Abstract
Aim:
The aim of this study is to evaluate the surface roughness of four flowable resin composites following exposure to acidic and alcoholic drinks.
Materials and Methods:
SureFil SDR flow, TetricEvoFlow, Esthet-X Flow and Amaris Flow HT samples were immersed in artificial saliva, Coca Cola and Chivas Regal Whisky. Each specimen was examined using a Leica DCM 3D microscope: Arithmetical mean height of the surface profiles was measured (Sa).
Results:
Kruskal-Wallis test showed significant differences among various groups (P<0,001). Mann Whitney test was applied and control groups showed significantly lower Sa values than other groups (P=0,008). Coca Cola groups showed highest Sa values (P<0,021). No significant differences (P=0,14) in surface texture were found among the specimens of the different materials. No significant differences were found among TetricEvoFlow, Esthet-X Flow and Amaris Flow under control conditions nor after Coca Cola application. Under control condition and after Coca Cola application SureFil SDR flow showed significantly higher Sa values. Moreover, after whisky application Amaris Flow showed significantly lower Sa values then the other three groups that showed no significant differences among them.
Conclusions:
Acidic and alcoholic drinks eroded the surface roughness of all evaluated flowable resin composites.
Keywords: Acidic and alcoholic drinks, flowable resin composites, surface roughness
INTRODUCTION
Acidic beverages, such as soft drinks (orange juice, and cola), or ethanol (whisky), can produce erosion of resin composites.[1,2] The surface degradation of resin materials is related to the content and distribution of the fillers, the composition of the matrix resin, and the effect of silane surface treatment on the fillers.[3,4] Direct Class II composite restorations can be placed at an acceptable standard if the cervical margin is in sound enamel. When the adhesive restorations are located below the cement-enamel junction (CEJ) and cervical lesions have no enamel, the quality of the marginal integrity is questionable.[5] Below the CEJ, the bond with dentin is weaker: The polymerization shrinkage can result in gap formation between composite resin and the cavity walls. Marginal gap formation contributes to microleakage and permits the passage of oral fluids and bacteria from the oral cavity. It becomes a source of post-operative sensitivity, pulpal inflammation and recurrent caries.[6,7] To reduce these effects, a better option to the conventional resin technique has been suggested: The Class II open-sandwich restorations: Glass-ionomer cement (GIC) or resin-modified glass-ionomer cement (RMGIC) is placed between the dentin cervical margins and occlusal composite restoration.[8,9] GICs and RMGICs have been shown to be less able to seal margins and can dissolve over time in the oral environment.[10,11] Recently, flowable resin composites with lower filler content andfar lower viscosity have been recommended as liners at CEJ margins of the proximal box of Class II composite restorations:[6,12] A layer of flowable materials at the gingival floor in cementum margins of Class II composite restorations gets a better marginal seal of the restoration, and is an ideal choice for use in a open-sandwich technique.[13,14]
Although the mechanical properties of these materials have been improved substantially, their antibacterial properties are still limited.[15,16] The bacterial accumulation on the surfaces of restorative materials can provide the bacterial source leading to the development of secondary caries and periodontal diseases. Bacterial accumulation is highly dependent on the characteristics of the material surface and the roughness of resin composites can influence the oral biofilm adherence.[16] Biofilm formation is a stepwise process initiated by adhesion of planktonicbacteria onto the surface of a tooth or other structures in the oral cavity. This process progresses from colonization and coadhesion, via growth and maturation, to detachment and spread of the microorganisms from the biofilm.[17] The physical and chemical properties of the surface affect the feasibility of bacterial infection. Correlations between bacterial adhesion and various surface characteristics (chemical composition, surface energy, surface roughness and presence of functional groups on the surface) have been intensively investigated in an attempt to reduce bacterial adhesion through surface modification.[17–19]
The objective of this in vitro study was to evaluate the surface roughness of four flowable composites (SureFil SDR flow, TetricEvoFlow, Esthet-X Flow, Amaris Flow HT) after exposure to acidic and alcoholic drinks using a Leica DCM (Dual Core Measuring) 3D microscope. The null hypothesis of the study was that there is no significant difference in surface roughness of the evaluated flowable composites.
MATERIALS AND METHODS
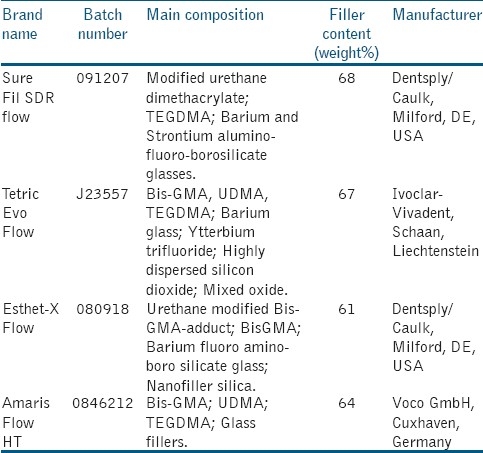
Four flowable resin composites were selected for the study: SureFil SDR flow (Dentsply/Caulk, Milford, DE, USA), TetricEvoFlow (Ivoclar-Vivadent, Schaan, Liechtenstein), Esthet-X Flow (Dentsply/Caulk, Milford, DE, USA), Amaris Flow HT (Voco GmbH, Cuxhaven, Germany). Details are listed in Table 1. All materials were polymerized according to manufacturers’ instructions into plastic rings (diameter=7 mm and thickness=2 mm) to obtain specimens identical in size. Cavities of these rings were slightlyoverfilled with the material, covered by a Mylar strip (Henry Schein; Melville, NY), placed between 2 glass slides and polymerized on each side(exposure time 40 seconds) with a visible light curing unit (EliparTrilight 3M ESPE, St. Paul, MN, USA). The intensity of the light was verified with a radiometer (SDS Kerr, Orange, CA). The light was placed perpendicular to the specimen surface, at a distance of 1.5 mm. Thirty cylindrical specimens of each material were prepared in this manner, for a total of 120 specimens. After polymerization and during the experimentation, the specimens were stored in artificial saliva (Bioxtra, Biopharm, PeschieraBorromeo, MI, Italy). All specimens were subjected to 1500 thermal cycles between 5°C (±5°C) and 50°C (±5°C) with a dwell time of 30 seconds in each water bath in order to simulate aging conditions.[16] The specimens were randomly assigned to 3 groups (artificial saliva, acidic drink and alcoholic drink) of 10 for each type of flowable resin composite and subjected to the action of drinks. In addition, the pH values of the test drinks were checked using a pH meter (Mettler Toledo MP230, Sigma-Aldrich, NY).
Table 1.
Composition and specifications of flowable resin composites evaluated in the present study
The specimens were immersed in drinks at room temperature over a 14-day test period:
Group A: Specimens immersed in 50 ml of artificial saliva (control),
Group B: Specimens immersed in 50 ml of acidic drink (Coca Cola, Italy),
Group C: Specimens immersed in 50 ml of alcoholic drink(Chivas Regal Whisky, Aberdeen, Scotland, UK).
Table 2 shows the pH values of the drinks. Solutions were changed daily and put in vials with cover that prevent evaporation of solutions. After, the specimens were rinsed with distilled water, air-dried and placed in physiological solution at 37°C. Each specimen was dried with absorbent paper points and examined using a Leica DCM 3D microscope (Leica Microsystems, Schweiz, AG - CH): Arithmetical mean height of the surface profiles was measured (Sa). The DCM 3D microscope combines confocal, interferometry and color imaging in technology for high speed and high-resolution measurements down to 0.1 nm. On each evaluated surface, five random traces along its length were performed to assure a linear profile pattern. Baseline surface height parameters was obtained by the arithmetic mean of these five readings. The results of arithmetical mean height along the z axis of the surface values (Sa) were submitted to statistical analysis using computer software “Stata 7.0” (Stata Corp., Station College, TX). A Kruskal-Wallis test and a Mann-Whitney U-test were performed. Significance was predetermined at P<0.05.
Table 2.
Solutions used in the present study

RESULTS
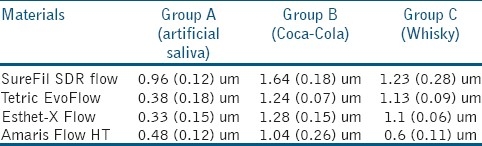
DCM 3D images of SureFil SDR flow, TetricEvoFlow, Esthet-X Flow, Amaris Flow HT. Arithmetical mean height values (Sa) are reported in Table 3. Kruskal-Wallis test showed significant differences among various groups (P<0.001). Mann Whitney test was then applied and control groups showed significantly lower Sa values than other groups (P=0.008) for all the flowable composites tested. Coca Cola groups showed highest Sa values (P<0.021). When analyzing the four different composites both under artificial saliva and acidic drink immersion, Surefil SDR flow expressed significantly higher Sa values (P<0,001) than other materials tested, that did not show significant differences among them (P>0,05). Moreover Amaris Flow HT after immersion in alcoholic drinkshowed significantly lower (P<0,001) Sa values than other three groups that did not show any significant differences (P>0,05) in surface texture [Table 3].
Table 3.
Arithmetical mean height (and Standard Deviation) along the z axis of the surface values (Sa)
DISCUSSION
A layer of flowable materials at the gingival floor (in cementum margins) of Class II composite restorations is an ideal choice for use in a sandwich technique.[13,14] The longevity of a resin restoration is influenced by many factors, such as, the various properties of composite resins and the restorative techniques implemented. These factors have a significant impact on the marginal integrity and adaptation of the resin-cavity interface. Excellent marginal adaptation extends the longevity of restorations. On the contrary, inadequate adaptation results in interfacial gap formation and marginal microleakage, which in turn induces recurrent caries. In the same way, the formation of a dental biofilm plays an important role. Dental hard surfaces, including restorative materials, can be modified either mechanically, by brushing and polishing actions, or chemically, by acidic or oxidizing bleaching agents.[20] Increase of surface roughness facilitates bacterial adherence, expecially in the cervical margin of direct class II composite restorations. Bacterial accumulation on the surfaces of restorative materials serves as source of bacteria in the oral cavity and may lead to secondary caries formation. Flowable composites are characterized by lower filler loading and by greater proportion of diluent monomers in the formulation. These flowables were traditionally created by retaining the same small particle size of the conventional hybrid composites, but reducing the filler content, and allowing the increased resin content to reduce the viscosity of the mixture. However their various mechanical properties such as flexural strength and wear resistance have been reported to be generally inferior if compared to those of the conventional composites. For these reasons, flowable composites have been suggested to be filling materials for low-stress applications and in situations with difficult access or those requiring good penetration.[21] Ikeda et al.[21] outlined some of the clinical indications for flowable resin composites: Composite or crown margin repairs; pit and fissure sealing; preventive resin restorations; air abrasion cavity preparations; cavity lining; porcelain repairs; enamel defects; incisal edge repairs in anterior sites; small Class III and Class V restorations. In all these cases, resin composites are subjected to action of acidic substances, which may affect the behaviour of restorative materials.[21–23] Previous studies have also shown such a tendency of surface degradation of flowable composites exposed to acidic and alcoholic drinks. Neamat et al.[1] have shown that fillers tend to fall out from resin materials and the matrix component decomposes when exposed to low pH environments. Many soft drinks are acidic and the pH is 3.0 or lower. This means that drinking acidic drinks over a long period and with continuous sipping can erode the tooth enameland the resin material as well.[1] Han et al.[23] evaluated the surface degradation of flowable resins exposed to different acidic drinks (orange juice, whisky and wine), and concluded that a relationship was observed between filler volume and the surface degradation of flowable resins; and, distribution density of fillers on resin surface was related to the surface degradation of flowable resins and surface degradation of flowable resins was also related to the surface treatment of fillers with silane. This means that the lower is the filler loading, the greater is the surface degradation.
The objective of this in vitro study was to evaluate the surface roughness, using a Leica DCM 3D microscope, of four flowable resin composites (SureFil SDR flow, TetricEvoFlow, Esthet-X Flow, Amaris Flow HT), in order to establish if exposure to acidic and alcoholic drinks is able to modify their surface roughness. DCM 3D measurements of surface profiles did not reveal surface roughnessin the specimens of control group immersed in artificial saliva.These specimens showed significantly lower Sa values than other groups and a smooth surface. Coca Cola and whisky immersion determined significant rising of Sa values for all the materials tested. Specimens eroded with acidic drinks showed greater surface roughness than specimens eroded with alcoholic drinks. In fact flowable resins are expected to undergo considerable degradation in the complex environment of the oral cavity (with exposure to alcohol, acids, mechanical abrasion and temperature changes).[23–25] Moreover in the present investigation no significant differences in Sa values were found among TetricEvoFlow, Esthet-X Flow and Amaris Flow HT or under control conditions, or after Coca Cola application. Under control conditions and after Coca Cola immersion SureFil SDR flow showed significantly higher Sa values then the other three materials tested. Moreover after whisky application, Amaris Flow HT showed significantly lower Sa values then the other three groups that showed no significant differences among them. This is probably due to carbonic acid present in the beverage formulation. Even though organic acids do not affect roughness, they cause a remarkable decrease in the resin composite hardness.[2]
The differences showed in the present study suggested different clinical performances of the flowable resin composites: Lower surface roughness improves the marginal integrity of Class II restorations in open sandwich technique
CONCLUSIONS
Within the limitations of this in vitro study, it can be concluded that exposure to acidic and alcoholic drinks, altered in different degrees, the surface roughness of all evaluated flowable resin composites. Moreover, under artificial saliva and acidic drink immersion, Surefil SDR Flow showed significantly higher Sa values than all other materials tested. On the contrary, under alcoholic drink immersion, Amaris Flow HT showed significantly lower Sa values than all other materials tested.
Each author affirms that had no financial affiliation (e.g., employment, direct payment, stock holdings, retainers, consultantships, patent licensing arrangements or honoraria), or involvement with any commercial organization with direct financial interest in the subject or materials discussed in this manuscript, nor had any such arrangements exist in the past three years. Any other potential conflict of interest is disclosed.
Footnotes
Source of Support: Nil,
Conflict of Interest: None declared.
REFERENCES
- 1.Neamat AB, Han L, Okamoto A, Iwaku M. Effect of alcoholic and low pH soft drinks on fluoride release from compomer. J Esthet Dent. 2000;12:97–104. doi: 10.1111/j.1708-8240.2000.tb00206.x. [DOI] [PubMed] [Google Scholar]
- 2.Badra VV, Faraoni JJ, Ramos RP, Palma-Dibb RG. Influence of different beverages on the microhardness and surface roughness of resin composites. Oper Dent. 2005;30:213–9. [PubMed] [Google Scholar]
- 3.Assmussen E. Softening of BISGMA-based polymers by ethanol and by organic acids of plaque. Scand J Dent Res. 1984;92:257–61. doi: 10.1111/j.1600-0722.1984.tb00889.x. [DOI] [PubMed] [Google Scholar]
- 4.Lee SY, Greener EH, Mueller HJ, Chiu CH. Effect of food and oral simulating fluids on dentine bond and composite strength. J Dent. 1994;22:352–9. doi: 10.1016/0300-5712(94)90088-4. [DOI] [PubMed] [Google Scholar]
- 5.Yip KH, Poon BK, Chu FC, Poon EC, Kong FY, Smales RJ. Clinical evaluation of packable and conventional hybrid resin-based composites for posterior restorations in permanent teeth: Results at 12 months. J Am Dent Assoc. 2003;134:1581–9. doi: 10.14219/jada.archive.2003.0103. [DOI] [PubMed] [Google Scholar]
- 6.Sadeghi M. The effect of fluid composite as gingival layer on microleakage of Class II composite restorations.Dent. Res J. 2007;4:40–7. [Google Scholar]
- 7.Attar N, Korkmaz Y. Effect of two light-emitting diode (LED) and one halogen curing light on the microleakage of Class V flowable composite restorations. J Contemp Dent Pract. 2007;8:80–8. [PubMed] [Google Scholar]
- 8.Loguercio AD, Reis A, Mazzocco KC, Dias AL, Busato AL, Singer JM, et al. Microleakage in Class II composite resin restorations: Total bonding and open sandwich technique. J Adhes Dent. 2002;4:137–44. [PubMed] [Google Scholar]
- 9.Besnault C, Attal JP. Simulated oral environment and microleakage of Class II resin-based composite and sandwich restorations. Am J Dent. 2003;16:186–90. [PubMed] [Google Scholar]
- 10.Welbury RR, Murray JJ. A clinical trial of the glass-ionomer cement-composite resin “sandwich” technique in Class II cavities in permanent premolar and molar teeth. Quintessence Int. 1990;21:507–12. [PubMed] [Google Scholar]
- 11.Stockton LW, Tsang ST. Microleakage of Class II posterior restorations with gingival margins entirely within dentin. J Can Dent Assoc. 2007;73:255. [PubMed] [Google Scholar]
- 12.Neme AM, Maxson BB, Pink FE, Aksu MN. Microleakage of Class II packable resin composites lined with flowables: An in vitro study. Oper Dent. 2002;27:600–5. [PubMed] [Google Scholar]
- 13.Sadeghi M. Influence of flowable materials on microleakage of nanofilled and hybrid Class II composite restorations with LED and QTH LCUs. Indian J Dent Res. 2009;20:159–63. doi: 10.4103/0970-9290.52891. [DOI] [PubMed] [Google Scholar]
- 14.Fabianelli A, Sgarra A, Goracci C, Cantoro A, Pollington S, Ferrari M. Microleakage in Class II restorations: Open vs closed centripetal build-up technique. Oper Dent. 2010;35:308–13. doi: 10.2341/09-128-L. [DOI] [PubMed] [Google Scholar]
- 15.Montanaro L, Campoccia D, Rizzi S, Donati ME, Breschi L, Prati C, et al. Evaluation of bacterial adhesion of streptococcus mutans on dental restorative materials. Biomat. 2004;25:4457–63. doi: 10.1016/j.biomaterials.2003.11.031. [DOI] [PubMed] [Google Scholar]
- 16.Da Crus A, Cogo K, Bergamaschi CC, Boscolo FN, Groppo FC, Almeida SM. Oral Streptococci growth on aging and non-aging esthetic restorations after radiotherapy. Braz Dent J. 2010;21:346–50. doi: 10.1590/s0103-64402010000400010. [DOI] [PubMed] [Google Scholar]
- 17.Gyo M, Nikaido T, Okada K, Yamauchi J, Tagami J, Matin K. Surface response of fluorine polymer-incorporated resin composites to cariogenic biofilm adherence. ApplEnvirMicrobiol. 2008;74:1428–35. doi: 10.1128/AEM.02039-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Verma P, Wadhwani KK. Surface finish of esthetic materials - an in vitro SEM study. J Conserv Dent. 2005;8:60–6. [Google Scholar]
- 19.Bashetty K, Joshi S. The effect of one-step and multi-step polishing systems on surface texture of two different resin composites. J Conserv Dent. 2010;13:34–8. doi: 10.4103/0972-0707.62637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Steinberg D, Mor C, Dogan H, Zacks B, Rotstein I. Effect of salivary biofilm on the adherence of oral bacteria to bleached and non-bleached restorative material. Dent Mater. 1999;15:14–20. doi: 10.1016/s0109-5641(99)90026-x. [DOI] [PubMed] [Google Scholar]
- 21.Ikeda I, Otsuki M, Sadr A, Nomura T, Kishikawa R, Tagami J. Effect of filler content of flowable composites on resin-cavity interface. Dent Mater J. 2009;28:679–85. doi: 10.4012/dmj.28.679. [DOI] [PubMed] [Google Scholar]
- 22.Lee YK, Powers JM. Discoloration of dental resins composites after immersion in a series of organic and chemical solutions. J Biomed Mater Res B ApplBiomater. 2005;73B:361–7. doi: 10.1002/jbm.b.30216. [DOI] [PubMed] [Google Scholar]
- 23.Han L, Okamoto A, Fukushima M, Okiji T. Evaluation of flowable resins composite surface eroded by acidic and alcoholic drinks. Dent Mater J. 2008;27:455–65. doi: 10.4012/dmj.27.455. [DOI] [PubMed] [Google Scholar]
- 24.Wongkhantee S, Patanapiradej V, Maneenut C, Tantbirojn D. Effect of acidic food and drinks on surface hardness of enamel, dentine, and tooth-coloured filling materials. J Dent. 2006;34:214–20. doi: 10.1016/j.jdent.2005.06.003. [DOI] [PubMed] [Google Scholar]
- 25.Valinoti AC, Neves BG, da Silva EM, Maia LC. Surface degradation of composite resins by acidic medicines and pH-cycling. J Appl Oral Sci. 2008;16:257–65. doi: 10.1590/S1678-77572008000400006. [DOI] [PMC free article] [PubMed] [Google Scholar]


