Abstract
Aim:
The purpose of this study is to evaluate the resin-dentin interface, quality of the hybrid layer of total-etching and self-etching adhesive systems under scanning electron microscopy (SEM).
Materials and Methods:
Class V cavities were prepared in 40 extracted human molars. In Group I XP bond (Dentsply), in Group II Adper Single Bond II (3M ESPE), in Group III Adper Easy One (3M ESPE), and in Group IV Xeno V (Dentsply) were applied. Teeth were restored with resin composite, subjected to thermocycling, and sectioned in Buccolingual plane. The samples were demineralized using 6N HCl, for 30 sec, and deproteinized with 2.5% NaOCl for 10 min, gold sputtered, and viewed using a scanning electron microscope.
Results:
Among the total-etch systems used, the XP Bond showed a clear, thick hybrid layer, with long resin tags and few voids. Among the self-etch adhesive systems, the Xeno V did not show a clearly recognizable hybrid layer, but there were no voids and continuous adaptation was seen with the dentin.
Conclusion:
The adaptation of self-etch adhesives to the resin-dentin interface was good without voids or separation of phases; showing a thin, continuous hybrid layer.
Keywords: Hybrid layer, morphology, resin tags, scanning electron microscopy, self-etch, total-etch
INTRODUCTION
Reality always seems to fall short of conceptualization, a variety of researchers in dentistry, brought a longstanding dream to fruition by providing adhesives that could effectively bond composites to dentin as well as enamel.
However, it is difficult to achieve, as the bonding process to dentin is very different from that for enamel. Dentin is more humid and more organic than enamel, which makes bonding a difficult task, unlike enamel, which is mostly inorganic in nature.
In an increasing attempt to simplify clinical procedures in adhesive dentistry, the most common approach was to shorten the adhesive system's application time and reduce the number of steps. The three-step etch-and-rinse systems have proved to be a gold standard. Self-etching systems were introduced and purported important advantages compared to the etch-and-rinse systems. These systems were often described as using the most promising adhesive approach. The intensity of the interaction of self-etching adhesive systems with dentin was mostly dependant on the acidity and aggressiveness of the primer used.[1]
The total-etch technique simultaneously removes the smear layer from both the enamel and dentin surface, followed by the application of one bottle agent that combines the primer and adhesive in one solution.[2]
The self-etch adhesives use acidic adhesive comonomers that simultaneously demineralize and infiltrate into the dentin; adhesive stability is related to the effective coupling of the comonomers that simultaneously demineralize and infiltrate into the dentin. Self-etching systems are less sensitive to technique as compared to systems requiring separate acid conditioning and rinsing steps. Despite the presence of short resin tags, a good seal is achieved as the smear plugs are left intact.[3] The present day, all-in-one single bottle of self-etching adhesive systems are highly hydrophilic polymers, which are permeable to water movement and are said to be using the most promising adhesive approach.[4]
Hence, the purpose of this study is to evaluate the morphology of the resin-dentin interface of the new total- and self-etching systems and to assess the quality of the hybrid layer formed.
MATERIALS AND METHODS
The present study was conducted at the Department of Conservative Dentistry and Endodontics, A.B. Shetty Memorial Institute of Dental Sciences, Mangalore.
Forty, freshly extracted caries-free human molars were collected and stored in saline at room temperature. All the teeth were examined and the teeth with caries or cracks were discarded. The teeth were then randomly and equally divided into four groups. Protocols in cross-infection control as per Occupational Safety and Health Administration regulations in storing, surfacing, and utilization were observed.
The 40 extracted teeth stored in saline were retrieved and Class V cavities (2 mm × 1.5 mm × 1.5 mm) were prepared in selected teeth using a No. 245 tungsten carbide bur; the length of the cutting end of the bur was approximately 3 mm. After preparation of Class V cavities in the 40 teeth, they were randomly divided into four groups of 10 specimens each. In Group I, the prepared dentinal surfaces of the teeth were etched with etchant, 35% phosphoric acid, for 30 sec, and washed. The surface was slightly blotted with gauze to produce a visible moist dentin surface to produce moist dentin. The resultant moist surfaces were treated with a total etching bonding agent (XP Bond, Dentsply) and cured as per manufacturer's instructions. In Group II, the prepared dentinal surfaces of the teeth were etched with etchant, 35% phosphoric acid, for 30 sec, and washed. The surface was slightly blotted with gauze to produce a visible moist dentin surface to produce moist dentin. The resultant moist surfaces were treated with total etching bonding agent (Adper Single Bond 2, 3M ESPE) and cured as per manufacturer's instructions. In Group III, the resultant moist dentin surfaces were treated with One-Step Self-Etching Bonding Agent (Adper Easy One, 3M ESPE), by applying a uniform layer, avoiding pooling, gently agitating with a microbrush for 20 sec, and allowing them to dry in a mild air stream for 10 sec. A second layer was applied with a microbrush, avoiding any pooling, and it was allowed to dry in a mild air stream for 10 sec. The bonding agent was light cured for 20 sec. In Group IV, all the prepared dentinal surfaces of the teeth were cleaned and blotted with gauze to produce visible moist dentin. The resultant moist dentin surfaces were treated with a One-Step Self-Etching Bonding Agent (Xeno V, Dentsply) by applying a uniform layer, avoiding pooling, and allowing them to dry in a mild air stream for 10 sec. The bonding agent was light cured for 20 sec.
The Class V preparations were then restored with hybrid composite in an incremental technique of 1 mm each, curing each layer for 40 sec. Specimens were then stored in distilled water under room temperature.
The restored specimens were then subjected to thermocycling (500 cycles, 5 to 55 C, 30 sec dwell time). Following thermocycling, the teeth were sectioned longitudinally through the restoration in a Buccolingual plane. Two sections were made per tooth, producing two specimens, which were polished with an increased grit of silicon carbide paper, under running water. A total of 20 specimens from each group resulting in 80 specimens were prepared.
The specimens were demineralized using 6N HCl for 30 sec and deproteinized with 2.5% NaOCl (Sodium hypochlorite) for 10 min, to enable examination of the interface and left to dry for 24 h. The specimens were gold sputtered and morphological evaluation of the resin-dentin interface was conducted using scanning electron microscopy under various magnifications.
RESULTS
Qualitative evaluation of the resin-dentin interface (SEM images)
Group I — Scanning electron microscopic evaluation showed a clearly recognizable hybrid layer, which was thick, defined, and continuous. The thickness of the hybrid layer ranged between 3 - 8 μm. There was no adhesive failure seen between the resin-dentin interface. However, few areas showing cohesive failure were seen within the resin and the resin-composite interface. The length of the resin tags ranged between 10 - 25 μm. The resin tags showed numerous lateral tags. Few voids were noticed on the resin tags [Figure 1].
Figure 1.
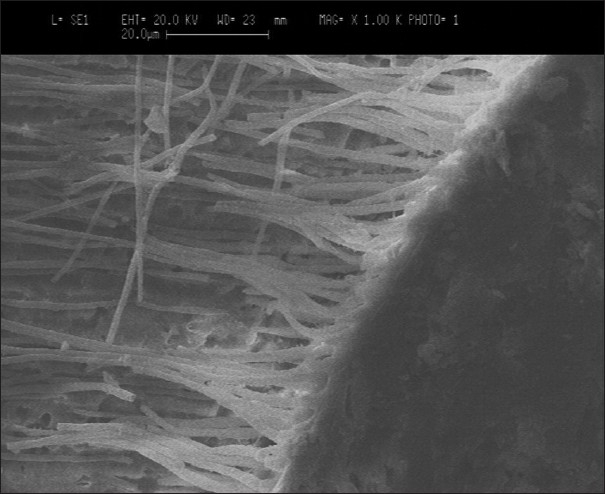
Group I — SEM image of XP Bond with dentin
Group II — A scanning electron microscopic evaluation of the specimens showed a clearly recognizable thin hybrid layer. The hybrid was clearly defined and the thickness ranged between 3 - 6 μm. However, there were areas where there was a visible region of separated layers showing adhesive failure between the dentin and resin interface. There were few areas of separation between the composite and resin interface indicating a cohesive failure. The length of the resin tags ranged between 8 and 20 μm. The resin tags were in the shape of inverted cones. Few voids were seen on the resin tags and they showed few lateral tags [Figure 2].
Figure 2.

Group II — SEM image of Adper Single Bond 2 with dentin
Group III — A scanning electron microscopic evaluation of specimens showed a thin hybrid layer that was not clearly distinct and no discontinuity was noticed. However, there was no adhesive or cohesive failure observed. The thickness of the hybrid layer ranged between 0.8 - 1.4 μm. Few resin tags were observed with a range of 1 - 2 μm. There were no voids observed and few lateral tags were observed [Figure 3].
Figure 3.
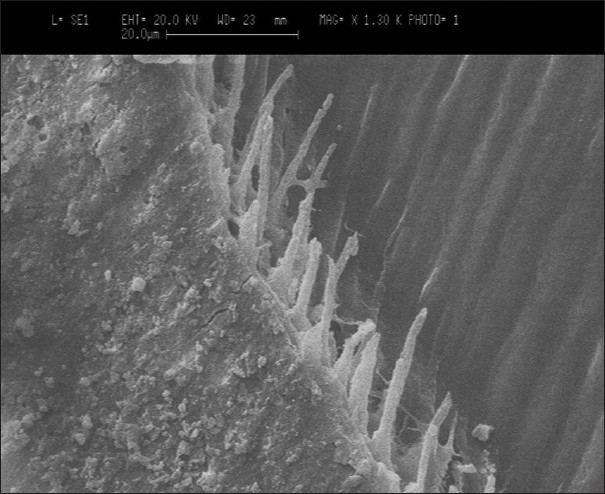
Group III — SEM image of Adper Easy One with dentin
Group IV — A scanning electron microscopic evaluation showed no evidence of a hybrid layer. The junction between the resin-dentin appeared to be tight, thin, and continuous with no adhesive or cohesive failure. Minimal formation of microtags was noticed under higher magnification with a range of 0.6 - 1.5 μm. There were no evident lateral tags. Some of the dentinal tubules appeared to be obstructed by smear plugs [Figure 4].
Figure 4.

Group IV — SEM image of XENO V with dentin
DISCUSSION
Hybridization of dental hard tissues provides a new frontier in modern adhesive dentistry because: it creates a new, advanced, multifunctional dental material that can be prepared in the subsurface of teeth. The hybrid layer refers to the structure formed in the dental hard tissues by demineralization of the surface and sub-surface, followed by infiltration of monomers and subsequent polymerization.[5]
Dentin is the fundamental substrate of restorative dentistry, and its properties and characteristics are key determinants of nearly all restorative, preventive, and disease processes of the teeth. Dentin is a complex hydrated composite of dentinal tubules, mineralized matrix, collagen, and dentinal fluid. Dentinal tubules are not smooth, and have many lateral branches and microchannels that connect to adjacent tubules and permit fluid movement. Thus, variations in permeability arise from regional differences, tubular irregularities, mineral deposits, organic components of odontoblastic processes, collagen, and dentinal fluid. From the bonding perspective, the hydrated structure and variability of the dentin in its characteristics of permeability, cause substantial difficulties. Unless the bonding agent can displace or is miscible in the fluid, it will be difficult to achieve a stable, long-lasting bond.[6]
As bonding is created by impregnation of dentin substrate by blends of resin monomers, the stability of the bonded interface relies on the creation of a compact and homogenous hybrid layer. In etch and rinse strategy, bonding monomers impregnate the porous etched substrate; and stable bonds can be achieved if the etched substrate is fully infiltrated by the monomers. Conversely, the self-etch approach uses acidic adhesive comonomers that simultaneously demineralize and infiltrate into the dentin. Adhesive stability is related to the effective coupling of the comonomers with an infiltrated substrate.[7]
Self-etching systems are generally less technique sensitive compared to systems that utilize separate acid conditioning and rinsing steps. Collapse of the air-dried, demineralized collagen is prevented as the smear layer-retained dentin is simultaneously demineralized and polymerized in situ. Despite the presence of short resin tags, a good seal is achieved as the smear plugs are left intact.[8]
The specimen preparation in the present study involved the preparation of Class V cavities for three reasons: First, because it involves three dental hard structures, that is, enamel, dentin, and cementum. Second, a Class V cavity simulates a clinical situation of higher stress due to a higher C-factor, and third, due to exposure to varied fluids in the cervical region of the tooth. Radovic I, Vulicevic ZR, and Godoy GF chose Class V cavities, because samples prepared in this manner involved the difficulties in achieving the suggestive width of the adhesive layer with a higher C-factor and higher stress contraction stress. The other rationale was to make the microleakage studies of adhesive systems more relevant.[1]
The most widely used method for artificial aging and thermocycling was performed. The number of cycles used in the present study was 500 (5 to 55 C with 30 sec dwell time). Radovic I, Vulicevic ZR, and Godoy GF also used 500 cycles, as it was based on the current ISO standard.[1] In the present study, 6N Hydrochloric acid was used for demineralization and 2.5% sodium hypochlorite for deproteinization. This ensured optimal exposure of the resin tags and hybrid layer and enabled viewing under the scanning electron microscope. Nakabayashi and Pashley also stated that the study of the usual procedure for specimen preparation under SEM involved preparation of polished cross-sections of the bonded interface followed by an acid challenge with 5N Hydrochloric acid for 30 sec to demineralized, followed by 1% sodium hypochlorite for 10 min. This procedure exposed the resin tags to their full length and demarcated the bottom of the hybrid layer.[5] The present study is in accordance with the study done by Radovic, Vulicevic, and Garcia Godoy, who also used 6N Hydrochloric acid and 2.5% sodium hypochlorite for demineralization and deproteinization.[1]
Scanning electron microscopy is one of the first and most widely used tools to study the interfacial characteristics and process of bonding. Scanning electron microscopy, although very useful, induces shrinkage artifacts in the specimens during processing and in the high vacuum of the microscope, unless the specimens in the resin are perfectly infiltrated with resin. The usual procedure involves the preparation of polished cross-sections of the bonded interface followed by an acid challenge with 6N Hydrochloric acid for 30 sec to demineralize, followed by 2.5% sodium hypochlorite for 10 min. This procedure exposes the resin tags to their full length and demarcates the bottom of the hybrid layer.[5]
In the present study it was observed that, there was shrinkage artifacts created, which was attributed to the fact that the specimen preparation procedures in scanning electron microscopy induced artifacts. Perdigao and Carvalho had investigated and found that specimens prepared for scanning electron microscopy underwent air drying, Hexamethyldisilazane, or Peldri II drying. It was concluded that specimens prepared by air drying showed shrinkage that occurred during specimen preparation and this influenced the exact dimensions of the morphology.[1]
Voids seen in the resin tags of the specimens in Group I and Group II represent remnants of water, in the form of droplets, which were trapped in the adhesive before polymerization. Air drying over a long time reduces the number of water droplets, but total drying is difficult to achieve. During the desiccation procedure, for gold sputtering the specimens, water droplets might have evaporated, leaving voids. Van Landuyt et al, in their investigation, found that the number of water droplets was significantly reduced after strong air drying, although they did not completely disappear.[1,9]
In the present study there is a highly significant difference in the thickness of the hybrid layer and the length of the resin tags formed between the total-etch adhesives (group I and group II) and self-etch adhesives (group III and group IV). The total-etch adhesives use the etch-and-rinse strategy, thus removing the smear layer and subsequent infiltration of monomers and polymerization. The primary bonding mechanism is a diffusion-based process leading to the production of a hybrid resin-dentin interdiffusion zone. The most critical step in the total-etch technique is the priming stage. In Group I, XP Bond has been used, which contains t- butanol; and in Group II, Adper Single Bond 2 ethanol is used, which requires the ‘wet bonding’ technique and thus there are lesser regions of adhesive failure seen. This explains that although the adhesive (XP Bond and Adper Single Bond 2) produces resin tags that are long, with thicker hybrid layers, adhesive failure and cohesive failure are seen due to the technique sensitivity of the bonding mechanism. Gentle postconditioning drying of the etched dentin still guarantees effective bonding for ethanol-based adhesives. However, true chemical bonding is unlikely, because the functional monomers have a weak affinity to the hydroxyapatite-depleted collagen.[10]
Group III and Group IV, self-etch adhesives showed thin hybrid layers, with fewer microtags. However, there is no evidence of adhesive or cohesive failures observed with self-etch adhesives.
There was a thin, continuous adaptation of the resin with the dentin interface. The self-etch approach had an important advantage in that the infiltration of the resin occurred simultaneously with the self-etching process resulting in a uniform interdiffusion zone. This superficial demineralization kept the residual hydroxyapatite still attached to the collagen. Nevertheless, sufficient porosity was created to obtain micromechanical interlocking. De Munck et al, in their study concluded that the thickness of the hybrid layer was, however, much smaller than that produced by the strong self-etch adhesive or the etch-and-rinse total-etch approach. It was proven to be minor in importance with regard to the actual bonding effectiveness.[11]
However, the drawback of the all-in-one adhesive is its behavior as a semi-permeable membrane allowing movement of water across bonded interfaces, which may in the long term lead to potential hydrolytic degradation.[12]
The limitations of conventional scanning electron microscopy compared to more sophisticated methods of examining tooth restoration interfaces have been recognized. However, regardless of the method used (SEM / TEM), no correlation between the hybrid layer width and bond strength of the adhesive systems could be determined.[1,13,14]
Within the limitations of the present study, however, the scanning electron microscopy method can provide basic information, which points to the possible specific features of the interface, which need to be examined closely. In vitro studies depend on variable parameters, such as, sample collection, surfacing, aging, thermocycling, and hurdles in specimen preparation, for scanning electron microscopy. There being a clinical correlation with long-term studies on the effects of aging, thermal effects on the bonding process need to be examined closely.
CONCLUSION
From the present study it is concluded that: XP Bond, among the total-etch bonding agents, showed good penetration of resin into the dentin by the presence of long resin tags and a thick hybrid layer.
Xeno V, among the self-etch bonding agents, did not show a clear hybrid layer with few resin tags, but showed a continuous adaptation along the dentin surface.
The adaptation of self-etch adhesives to the resin- dentin interface was good without voids or separation of phases; showing a thin, continuous hybrid layer. It was concluded that such an adaptation of resin with dentin tissue, as in the case of self-etch adhesives, was required for better treatment outcome to reduce postoperative sensitivity.
Footnotes
Source of Support: Nil,
Conflict of Interest: None declared.
REFERENCES
- 1.Radovic I, Vulicevic ZR, Garcia-Godoy F. Morphological evaluation of 2- and 1-step self-etching system interfaces with dentin. Oper Dent. 2006;31:710–8. doi: 10.2341/05-145. [DOI] [PubMed] [Google Scholar]
- 2.Ceballos L, Camejo DG, Victoria Fuentes M, Osorio R, Toledano M, Carvalho RM, et al. Microtensile bond strength of total-etch and self-etching adhesives to caries-affected dentin. J Dent. 2003;31:469–77. doi: 10.1016/s0300-5712(03)00088-5. [DOI] [PubMed] [Google Scholar]
- 3.Wang Y, Spencer P. Hybridization efficiency of the adhesive/ dentin interface with wet bonding. J Dent Res. 2003;82:141–5. doi: 10.1177/154405910308200213. [DOI] [PubMed] [Google Scholar]
- 4.Eliades G, Vougiouklakis G, Palaghias G. Heterogenous distribution of single-bottle adhesive monomers in the resin-dentin interdiffusion zone. Dent Mater. 2001;17:277–83. doi: 10.1016/s0109-5641(00)00082-8. [DOI] [PubMed] [Google Scholar]
- 5.Nakabayashi N, Pashley DH, Nubuo, David H. Hybridization of Dental Hard Tissues. Hybridization of Dental Hard Tissues. Tokyo: Quint Int. Pub. Co; 1998. [Google Scholar]
- 6.de Alexandre RS, Sundfeld RH, Giannini M, Lovadino JR. The influence of temperature of three adhesive systems on bonding to ground enamel. Oper Dent. 2008;33:272–81. doi: 10.2341/07-79. [DOI] [PubMed] [Google Scholar]
- 7.Breschi L, Mazzoni A, Ruggeri A, Cadenaro M, Di Lenarda R, De Stefano Dorigo E. Dental adhesion review: Aging and stability of bonded interface. Dent Mater. 2008;24:90–101. doi: 10.1016/j.dental.2007.02.009. [DOI] [PubMed] [Google Scholar]
- 8.Tay FR, Pashley DH. Aggressiveness of contemporary self-etching systems.I: Depth of penetration beyond dentin smear layers. Dent Mater. 2001;17:296–308. doi: 10.1016/s0109-5641(00)00087-7. [DOI] [PubMed] [Google Scholar]
- 9.Van Landuyt KL, De Munck J, Snauwaert J, Coutinho E, Poitevin A, Yoshida Y, et al. Monomer-solvent phase separation in one-step self-etch adhesives. J Dent Res. 2005;84:183–8. doi: 10.1177/154405910508400214. [DOI] [PubMed] [Google Scholar]
- 10.Van Meerbeek B, De Munck J, Yoshida Y, Inoue S, Vargas M, Vijay P, et al. Adhesion to enamel and dentin: Current status and future challenges. Oper Dent. 2003;28:215–35. [PubMed] [Google Scholar]
- 11.De Munck J, Van Meerbeek B, Yoshida Y, Inoue S, Vargas M, Suzuki K, et al. Four-year water degradation of total etch adhesives bonded to dentin. J Dent Res. 2003;82:136–40. doi: 10.1177/154405910308200212. [DOI] [PubMed] [Google Scholar]
- 12.Perdigao J. New developments in dental adhesion. Dent Clin North Am. 2007;51:333–57. doi: 10.1016/j.cden.2007.01.001. [DOI] [PubMed] [Google Scholar]
- 13.Perdigao J, May KN, Wilder AD, Jr, Lopes M. The effect of depth of dentin demineralization on bond strengths and morphology of the hybrid layer. Oper Dent. 2000;25:186–94. [PubMed] [Google Scholar]
- 14.Nakabayashi N, Saimi Y. Bonding to intact dentin. J Dent Res. 1996;75:1706–15. doi: 10.1177/00220345960750091401. [DOI] [PubMed] [Google Scholar]


