantness ratings of the contact heat and waterbath protocols may be related to characteristic differences in the psychophysical ratings of these different painful stimuli [Rainville et al. 1992]. Similarly, these protocols evoked intensity and unpleasantness ratings which differed when presented to the same subject, in the current study. We propose that the post-operative changes observed with water bath testing reflect a true difference in the subject’s perceptual system, which was not captured using small area contact stimuli [Rainville et al. 1992].
The patient showed non-significant increases in unpleasantness ratings for cold water bath temperatures bilaterally. The lack of a significant change may be a result of the high preoperative ratings which precluded a significant increase postoperatively (Figure 2B). These unpleasantness ratings were obtained at temperatures which were not rated as painful (Figure 2A), as previously described for healthy volunteers [Greenspan et al. 2003]. Innocuous, thermal intensity ratings changed little post-operatively (VAS change ≤ 10/100) and always fell within the normal range.
Prior to cingulotomy, pain-related PET activations were identified in the bilateral MCC (post ACC) and adjacent supplementary motor area (SMA) bilaterally, referred to as MCC/SMA. A second region of activation was identified in the left (contralateral) fronto-parietal operculum centered on the central sulcus (white arrow in Figure 1, Table 2). Based on a repeat PET scan ten days after cingulotomy, the global analysis of pain-related activations identified significant activations in the ipsilateral (right) frontal operculum, putamen and thalamus, portions of the parietal operculum in the vicinity of ipsilateral S2, and the posterior insula. The contralateral S2 showed no evidence of pain related activation postoperatively, even at less conservative Z- scores, Activation within the MCC/SMA was significantly decreased by cingulotomy (t=4.18, p<0.01, ROI analysis) consistent with the lack of postoperative activation within in MCC/SMA in our global search (Figure 1).
Table 2.
Activation by painful heating of the right forearm: Pre- and Post-operative
| PREOPERATIVE | |||||
|---|---|---|---|---|---|
| Z score | X tal | Y tal | Z tal | Side | Region |
| 4.543 | −2.00 | −6.00 | 70.00 | Mid | MCC/SMA BA 6/24 |
| 3.624 | 2.00 | 2.00 | 62.00 | Mid | SMA, BA 6 |
| 5.593 | −48.00 | 4.00 | 6.00 | Left | Fronto-parietal operculum, BA 6 |
| 4.817 | 4.00 | −56.00 | −26.00 | Right | Cerebellar vermis |
| 3.854 | 0.00 | −62.00 | −42.00 | Mid | Cerebellar vermis |
| 3.751 | 28.00 | −48.00 | −26.00 | Right | Cerebellar hemisphere |
| POSTOPERATIVE | |||||
| 4.159 | 52.00 | −28.00 | 28.00 | Right | Parietal operculum, SII |
| 4.051 | 54.00 | 6.00 | 12.00 | Right | Frontal operculum, BA 6/44 |
| 3.615 | 34.00 | −28.00 | 18.00 | Right | Post insula |
| 3.555 | 32.00 | −28.00 | 16.00 | Right | Post insula |
| 5.233 | 46.00 | −8.00 | 26.00 | Right | Precentral gyrus, BA6 |
| 5.133 | 24.00 | −6.00 | 6.00 | Right | Putamen |
| 3.728 | 22.00 | 8.00 | −4.00 | Right | Putamen/GP |
| 3.718 | 24.00 | 6.00 | −2.00 | Right | Putamen/GP |
| 3.389 | 32.00 | 10.00 | 4.00 | Right | Putamen |
| 3.310 | 30.00 | 8.00 | 4.00 | Right | Putamen |
| 4.262 | 50.00 | −58.00 | −34.00 | Right | Cerebellar hemisphere |
Mid: bilateral activation at the midline.
Neither pain intensity nor pain unpleasantness ratings obtained with a contact thermal stimulator, during PET scanning, changed significantly after surgery (pain intensity: preoperatively 8.6, post-op 7.7; pain unpleasantness: preoperative 8.9, post-op 8.3).
DISCUSSION
The hypothesis that pain-related activation of the MCC is related to the unpleasantness of acute pain predicts that MCC lesions will decrease the unpleasantness of pain [Apkarian, Bushnell, Treede, and Zubieta 2005; Rainville et al. 1997; Davis et al. 1997]. However, our psychophysical rating studies showed that the unpleasantness and intensity of acute thermal pain were increased after cingulotomy. Our postoperative imaging studies showed decreased pain-evoked activation of the MCC/SMA and increased activation of the ipsilateral parasylvian cortical structures (Figure 1) which are connected with the MCC [Van Hoesen et al. 1993] [Vogt 2005]. These results suggest that lesions of MCC disinhibit parts of ipsilateral parasylvian cortex, and so are independent evidence of functional connectivity. This connectivity can be considered a defining characteristic of the pain network suggested by analysis of synchrony of local field potentials [Ohara, Crone, Weiss, and Lenz 2006].
Perceptual Changes following Lesions of the Frontal Lobe
Our results are congruent with those of the only prior psychophysical study of changes in pain and temperature perception after cingulotomy [Davis, Hutchinson, Lozano, and Dostrovsky 1994]. Both studies showed increased ratings of the intensity and unpleasantness of heat pain. The prior study showed increased ratings of the intensity and unpleasantness for cold pain, while the present study showed significantly increased ratings only for the intensity of cold pain. The lack of a significant increase in cold unpleasantness may result from a ceiling effect, since unpleasantness ratings for cold stimuli were high enough preoperatively to preclude a significant increase in ratings postoperatively (Figure 2B). None of the thermal thresholds (warm, cool, heat pain or cold pain) in either study demonstrated a significant change within two weeks of surgery. However, the large postoperative increase in cold pain threshold (almost 10°C) may be biologically significant. Nevertheless, the most consistent finding between these two studies is the significantly increased ratings of intensity and unpleasantness of thermal pain.
One psychophysical study has examined the effects of anterior capsulotomy upon the perception of acute pain in patients without any MRI abnormality. Anterior capsulotomy is a rare procedure for psychiatric disease which produces a more extensive lesion than cingulotomy by interrupting afferent and efferent fibers to the anterior portion of the MCC and other frontal lobe structures [Talbot et al. 1995]. This study found decreased ratings for painful stimuli, yet decreased tolerance post-capsulotomy, in contrast to the effects of cingulotomy (see above). Anterior capsulotomy partially disconnects and disinhibits, but not does not destroy, the MCC, perhaps leading to physiological and psychophysical changes opposite those of cingulotomy [Talbot, Villemure, Bushnell, and Duncan 1995].
Both cingulotomy and frontal leukotomy, a more extensive lesion than capsulotomy, have been reported to make chronic or cancer pain less unpleasant but not necessarily less intense [Foltz and White 1962]. More recent studies have reported the effect of cingulotomy upon chronic pain (reviewed by [Abdelaziz and Cosgrove 2002]) report a decrease in chronic pain, but not a selective decrease in the unpleasantness of pain. Therefore effect of cingulotomy upon chronic pain is clearly different from the effect upon experimental pain (see above) [Davis, Hutchinson, Lozano, and Dostrovsky 1994], Differences of this type also are seen in imaging studies of acute and chronic pain [Apkarian, Bushnell, Treede, and Zubieta 2005] and demonstrate that the present results can only be interpreted in case of acute pain.
Changes in Pain-related blood flow in the MCC/SMA, Insula, and Parietal Operculum
Our preoperative scans demonstrate a pain-related response in the contralateral frontal-parietal operculum, the bilateral caudal MCC/SMA, including Brodmann’s cortical area 24. This is consistent with many functional imaging studies [Apkarian, Bushnell, Treede, and Zubieta 2005; Davis 2000; Rainville et al. 2000] and with electrophysiologic evidence of nociceptive input to these areas [Hutchinson et al. 1999; Lenz et al. 1998b; Lenz et al. 1998a; Ohara et al. 2004]. However, the present results demonstrate no pain-related MCC/SMA activation after the cingulotomy.
The cingulotomy lesions were consistent with those of previous reports [Abdelaziz and Cosgrove 2002], and were located 3 cm anterior to the preoperative pain-related activation on the medial frontal lobe (Figure 1). Therefore, the position of the lesion relative to the preoperative activation suggests that the decreased blood flow was not a result of direct tissue injury (see also {Peyron, 2000 3958/id}). However, lesions of the cingulum bundle lead to widespread disruption of connections between different parts of the MCC, and to widespread effects on the MCC. These lesions may also interrupt input arising from the pain-related structures in the thalamus and amygdalae, and thereby eliminate pain-related activation in the MCC, which was seen preoperatively in this case [Mufson and Pandya 1984; Bernard et al. 1993].
Pain-related activations were on the right (ipsilateral, left side of images) opercular and insular cortex after cingulotomy, but the left preoperatively. This pattern would be unusual in a population study protocol of healthy controls [Apkarian, Bushnell, Treede, and Zubieta 2005], although the ipsilateral activations occurred commonly in an fMRI study of healthy single subjects [Davis et al. 1998]. Ipsilateral parasylvian activations have also been observed during the increased (allodynic) responses to thermal stimuli, in patients with central pain related to lesions of the brain or spinal cord {Peyron, 2000 3958/id}{Peyron, 2004 7557/id}{Ducreux, 2006 7429/id}. These results and the present postoperative results suggest that ipsilateral activations are a common factor in increased ratings of pain following brain lesions, whether clinically significant (allodynic) or not.
The mechanism of the increased activation of ipsilateral parasylvian structures postoperatively may be disinhibition of pain-related inputs to these structures by the cingulotomy [Lenz, Rios, Chau, Krauss, Zirh, and Lesser 1998a; Van Hoesen, Morecraft, and Vogt 1993; Vogt 2005]. This disinhibition could lead to pain-related increased synaptic activity and bloodflow. In addition, postoperative pain-related activations of the right (ipsilateral) parietal and insular cortex after cingulotomy (Figure 1) might be consistent with reports of activation of right inferior parietal cortex following stimulation of either side [Coghill, Gilron, and Iadarola 2001].
In addition to these cortical activations we describe ipsilateral putaminal activation postoperatively but no activation was detected preoperatively. Again this pattern would be unusual in a population study protocol of healthy controls [Apkarian, Bushnell, Treede, and Zubieta 2005] but not in single subject fMRI studies of healthy controls [Davis, Kwan, Crawley, and Mikulis 1998]. Therefore, the observations on this patient are not unusual results for a single subject analysis of normals. Putaminal activation ipsilateral to the increase in cortical activation postoperatively may be related the powerful inputs to the putamen from cortex, including ipsilateral parasylvian cortex [Alexander et al. 1986; Albin et al. 1989].
Ipsilateral parietal and insular activations and increased pain ratings following bilateral cingulotomy might be interpreted as disinhibition resulting from a loss of an analgesic influence from the MCC [Van Hoesen, Morecraft, and Vogt 1993]. This analgesic influence may be related to opioid-mediated influence of MCC which binds to and is activated by μ-opioids, upon the parietal operculum and insula, which show neither μ-opioid binding nor activation. This disinhibition may also be interpreted as evidence of pain-related functional connectivity consistent with a pain network [Ohara, Crone, Weiss, and Lenz 2006].
Implications for Pain Networks
Lesion studies can be help to identify the networks related to neurologic function. For example, patients with lesions of the principle sensory nucleus of thalamus, and the parietal operculum demonstrate impaired discrimination of acute pain [Kim et al. 2007; Greenspan et al. 1999]. Patients with lesions of the insula which spare the parietal operculum, show normal discrimination but elevated tolerance for acute pain [Greenspan, Lee, and Lenz 1999; Berthier et al. 1988]. Pain tolerance incorporates the motivational, cognitive and affective components of pain [Blitz and Dinnerstein 1968; Chen et al. 1989]. The present and the prior studies independently demonstrate that cingulotomy increases the ratings of the intensity and unpleasantness of acute pain. Therefore, lesions of parietal operculum, insula, and MCC each produce a different effect on pain perception, which may reflect discrete roles that they play in the pain network defined by analysis of synchrony [Ohara, Crone, Weiss, and Lenz 2006].
If lesions of different structures produce different psychophysical effects upon some neurologic function, then these lesion effects are referred to as ‘double dissociation’ [Bullinaria 2002]. ‘Double dissociation’ is characteristic of hierarchical networks in which component structures, such as individual cortical areas, are local networks, each serving a different function. Hierarchical networks are assumed to account for many neurologic functions such as language, in which two different modules (inferior frontal and superior temporal areas) may subserve speech production and reception respectively [Bullinaria2002].
The psychophysical effects of lesions involving parietal operculum, insula, and MCC suggest that they are separate modules in a hierarchical pain network. Recent evidence of synchrony between local cortical field potentials suggests the presence of functional connectivity between MCC and parietal opercular and insular cortex [Ohara, Crone, Weiss, and Lenz 2006]. The functional connectivity within such a network may be conceived of as the network properties which enable its modules jointly to process inputs or outputs or both [Churchland and Sejnowski 1992]. Since a lesion of one (MCC) has a dramatic effect on the other two, these imaging results suggest that the MCC, parietal operculum and insula jointly process nociceptive signals. Therefore, analysis of imaging, lesion and synchrony suggest the presence of a hierarchical pain network which may facilitate modeling studies of the pain network, as in the case of the visual system [Churchland and Sejnowski 1992].
Acknowledgments
This work is supported by the National Institutes of Health – National Institute of Neurological Disorders and Stroke (NS38493 and NS40059 to F.A.L. NS-39337 to JDG., and NS 39426 to RCC). We thank L. H. Rowland, C. Cordes, and Elisa M. Rosier for excellent technical assistance. We also thank Dr. Michael Iadarola for his support of the imaging component of this project. We also thank Dr. M Backonja for providing test-retest psychophysical data prior to its publication.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Reference List
- Abdelaziz OS, Cosgrove GR. Stereotactic cingulotomy for the treatment of chronic pain. In: Burchiel KJ, editor. Surgical management of pain. New York: Thieme; 2002. pp. 812–20. [Google Scholar]
- Albin RL, Young AB, Penney JB. The functional anatomy of basal ganglia disorders. Trends Neurosci. 1989;12:366–375. doi: 10.1016/0166-2236(89)90074-x. [DOI] [PubMed] [Google Scholar]
- Alexander GE, DeLong MR, Strick PL. Parallel organization of functionally segregated circuits linking basal ganglia and cortex. Annu Rev Neurosci. 1986;9:357–381. doi: 10.1146/annurev.ne.09.030186.002041. [DOI] [PubMed] [Google Scholar]
- Apkarian AV, Bushnell MC, Treede R-D, Zubieta JK. Human brain mechanisms of pain perception and regulation in health and disease. European Journal of Pain. 2005;9:463–484. doi: 10.1016/j.ejpain.2004.11.001. [DOI] [PubMed] [Google Scholar]
- Bernard JF, Alden M, Besson JM. The organization of the efferent projections from the pontine parabrachial area to the amygdaloid complex: a Phaseolus vulgaris leucoagglutinin (PHA-L) study in the rat. J Comp Neurol. 1993;329:201–229. doi: 10.1002/cne.903290205. [DOI] [PubMed] [Google Scholar]
- Berthier M, Starkstein S, Leiguarda R. Asymbolia for pain: a sensory-limbic disconnection syndrome. Ann Neurol. 1988;24:41–49. doi: 10.1002/ana.410240109. [DOI] [PubMed] [Google Scholar]
- Blacker D. Psychiatric rating scales. In: Sadock BJ, Sadock VA, editors. Comprehensive textbook of psychiatry. Philadelphia: Lippincott, Williams, and Wilkins; 1999. pp. 755–83. [Google Scholar]
- Blitz B, Dinnerstein AJ. Effects of different types of instructions on pain parameters. J Abnorm Psychol. 1968;73:276–280. doi: 10.1037/h0025745. [DOI] [PubMed] [Google Scholar]
- Bullinaria JA. Lesioned networks as models of neuropsychological deficits. In: Arbib MA, editor. The handbook of brain theory and neural networks. Cambridge: The MIT Press; 2002. pp. 635–8. [Google Scholar]
- Chen AC, Dworkin SF, Haug J, Gehrig J. Human pain responsivity in a tonic pain model: psychological determinants. Pain. 1989;37:143–160. doi: 10.1016/0304-3959(89)90126-7. [DOI] [PubMed] [Google Scholar]
- Chmielowska J, Coghill RC, Carson RE, et al. Comparison of PET [Oxygen 15]water studies with 6 min and 10 min interscan intervals: single subject and group analyses. J Cereb Blood Flow and Metabol. 1999;19:570–582. doi: 10.1097/00004647-199905000-00011. [DOI] [PubMed] [Google Scholar]
- Chmielowska J, Coghill RC, Maisog JM, et al. Positron emission tomography [15O]water studies with short interscan interval for single-subject and group analysis: influence of background subtraction. J Cereb Blood Flow Metab. 1998;18:433–443. doi: 10.1097/00004647-199804000-00012. [DOI] [PubMed] [Google Scholar]
- Churchland PS, Sejnowski TJ. The computational brain. Cambridge: MIT Press; 1992. [Google Scholar]
- Coghill RC, Gilron I, Iadarola MJ. Hemispheric lateralization of somatosensory processing. J Neurophysiol. 2001;85:2602–2612. doi: 10.1152/jn.2001.85.6.2602. [DOI] [PubMed] [Google Scholar]
- Davis KD. Studies of pain using functional magnetic resonance imaging. In: Casey KL, Bushnell MC, editors. Pain Imaging. Seattle: IASP Press; 2000. pp. 195–210. [Google Scholar]
- Davis KD, Hutchinson WD, Lozano AM, Dostrovsky JO. Altered pain and temperature perception following cingulotomy and capsulotomy in a patient with schizoaffective disorder. Pain. 1994;59:189–199. doi: 10.1016/0304-3959(94)90071-X. [DOI] [PubMed] [Google Scholar]
- Davis KD, Kwan CL, Crawley AP, Mikulis DJ. Functional MRI study of thalamic and cortical activations evoked by cutaneous heat, cold, and tactile stimuli. J Neurophysiol. 1998;80:1533–1546. doi: 10.1152/jn.1998.80.3.1533. [DOI] [PubMed] [Google Scholar]
- Davis KD, Taylor SJ, Crawley AP, Wood ML, Mikulis DJ. Functional MRI of pain- and attention related activation in the human cingulate cortex. J Neurophysiol. 1997;77:3370–3380. doi: 10.1152/jn.1997.77.6.3370. [DOI] [PubMed] [Google Scholar]
- Dougherty DD, Baer L, Cosgrove GR, et al. Prospective long-term follow-up of 44 patients who received cingulotomy for treatment-refractory obsessive-compulsive disorder. Am J Psychiatry. 2002;159:269–275. doi: 10.1176/appi.ajp.159.2.269. [DOI] [PubMed] [Google Scholar]
- Foltz EL, White LE. Pain “relief” by frontal cingulumotomy. J Neurosurg. 1962;19:89–100. doi: 10.3171/jns.1962.19.2.0089. [DOI] [PubMed] [Google Scholar]
- Friston KJ, Worsley KJ, Frackowiak RSJ, Mazziotta JC, Evans AC. Assessing the significance of focal activations using their spatial extent. Hum Brain Mapp. 1994;1:210–220. doi: 10.1002/hbm.460010306. [DOI] [PubMed] [Google Scholar]
- Fruhstorfer H, Lindblom U, Schmidt WC. Method for quantitative estimation of thermal thresholds in patients. J Neurol Neurosurg Psychiatry. 1976;39:1071–1075. doi: 10.1136/jnnp.39.11.1071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenspan JD, Lee RR, Lenz FA. Pain sensitivity alterations as a function of lesion location in the parasylvian cortex. Pain. 1999;81:273–282. doi: 10.1016/S0304-3959(99)00021-4. [DOI] [PubMed] [Google Scholar]
- Greenspan JD, Roy EA, Caldwell PA, Farooq NS. Thermosensory intensity and affect throughout the perceptible range. Somatosens Mot Res. 2003;20:19–26. doi: 10.1080/0899022031000083807. [DOI] [PubMed] [Google Scholar]
- Hutchinson WD, Davis KD, Lozano AM, Tasker RR, Dostovsky JO. Pain-related neurons in the human cingulate cortex. Nature Neurosci. 1999;2:403–405. doi: 10.1038/8065. [DOI] [PubMed] [Google Scholar]
- Jenkinson M, Bannister P, Brady M, Smith S. Improved optimization for the robust and accurate linear registration and motion correction of brain images. Neuroimage. 2002;17:825–841. doi: 10.1016/s1053-8119(02)91132-8. [DOI] [PubMed] [Google Scholar]
- Kim JH, Greenspan JD, Coghill RC, Ohara S, Lenz FA. Lesions limited to the human thalamic principal somatosensory nucleus (ventral caudal) are associated with loss of cold sensations and central pain. J Neurosci. 2007;27:4995–5004. doi: 10.1523/JNEUROSCI.0716-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lenz FA, Rios M, Chau D, Krauss GL, Zirh TA, Lesser RP. Painful stimuli evoke potentials recorded from the parasylvian cortex in humans. J Neurophysiol. 1998a;80:2077–2088. doi: 10.1152/jn.1998.80.4.2077. [DOI] [PubMed] [Google Scholar]
- Lenz FA, Rios MR, Zirh TA, Krauss G, Lesser RP. Painful stimuli evoke potentials recorded over the human anterior cingulate gyrus. J Neurophysiol. 1998b;79:2231–2234. doi: 10.1152/jn.1998.79.4.2231. [DOI] [PubMed] [Google Scholar]
- Mufson EJ, Pandya DN. Some observations on the course and composition of the cingulum bundle in the rhesus monkey. J Comp Neurol. 1984;225:31–43. doi: 10.1002/cne.902250105. [DOI] [PubMed] [Google Scholar]
- Ohara S, Crone NE, Weiss N, Lenz FA. Analysis of synchrony demonstrates ‘pain networks’ defined by rapidly switching, task-specific, functional connectivity between pain-related cortical structures. Pain. 2006;123:244–253. doi: 10.1016/j.pain.2006.02.012. [DOI] [PubMed] [Google Scholar]
- Ohara S, Crone NE, Weiss N, Treede RD, Lenz FA. Cutaneous painful laser stimuli evoke responses recorded directly from primary somatosensory cortex in awake humans. J Neurophysiol. 2004;91:2734–2746. doi: 10.1152/jn.00912.2003. [DOI] [PubMed] [Google Scholar]
- Rainville P, Bushnell MC, Duncan GH. PET studies of the subjective experience of pain. In: Casey KL, Bushnell MC, editors. Pain Imaging. Seattle: IASP Press; 2000. pp. 123–56. [Google Scholar]
- Rainville P, Duncan GH, Price DD, Carrier B, Bushnell MC. Pain affect encoded in human anterior cingulate but not somatosensory cortex. Science. 1997;277:968–971. doi: 10.1126/science.277.5328.968. [DOI] [PubMed] [Google Scholar]
- Rainville P, Feine JS, Bushnell MC, Duncan GH. A psychophysical comparison of sensory and affective responses to four modalities of experimental pain. Somatosens Mot Res. 1992;9:265–277. doi: 10.3109/08990229209144776. [DOI] [PubMed] [Google Scholar]
- Sarlani E, Farooq N, Greenspan JD. Gender and laterality differences in thermosensation throughout the perceptible range. Pain. 2003;106:9–18. doi: 10.1016/s0304-3959(03)00211-2. [DOI] [PubMed] [Google Scholar]
- Talairach J, Tournoux P. Coplanar stereotactic atlas of the human brain. Stuttgart: Thieme; 1988. [Google Scholar]
- Talbot JD, Villemure JG, Bushnell MC, Duncan GH. Evaluation of pain perception after anterior capsulotomy: a case report. Somatosens Mot Res. 1995;12:115–126. doi: 10.3109/08990229509101503. [DOI] [PubMed] [Google Scholar]
- Van Hoesen GW, Morecraft RJ, Vogt BA. Connections of the monkey cingulate cortex. In: Vogt BA, Gabriel M, editors. Neurobiology of cingulate cortex and limbic thalamus: a comphrehensive handbook. Boston: Birkhäuser; 1993. pp. 249–84. [Google Scholar]
- Vogt BA. Pain and emotion interactions in subregions of the cingulate gyrus. Nat Rev Neurosci. 2005;6:533–544. doi: 10.1038/nrn1704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogt BA, Derbyshire S, Jones AK. Pain processing in four regions of human cingulate cortex localized with co-registered PET and MR imaging. Eur J Neurosci. 1996;8:1461–1473. doi: 10.1111/j.1460-9568.1996.tb01608.x. [DOI] [PubMed] [Google Scholar]