Abstract
Objectives:
The objective of this study was to evaluate the effectiveness of manual, sonic-air and ultrasonic instrumentation with varying irrigation protocols on removal of the smear layer from root canal walls.
Study Design:
Sixty extracted single rooted human teeth stored in 0.5% saline were used. Periodontal soft tissues were removed followed by crown separation at the cementoenamel junction (CEJ). All the teeth were randomly divided into three groups. Group I was manually instrumented and irrigated with 5.25% sodium hypochlorite (NaOCl) alone and 17% Ethylenediaminetetraacetic acid (EDTA) alternately, same as sonically instrumented Group II and ultrasonically instrumented Group III. The controls for all groups were irrigated with saline solution.
Results:
Ultrasonic over the sonic-air and manual technique, and the use of a combination of two different solutions (17% EDTA and 5.25% NaOCl) alternatively yielded better outcome.
Conclusions:
Ultrasonic, sonic-air and manual instrumentation of the root canal and irrigation with combined solutions is effective in removal of the smear layer from the instrumented walls of the root canal.
Keywords: Cleaning, irrigation, instrumentation, manual, smear layer, ultrasonic instruments
INTRODUCTION
Instrumentation of the root canal system is recognized as being one of the most important stages in root canal treatment. Endodontic treatment includes removal of vital and necrotic tissues from the root canal system, along with infected root dentine.[1] Finally, the root canal must be cleaned, shaped and obturated accurately for a successful outcome.[2] The smear layer created during root canal instrumentation should be removed from the dentine surface of the canal wall and dentine tubules. This is because the smear prevents medicaments from penetrating dentinal tubules.[3] On the other hand, complete removal of the smear layer improves the adaptation of filling materials to the root canal,[4] while reducing apical and coronal microleakage of the root canal sealant materials.[5]
Success of the smear layer removal depends on the instrumentation techniques employed and irrigation solvents used.[6] Sodium hypochlorite solution has been the commonly used irrigant, especially due to its effective antibacterial properties and its excellent action as an organic material solvent.[7] The use of only one irrigant is not sufficient to achieve smear layer removal. Many reports have demonstrated the decalcifying capacity of agents such as 10-17% EDTA,[8] 17% ethylene glycol tetraacetic acid ( EGTA),[9] MTAD [mixture of tetracycline isomer (doxycycline), an acid (citric acid), and a detergent (Tween 80)],[10] sodium hypochlorite, citric acid,[11] 7% maleic acid,[12] 9% and 18% HEBP (etidronic acid)[13] and dimercaptosuccinic acid (DMSA).[14] These agents bring about the removal of inorganic components and debris from the instrumented canals. The decalcifying efficacy of these acidic and chelating agents depends on the root length, application time, diffusion in the dentine and solution pH.R Sudha et al.,[8] had obtained optimum results with EDTA at a pH of 9 with 10% concentration and application for 1 minute. The use of a neutral pH of around 7.3 is recommended for EDTA solutions.[15]
Currently, there are a number of techniques available for root canal instrumentation. Ultrasonic and manual instrumentation in combination with various irrigating solutions enhance smear layer removal.[16,17] Moreover, Abbott et al.[18] reported that NaOCl in combination with ethylenediamine tetra-acetic acid plus Cetavlon (EDTAC) produced clean canal walls; however, use of ultrasonic instruments did not enhance the cleaning action of the respective solutions. Ahmad[19] in his comparative study between manual and ultrasonic instrumentation techniques indicated a quantitative difference between the two. In fact, manual instrumentation and irrigation with 2.5% NaOCl demonstrated a lesser amount of smear deposition in the apical thirds of the root canal. Brannstrom[20] provided data on the effectiveness of sonic and ultrasonic preparation of the root canals including the advantages of manual shaping of the root canal system. Passive ultrasonic instrumentation combined with syringe irrigation with 1% NaOCl are capable of significantly removing the artificially placed dentine debris from simulated root canals[21] Yamashita[22] performed a scanning electron microscope (SEM) analysis for evaluating the cleanliness of the root canal systems, following their irrigation with 2% chlorhexidine, 2.5% NaOCl and a combination of 2.5% NaOCl and EDTA. They conclusively reported the inferiority of all cleaning, shaping and irrigation techniques in achieving complete smear layer removal, especially at the apical thirds of the root canal systems.
MATERIALS AND METHODS
Sixty freshly extracted single rooted human teeth (n=60) stored in 0.5% saline solution were used as a study material. They were mainly upper incisors and mandibular premolars (teeth with single root canal), which were extracted due to their poor periodontal prognosis and orthodontic indications. The periodontal soft tissues were removed, followed by crown separation at the cement-enamel junction (CEJ) using high-speed fissure burs (Bien-Air, Bienne, Switzerland) under water spray. All the teeth were randomly divided into three groups [Table 1].
Table 1.
Distribution of samples of teeth depending on instrumentation technique and irrigation protocol in the study
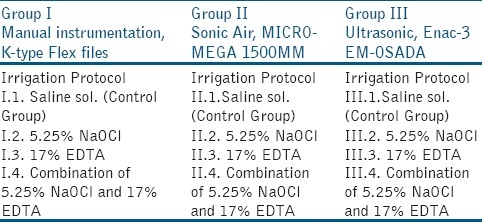
Group I was manually instrumented, while Group II was sonically and Group III was ultrasonically instrumented. The control groups were irrigated with saline solution. Group I was divided into subgroups, according to the irrigation protocol followed, as elaborated: I.1. irrigated with saline solution (Control Group); I.2. irrigated with 5.25% NaOCl (ADD Vision, Germany); I.3. Irrigated with 17% EDTA (Calcinase, LegeArtisPharmaDettenhausen, Germany); and, I.4. Irrigated with a combination of 17% EDTA and 5,25% NaOCl solutions. Group II was sonically instrumented and divided into the following subgroups: II.1. Irrigated with saline solution; II.2. Irrigated with 5.25% NaOCl; II.3. Irrigated with 17% EDTA; and, II.4. Irrigated with 17% EDTA and 5.25% NaOCl solutions.
Group III was ultrasonically instrumented and divided into the following subgroups: III.1. Irrigated with saline solution; III.2. Irrigated with 5.25% NaOCl; III.3. Irrigated with 17% EDTA; and, III.4. Irrigated with 17% EDTA and 5,25% NaOCl solutions. Pulp tissue was removed using a barbed broach (Dentsply, Tulsa, USA). Manual instrumentation of the root canal systems was performed using K-type Flex files (Kerr Mfg. Co., Romulus, Michigan, USA). Working lengths were established at 0.5 mm short of the anatomical apex by visually identifying #10 K-file at the apical foramina. The roots were instrumented up to #45 K-file (according to the canal volume) using a step-back preparation technique followed by irrigation with 2.0 ml of the irrigating solution at each change of instrument. The canals were irrigated with EDTA for 20-30 seconds and with sodium hypochlorite for 1 minute. Sonic-air instrumentation was performed with SONIC AIR® MM 1500 (Micro-Mega, Besançon, France), with subsonic vibration frequency 1500-3000 Hz at an optimal amplitude of oscillation of 0.5 mm. Initial instrumentation was performed with a K-file #8, #10 or #15 depending on the canal volume, and continued with Rispi-Sonic file size 1-3 for apical third of the root canal, with vertical movement of 2-3 mm of the file. The middle and coronal third was instrumented with Shaper-Sonic file #15 to #40 with up-down movement of 3-5 mm of the file. Ultrasonic instrumentation was performed with Enac-3 EM-OSADA (Enac-3 EM-OSADA, Tokyo, Japan), that produces ultrasonic vibration with a frequency of over 20.000 Hz. The power adjustment of the unit was set at level 3.
Initial instrumentation (scouting) was performed with a K-file #8 or #10, depending on the canal volume. The ultrasonic file, connected to the handpiece, was placed in the canal to the measured length. Upon activation, the file was moved passively in up-down motion to ensure it did not bind to the root canal walls. The energized ultrasonic file was used continuously for 20-30 seconds. For each consecutive file, irrigation with 17% EDTA for 20-30 seconds and with sodium hypochlorite for 1 minute, was carried out. Irrigation solutions were delivered via a 10 mL syringe, at a rate of 15 mL for each canal. According to this protocol, samples of subgroup II.4.(combination of 17% EDTA and 5.25% sodium hypochlorite) were irrigated as follows: Each irrigation with EDTA was followed with equal amount of 5.25% sodium hypochlorite and final irrigation was done with 2 mL of 5.25% sodium hypochlorite solution.
Twenty teeth were used for each individual instrumentation technique. Irrigating solutions were delivered via a 23 gauge needle (Romed® Holland, CH Wilnis, NL) inserted into the canal without binding into the walls. After adhering to the above established instrumentation and irrigation protocols of the respective teeth, the canals were rinsed with 2 mL saline solution to avoid long-term action of the respective irrigation solutions. All teeth were dried with paper points (Dentsply, Maillefer, Switzerland) and split along the long axis in the bucco-lingual direction to expose the entire extent of the root canal.
The resulting specimens were prepared for Scanning Electron Microscopy JEOL JSM-6335F (Tokyo, Japan) study. The blocks of teeth were cut with the ISOMET 11-1180 Low Speed SAW at the predetermined coronal, middle and the apical thirds.
The cut samples were fixed in metallic blocks and put on the Sputter Coater S150 B EDWARDS for impregnation in gold (Au) at 10 mbar pressure. Internal parts of the root canal systems were specially microfilmed. After scanning and observing each third with the microscope, an image of the most representative area of that third was taken. Three pictures were obtained from each tooth, one for each third, to give a total of 180 pictures. The images were analyzed for the amount of smear layer, scored as: 1=no smear layer; 2=few areas covered by smear layer with many dentinal tubule openings visible; 3=most areas covered by smear layer, with few dentinal tubule openings visible; 4=all areas covered by smear layer, no dentinal tubule openings visible; 5=heavy, non-homogeneous smear layer covering the complete root canal wall. Statistical analysis was performed using Friedman's test to find if there was any difference in the effectiveness of instrumentation on removal of smear layer.
RESULTS
The level of smear layer remaining in each third, between different instrumentation techniques and irrigation protocol was as follows:
In the control group, manually, sonically or ultrasonically instrumented and irrigated with saline solution, there were no differences between the groups [Table 2]. Control Groups (I.1., II.1. and III.1.) produced a heterogeneous smear layer (score 5, with no differences between instrumentation technique) that coated the root canal walls. The smear layer also penetrated the dentin tubules. When comparing manual, sonic and ultrasonic instrumentation and irrigation with sodium hypochlorite [Table 3], smear layer removal at cervical and middle thirds was more effective with ultrasonic instrumentation (score 1.6 vs. 3.8) and the results were statistically significant (P<0.05). However, when comparing these techniques in the apical third, there was no statistical significance in their smear layer removal effectiveness. Group I.2. [Figure 1a], produced a smear layer that resembled a dry, cracked soil surface, and covered the dentin tubules. Group II.2. and III.2., [Figures 1b and c] revealed more specimens free of smear layer in their root canal walls. In some parts of the root canal walls, however, the dentine tubules were packed with smear.
Table 2.
Scores of smear layer formation within the group of the study, when irrigation was carried out with saline solution (control group)
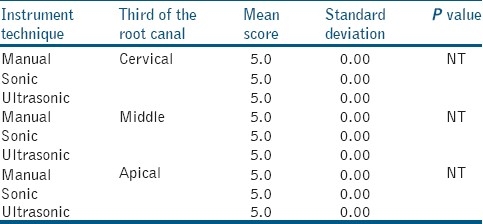
Table 3.
Scores of smear layer within the groups of the study when irrigation with 5.25% sodium hypochlorite was carried out
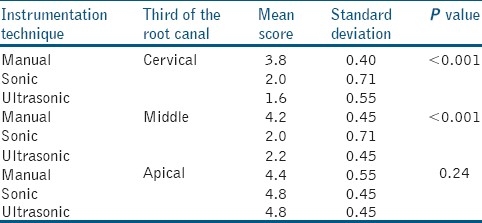
Figure 1.
Manual instrumentation (a), sonic-air (b) and Ultrasonically instrumentation (c), irrigation with 5.25% sodium hypochlorite, middle third, original magnification a: ×3000, b ×500 and c ×1500
In all the groups irrigated with 17% EDTA (Group I.3., II.3. and III.3.) there was a higher score of smear layer [Table 4] with no statistical differences between ultrasonic, sonic and manual instrumentation (ultrasonic on cervical and middle third 4.8 vs.5.0 sonic-air and manually), with P>0.05. Smear layer removal at the cervical and middle third is more effective after ultrasonic, sonic and manual instrumentation [Table 5] and irrigation with a combination of 17% EDTA and 5.25% NaOCl (score 1 vs. 1.4), as well slightly more effective with ultrasonics on apical thirds (score 1.8 vs.2). No statistical significant difference was found between the techniques of instrumentation and irrigation protocol (P>0.05). In Group I.4. [Figure 2a], in the apical third of the root canal, the orifices of the dentin tubules and the presence of smear in some parts is clearly visible. Additionally, debris is also present in this view. In Group II.4. [Figure 2b] the middle third of the sonic instrumented root canal yielded a slightly different view because here the orifices into the dentin tubules are visible, and debris is consistently present throughout. In Group III.4. [Figure 2c], the root canal walls and the orificesof the dentin tubules are clearly visible with bits of visible debris.
Table 4.
Scores of smear layer within the groups of the study when irrigation with 17% Ethylenediaminetetraacetic acid was carried out
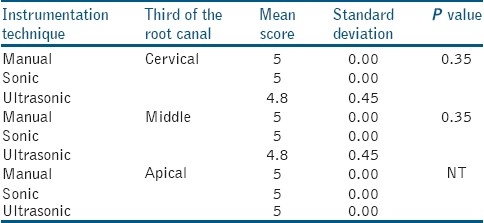
Table 5.
Scores of smear layer within the group, Irrigation with 5.25% sodium hypochlorite and 17% Ethylenediaminetetraacetic acid
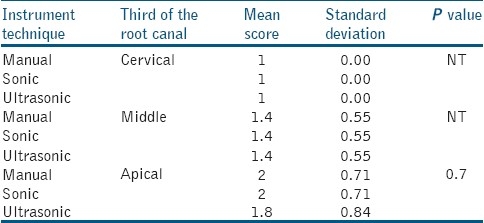
Figure 2.
Manually instrumentation (a), sonic-air (b) and Ultrasonically instrumentation (c), irrigation with 17% Ethylenediaminetetraacetic acid followed by 5.25% sodium hypochlorite, a; apical third, b and c: Middle third, original magnification a-b: ×3000 and c: ×10000
DISCUSSION
Our research shows that the teeth treated manually, sonically or with ultrasonic techniques and irrigated with saline solution tend to form an amorphous mass which covers the entire inner wall of the treated canal, and gets packed deep into the dentinal tubules.[16–17] These changes are a direct effect from the action between root canal instruments and the root canal inner wall. In the case of ultrasonic treatment, the smear layer has a different appearance; however, it covers the whole treated wall.[23] Ultrasonic instrumentation and irrigation with 5.25% NaOCl was successful in removing the smear layer from the surface of the root canal inner wall; however, it could not remove the smear layer entirely from the dentin tubules.[16] Berg et al.[24] obtained similar results when they compared the manual method with ultrasonic technique and irrigation with 5.25% NaOCl. Ahmad et al.[19] with ultrasonic instrumentation and irrigation with 5.25% NaOCl managed to remove the smear layer from the surface of the root canal wall. They also noticed a considerate amount of debris, mainly in the curved canals. Cameron,[17] using different concentrations of NaOCl noticed that, in cases when the concentration was higher than 2%, the removal of the smear layer from the surface of the root canal wall was successful.
Manual instrumentation and irrigation with EDTA solution produces a large amount of smear layer. In ultrasonic instrumentation combined with irrigation with EDTA, the smear layer of the root canal wall was partially removed in the cervical and middle thirds, and smear layer was visibly packed in the dentine tubules. These results demonstrated the effectiveness of EDTA and its demineralization potential.[25] Research on the evaluation of manual, sonic-air and ultrasonic instrumentation techniques with combined irrigation with 17% EDTA and 5.25% NaOCl demonstrated their effectiveness in complete removal of the smear layer from root canal walls, and from the orifice of dentin tubules in the cervical and middle thirds.[16,17] In cases when 5.25% NaOCl solution was used as a final irrigant, remnants of smear layer were occasionally visible.
CONCLUSIONS
Following manual instrumentation and irrigation with 17% EDTA and 5.25% NaOCl, the smear layer was absent in the coronal and middle thirds of the root canal walls. Ultrasonic, sonic and manual instrumentation, and irrigation with 17% EDTA followed by 5.25% NaOCl was successful in complete removal of the smear layer in the cervical and middle thirds of the root canals. Also, ultrasonic instrumentation was slightly more effective in the apical thirds of the root canals.
Footnotes
Source of Support: Nil,
Conflict of Interest: None declared.
REFERENCES
- 1.Peters L, Wesselink P, Moorer W. Penetration of bacteria in root dentine in vitro. Int Endod J. 2000;33:28–36. doi: 10.1046/j.1365-2591.2000.00268.x. [DOI] [PubMed] [Google Scholar]
- 2.Ruddle C. Cleaning and shaping the root canal system. In: Cohen S, Burns R, editors. Pathways of the Pulp. 8th ed. St Louis, MO: Mosby; 2002. p. 2002. [Google Scholar]
- 3.Clark-Holke D, Drake D, Walton R, Rivera E, Guthmiller J. Bacterial penetration through canals of endodontically treated teeth in the presence or absence of smear layer. J Dent. 2003;31:275–81. doi: 10.1016/s0300-5712(03)00032-0. [DOI] [PubMed] [Google Scholar]
- 4.Sen B, Wesselink P, Turkun M. The smear layer: A phenomenon in root canal therapy. Int Endod J. 1995;28:141–8. doi: 10.1111/j.1365-2591.1995.tb00289.x. [DOI] [PubMed] [Google Scholar]
- 5.Khayat A, Jahanbin A. The influence of smear layer on coronal leakage of Roth 801 and AH26 root canal sealers. Aust Endod J. 2005;31:66–8. doi: 10.1111/j.1747-4477.2005.tb00228.x. [DOI] [PubMed] [Google Scholar]
- 6.Yamada R, Armas A, Goldman M, Lin P. A scanning electron microscopic comparison of a high volume final flush with several irrigating solutions: Part 3. J Endod. 1983;9:137–42. doi: 10.1016/S0099-2399(83)80032-6. [DOI] [PubMed] [Google Scholar]
- 7.Moorer WR, Wesselink PR. Factors promoting the tissue dissolving capability of sodium hypochlorite. Int Endod J. 1982;15:187–96. doi: 10.1111/j.1365-2591.1982.tb01277.x. [DOI] [PubMed] [Google Scholar]
- 8.Sudha R, Sukumaran VR, Ranganathan J, Bharadwaj N. Comparative evaluation of the effect of two different concentrations of EDTA at two different PH and time periods on root dentin. J Conserv Dent. 2006;9:36–42. [Google Scholar]
- 9.Jhamb S, Nikhil V, Singh V. An in vitro study to determine the sealing ability of sealers with and without smear layer removal. J Conserv Dent. 2009;12:150–3. doi: 10.4103/0972-0707.58335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Torabinejad M, Khademi AA, Babagoli J, Cho Y, Johnson WB, Bozhilov K, et al. A new solution for the removal of smear layer. J Endod. 2003;29:170–5. doi: 10.1097/00004770-200303000-00002. [DOI] [PubMed] [Google Scholar]
- 11.Mamatha Y, Ballal S, Gopikrishna V, Kandaswamy D. Comparison of sodium hypochlorite and edtairrigants with an indigenous solution as an alternative to mtad. J Conserv Dent. 2006;9:48–52. [Google Scholar]
- 12.Ballal NV, Kandian S, Mala K, Bhat KS, Acharya S. Comparison of the Efficacy of Maleic Acid and Ethylenediaminetetraacetic Acid in Smear Layer Removal from Instrumented Human Root Canal: A Scanning Electron Microscopic Study. J Endod. 2009;35:1573–6. doi: 10.1016/j.joen.2009.07.021. [DOI] [PubMed] [Google Scholar]
- 13.Kandaswamy D, Venkateshbabu N, Arathi G, Roohi R, Anand S. Effects of various final irrigants on the shear bond strength of resin-based sealer to dentin. J Conserv Dent. 2011;14:40–2. doi: 10.4103/0972-0707.80737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rai B, Jain R, Kharb S, Miglani S, Anand SC. Comparative anti-microbial activity of 15% EDTA and 15% dimercaptosuccinic acid (DMSA) J Conserv Dent. 2006;9:32–5. [Google Scholar]
- 15.Zhang K, Kim YK, Cadenaro M, Bryan TE, Sidow SJ, Loushine RJ, et al. Effects of different exposure times and concentrations of sodium hypochlorite/ethylenediaminetetraacetic acid on the structural integrity of mineralized dentin. J Endod. 2010;36:105–9. doi: 10.1016/j.joen.2009.10.020. [DOI] [PubMed] [Google Scholar]
- 16.Cheung G, Stock C. In vitro cleaning ability of root canal irrigants with and without endosonics. Int Endod J. 1993;26:334–43. doi: 10.1111/j.1365-2591.1993.tb00766.x. [DOI] [PubMed] [Google Scholar]
- 17.Cameron JA. The choice of irrigant during hand instrumentation and ultrasonic irrigation of the root canal: A scanning electron microscope study. Aust Dent J. 1995;40:85–90. doi: 10.1111/j.1834-7819.1995.tb03121.x. [DOI] [PubMed] [Google Scholar]
- 18.Abbott P, Heijkoop P, Cardaci S, Hume W, Heithersay G. An SEM study of the effects of different irrigation sequences and ultrasonics. Int Endod J. 1991;24:308–16. doi: 10.1111/j.1365-2591.1991.tb00141.x. [DOI] [PubMed] [Google Scholar]
- 19.Ahmad M, Pit Ford T, Crum L. Ultrasonic debridement of root canals: An insight into the mechanism involved. J Endod. 1987;13:93–101. doi: 10.1016/S0099-2399(87)80173-5. [DOI] [PubMed] [Google Scholar]
- 20.Brännström M. Removal of the dentinal smear layer. Quintessence Int. 1990:21425–426. [Google Scholar]
- 21.Gaurav G, Sangeeta T. Comparison of the efficacy of ‘F-File’ with sonic and ultrasonic debridement to remove artificially placed dentine debris from human root canals - An in vitro study. Endodontology. 2010;22:39–47. [Google Scholar]
- 22.Yamashita J, Tanomaru-Filho M, Leonardo M, Rossi M, Silva L. Scanning electron microscopic study of the cleaning ability of chlorhexidine as a root-canal irrigant. Int Endod J. 2003;36:391. doi: 10.1046/j.1365-2591.2003.00656.x. [DOI] [PubMed] [Google Scholar]
- 23.Mc Comb D, Smith D, Beagrie G. The results of in vivo endodontic chemo mechanical instrumentation - A scanning electron microscopic study. J Br Endod Soc. 1976:911–8. doi: 10.1111/j.1365-2591.1976.tb01231.x. [DOI] [PubMed] [Google Scholar]
- 24.Berg M, Jacobsen E, Degole E, Remeikis N. A comparison of five irrigant solutions: A scanning electron microscopic study. J Endod. 1986;12:192–7. doi: 10.1016/S0099-2399(86)80153-4. [DOI] [PubMed] [Google Scholar]
- 25.Pérez-Heredia M, Ferrer-Luque CM, González-Rodríguez MP, Martín-Peinado FJ, González-López S. Decalcifying effect of 15% EDTA, 15% citric acid, 5% phosphoric acid and 2.5% sodium hypochlorite on root canal dentine. IntEndod J. 2008;41:418–23. doi: 10.1111/j.1365-2591.2007.01371.x. [DOI] [PubMed] [Google Scholar]




