Abstract
A natural TB infection model using guinea pigs may provide useful information for investigating differences in transmission efficiency and establishment of active disease by clinical TB strains in a highly susceptible host under controlled environmental conditions. We sought to examine the capacity of naturally transmitted multidrug-resistant M. tuberculosis to establish infection and produce active disease in guinea pigs. Guinea pigs were continuously exposed for 4 months to the exhaust air of a 6-bed multidrug-resistant tuberculosis inpatient hospital ward in South Africa. Serial tuberculin skin test reactions were measured to determine infection. All animals were subsequently evaluated for histologic disease progression at necropsy. Although 75% of the 362 exposed guinea pigs had positive skin test reactions [≥6mm], only 12% had histopathologic evidence of active disease. Reversions (≥ 6 mm change) in skin test reactivity were seen in 22% of animals, exclusively among those with reactions of 6 to 13 mm. Only two of 86 guinea pigs with reversion had histological evidence of disease compared to 47% (31/66) of guinea pigs with large, non-reverting reactions. Immunosuppression of half the guinea pigs across all skin test categories did not significantly accelerate disease progression. In guinea pigs that reverted a skin test, a second positive reaction in 27 (33%) of them strongly suggested re-infection due to ongoing exposure. These results show that a large majority of guinea pigs naturally exposed to human-source strains of multidrug-resistant tuberculosis became infected, but that many resolved their infection and a large majority failed to progress to detectable disease.
Keywords: MDR/XDR tuberculosis, guinea pig model, natural infection, skin test reactivity
INTRODUCTION
The propagation of multidrug-resistant (MDR) and extensively drug-resistant (XDR) tuberculosis (TB) threatens the success of global TB control and rapidly expanding antiretroviral treatment programs. In the widely publicized outbreak of XDR-TB in KwaZulu-Natal, South Africa, 55% of the patients had not been previously treated for TB, but two-thirds had been recently hospitalized and potentially exposed 1. Moreover, 85% of the XDR patients with genotyped isolates had similar strains, suggesting extensive transmission in hospitals, other congregate settings, and possibly in the community. Worldwide, more than 50% of currently reported MDR cases have not been treated previously, indicating the importance of transmission in propagating the epidemic 2. In Siberia, Russia, for example, a recent analysis found previous hospitalization for drug susceptible TB rather than treatment non-adherence as the predominant risk factor (RR>6) for acquisition of drug-resistant TB, further supporting the contribution of transmission and re-infection in driving the MDR-TB epidemic 3. In another study in Latvia, molecular fingerprinting of patient isolates revealed nosocomial transmission to be a highly significant risk factor for having MDR-TB (OR 18.33) 4. The emerging importance of M/XDR transmission contrasts sharply with the view promulgated early in the epidemic that drug resistance mutations conveyed a microbial fitness cost that would greatly limit propagation 5. A review of the TB fitness literature, however, suggests that drug-resistant strains exhibit heterogeneous fitness, due in part to compensatory mutations 6. In the context of such fitness heterogeneity, the capacity of drug-resistant strains to cause disease may be tied to transmission intensity and opportunity for re-infection as well as host susceptibility 7.
Here we present observations from the natural exposure of guinea pigs to the air from an experimental hospital ward in which patients infected with MDR/XDR strains of M. tuberculosis resided. The initial objective in this pilot study was limited to quantifying human to guinea pig transmission in anticipation of a series of studies to test air disinfection interventions such as ultraviolet air disinfection, surgical masks on patients, and room air filtration machines. The study was not designed or funded to characterize human transmission factors, strain transmissibility or virulence, animal host response, or the impact of treatment on transmission. However, faced with high rates of tuberculin skin test (TST) conversions, unexpected skin test reversions, and little evidence of disease progression in the guinea pigs, we modified the experiment after the initially planned skin testing was complete by immunosuppressing half the skin test positive guinea pigs with high-dose corticosteroids in an attempt to promote disease progression and potentially increase the microbiological yield of isolates from animal tissues to permit matching of guinea pig isolates with those in patient sputum samples. We also preserved animal tissue samples for future microbiological and pathological examination.
Guinea pigs have long been used as a surrogate animal model of TB. These animals have been shown to be uniformly susceptible to M. tuberculosis and to acquire infection after inhalation of a single droplet nucleus containing no more than a few virulent organisms. They develop strong delayed type hypersensitivity responses after infection, as detected by TSTs to purified protein derivative (PPD) 8. When challenged with virulent strains in laboratory aerosol infection studies, they develop disseminated, multi-organ disease and eventually succumb to infection after a period of several months 8. The disease that develops in guinea pigs replicates many important aspects of human TB, including pulmonary and extrapulmonary true granulomata with caseous necrosis. In contrast, however, the guinea pig model is not known to exhibit latency, nor does experimentally induced progressive disease typically lead to lung cavitation 9–11.
In this study, 75% of the exposed animals developed ≥ 6 mm TST reactions, but only 12% developed pathologic evidence of active disease despite lengthy follow up. This is an important observation, because in the laboratory-based low dose aerosol exposure model, typically, 100% of guinea pigs develop disease, even when challenged with MDR-TB 11, 12. In the natural exposure model described here, far more akin to TB transmission and propagation among humans, the majority of guinea pigs did not develop active disease. This latter model, an updated version of the classic studies by Riley and Wells five decades ago 13, 14, therefore has the potential to provide new insights into TB transmission and the complex relationship between naturally transmitted infections and re-infections, delayed-type hypersensitivity, and the establishment of active disease.
METHODS
Studies were performed at the Airborne Infections Research (AIR) Facility of the Medical Research Council (in eMahlahleni [formerly known as Witbank], South Africa), a facility designed to expose susceptible sentinel guinea pigs to airborne tuberculosis (infectious droplet nuclei) generated by patients with active, culture-proven, mostly sputum smear positive MDR pulmonary tuberculosis receiving standardized MDR-TB treatment. The AIR facility is part of the Mpumalanga provincial MDR-TB referral hospital and consists of a 6-bed inpatient MDR-TB ward connected to 2 guinea pig exposure chambers by an airtight ventilation system.
HEPA filter treated air enters the patient ward at a rate of 12 air changes per hour and is then delivered entirely through ventilation ductwork to each of two guinea pig chambers located in parallel with each other. Within each guinea pig exposure chamber, the air being delivered from the patient ward emerges from a grid of 60 individual ducts located on a side wall. Each of these 60 ducts supplies ward air to one cage of animals (1:1 duct to cage ratio). Each cage holds three animals. The cages are located 1 cm from the opening face of the duct supplying that cage. Air from the guinea pig chamber is then actively exhausted through a single exhaust duct located on the ceiling. The direction of air flow ensures that air emerges from the inlet duct and passes through the guinea pig cages prior to exiting the animal exposure chamber.
Twenty six patients (11 female, 15 male) with MDR-TB were recruited for this study from among the patients referred to the adjacent provincial MDR-TB hospital to begin standardized MDR-TB treatment according to South African guidelines. Informed consent was obtained for participation in the study. This study was approved by the human studies committees of the South African MRC, the US CDC, the Harvard School of Public Health, and the Brigham and Women’s Hospital. Of the 26 patients that had pulmonary MDR-TB confirmed by culture and first-line drug susceptibility tests performed in various South African TB reference laboratories, only 13 patients’ isolates were subsequently cultured from sputum samples obtained after transfer to the AIR facility. Although not an original aim of this study, these 13 isolates were tested for susceptibility to second line drugs and genotyped using published methods to allow for potential matching with guinea pig isolates 15–17.
Patients occupied the AIR Facility in groups of 6 men or 6 women during the 16 week (112 day) exposure phase of the study. These individuals received exactly the same care and treatment as in the main MDR facility. In order to maximize transmission for this pilot study, patients admitted to the main MDR hospital were recruited to the AIR facility study using the following inclusion criteria: 1) active cough (100%), 2) sputum smear positive for acid fast bacilli (76%), and 3) cavitary TB by chest radiograph (86%). Fourteen patients were known to be HIV+, but HIV status was not known in all patients in this pilot study. Although it takes 2 months on average for sputum to convert to smear negative on the South African standard MDR-TB regimen 18, patients in the AIR facility were replaced approximately every 2 weeks (pending suitable alternate subjects) to sample a wider range of patients. Compared to unselected patients admitted to the main MDR hospital, study patients were more likely to be female (42 vs. 32%) and to have lung cavitation (86 vs. 59%), but equally likely to be smear positive (both 76%) and culture positive (95 and 96%).
Outbred female, specific pathogen free, Dunkin-Hartley guinea pigs (NHLS, South Africa) were used in this study. Guinea pigs (n = 362) were acquired at 6 weeks of age weighing 250 – 300 g. Animals were maintained in the AIR Facility animal exposure chambers (3 per cage) under animal biosafety level 3 conditions at an ambient temperature of 22 ± 1°C, relative humidity of 50 ± 10% and day/night cycle of 12 hours. Sterile food supplemented with irradiated hay (NHLS, South Africa) and sterile water supplemented with water-soluble ascorbic acid (Kyron, South Africa) were provided ad libitum. Animals also underwent clinical surveillance three times a week and any animals that became ill by explicit criteria were removed and sacrificed for necropsy. Animal cages had wire mesh floors to reduce the risk of fecal-oral transmission of infection between animals, a phenomenon known to occur only late in the course of disseminated disease 19. Animal care was overseen by a licensed laboratory veterinarian and all protocols were approved by the Animal Use committees of the South African MRC, the US CDC, and Harvard Medical School.
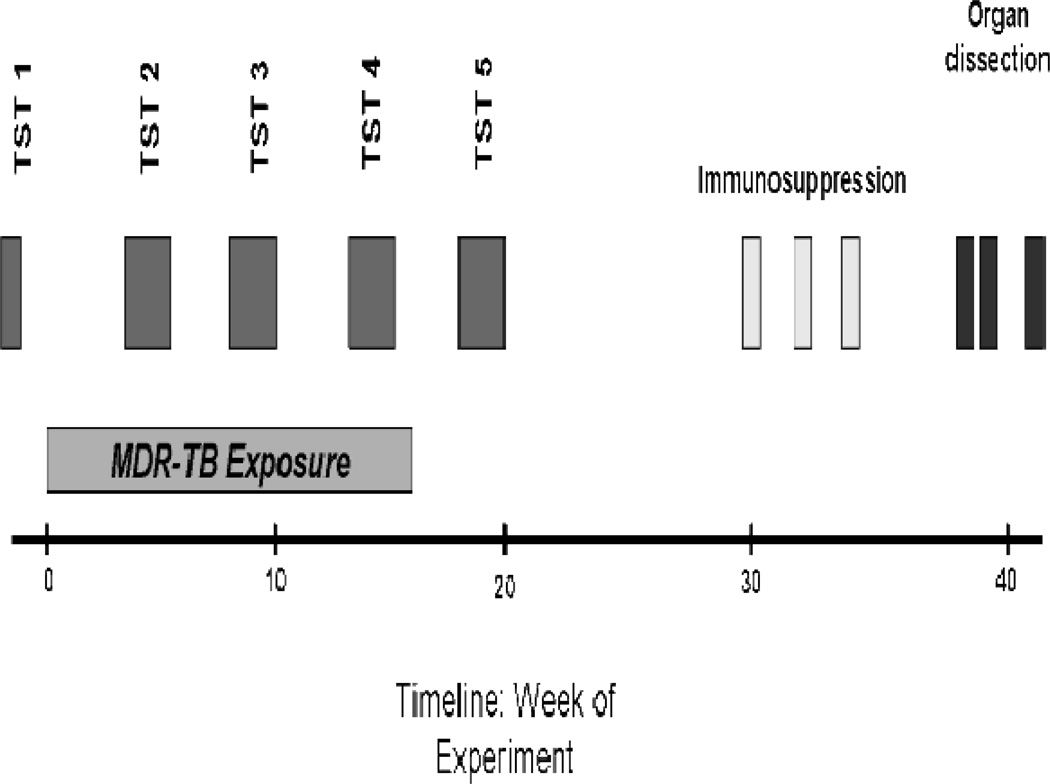
TST was performed with 100 tuberculin units (2 µg) of commercially produced PPD (Mycos Research LLC, Loveland, Colorado, USA). In addition to the baseline TST performed prior to exposure to infectious ward air, animals underwent monthly TSTs during and after MDR-TB exposure (Figure 1). PPD was diluted immediately before testing and was administered intradermally on a depilated area of the back. TSTs were read in a blinded, duplicate manner using digital calipers (Wilson Wolpert, The Netherlands). Two independent readings were taken at right angles to each other (longitudinal and transverse) at 24 hours post-administration by a single animal handler, and the results recorded separately in millimeters. The final diameter of induration was calculated as the average from these four readings. All reactions were monitored for necrosis. Based on preliminary studies, an induration diameter of ≥ 6 mm was considered indicative of infection 13, 20. Animals with TST 2 reactions less than 10 mm were re-tested at months 3 and 4. We defined TST reversion as a decrease in the TST diameter by at least 6 mm to a final diameter of less than 6 mm. For example, an 11 mm reaction which upon retesting was 5 mm or less would be considered a reversion.
Figure 1.
Design and time course of study
Based on historical and contemporary data from similar experiments using a comparable antigen dose, TST results were categorized into three groups: non-reactions (0 – 5 mm) unlikely to represent infection, small reactions (6 – 13 mm) likely to represent limited infection, or large reactions (≥ 14 mm) likely to represent progressive infection 13, 20. Serial TSTs for each animal were also categorized by the pattern of reaction through the course of the study: persistent non-reactions, persistent small reactions, small reactions with reversion, small reactions with reversion followed by a second positive reaction, and persistent large reactions.
Guinea pigs were exposed to ward air for 16 weeks. Given the large numbers of animals being tested, animals were divided into three cohorts separated by a week at each time point. TST measurements 2, 3, and 4 occurred between weeks 4 – 6, weeks 8 – 10, and weeks 12 – 14 of exposure, respectively (Figure 1). For an individual guinea pig, TST measurements 2 through 4 occurred exactly 4 weeks apart. A fifth TST was performed between weeks 18 – 20 (after exposure to ward air had ended).
At week 30, guinea pigs that had exhibited a ≥ 6 mm TST reaction at any time point were randomized to an immunosuppression or saline sham protocol, which consisted of either 0.265 mg Dexa-0.2-phenix/0.1 ml inoculum (equivalent to a dose of 100 mg/kg hydrocortisone) or saline sham injections subcutaneously 7 days/week on weeks 30, 32, and 34. All skin testing was completed 10 weeks prior to administration of steroids (Figure 1). A further 4 weeks were allowed for disease progression before the animals were euthanized and their organs harvested in a systematic fashion.
Guinea pigs were euthanized by intraperitoneal injection of 200 mg/kg sodium pentobarbital (Euthapent, Kyron Laboratories, SA). Lungs and spleen from all guinea pigs were aseptically removed. A small portion of tissue was reserved and the remainder was fixed in formalin. In addition, animals with grossly enlarged hilar lymph nodes or with liver lesions had samples of these tissues removed for fixation. Formalin preserved portions of organs were trimmed and processed for paraffin embedding. These paraffin embedded tissues were cut into 4 µm sections and stained with hematoxylin and eosin. A histopathological grading system modified and adapted from an acute exposure model was used to evaluate the extent of tuberculosis lesions in lungs, spleen, lymph nodes, and liver 21, 22. Using this method, lungs were scored for the presence and severity of seven features/lesions of TB as follows: (i) estimation of the percent of lung affected ranked at low magnification: 0 – no lesions in lung, 1 – up to 25% of lung involved, 2 – up to 50% of lung involved, 3 – up to 75% of lung involved, 4 – above 75% of lung involved. (ii) number of primary lesions: 0 – no primary lesions present, 1 – a single primary lesion present, 2 – two or more primary lesions present, multifocal, 3 – two or more primary lesions present, multifocal to coalescing, 4 – multiple primary lesions, coalescing and extensive. (iii) extent of secondary lesions: 0 – no secondary lesions present, 1 – up to 25% of lung involved, 2 – up to 50% of lung involved, 3 – up to 75% of lung involved, 4 – above 75% of lung involved. Necrosis (iv), cavitary lesions (v), mineralization (vi), and fibrosis (vii) were scored for severity on the following scale: 0 – none, 1 – minimal, 2 – mild, 3 – moderate, 4 – marked. Lung lesion morphology (viii) was graded from 1 – 4 based on the proportion of macrophages and lymphocytes present in granulomatous lesions estimated by light microscopic appearance at high magnification (1 represented lesions consisting of all lymphocytes and 4 represented lesions of all macrophages). All individual scores for these features were summed for a maximal total lung disease score of 32. The spleen, perihilar lymph nodes, and liver were scored on five features and their severity as described above: (i) percent organ involvement, (ii) degree of necrosis, (iii) fibrosis, (iv) mineralization, (v) lesion type. The maximal total score for each of these organs was 20. Since not all animals had lymph nodes or liver tissue available for histologic analysis, we used only the results from combined lung and spleen scores (maximum possible disease score of 52 per guinea pig) in analyses comparing mean disease severity, but did use presence of disease in any available organs for analyses on proportions of diseased animals in various groups.
Mean total disease scores were compared for the following categories: guinea pigs 1) with large and with small reactions; 2) with and without skin test reversion; 3) with large reactions with and without immunosuppression; and 4) with large reactions on the last skin test (TST 5) that converted their TST early (on TST 2 or 3) versus late (TST 4 or 5). Since total disease scores within some groups of guinea pigs were not normally distributed, the Wilcoxon Rank Sum Test with continuity correction for non-parametric data was used to compare group means. Skin reactions were analyzed using the Pearson’s product-moment correlation analysis for TST size and total disease score in the entire guinea pig cohort, as well as in two subgroups: large TST reactors that had received steroids and large TST reactors that had not received steroids. Statistical analyses were performed using R 2.7.0 (www.r-project.org).
Organs from 11 guinea pigs that were euthanized for routine health surveillance after TST 2 and from 19 guinea pigs that were euthanized due to overt illness during the study were evaluated histologically for TB by a local pathologist (they did not form part of the set of animals analyzed by the above referenced grading system for this manuscript) and were cultured immediately after necropsy on solid (LJ) agar and liquid based mycobacterial culture (MGIT) using published methods 17. Tissue samples from lung, spleen, and visibly abnormal liver or lymphatic tissue from the remaining guinea pigs that survived to the end of the exposure period (n = 332) were snap frozen at − 80° C and preserved. Although not an original aim of the experiment, solid (LJ) agar and liquid based mycobacterial cultures (MGIT) and genetic fingerprinting of M. tuberculosis isolates using spacer oligonucleotide genotyping were attempted on these frozen tissues in order to match patient and guinea pig isolates 17. For these frozen tissues, cultures were initially performed on organ homogenates of 34/40 guinea pigs that had histologic evidence of TB and 27/292 guinea pigs with small and large TST reactions but no demonstrable histologic evidence of TB. Since the culture yield (liquid and solid media) from these previously frozen tissues was low (even among histology positive, immunosuppressed animals), attempts at culturing remaining guinea pig tissues were not pursued. Viable isolates were obtained from 18/30 guinea pigs that were euthanized during the study and cultured immediately after death.
RESULTS
Tuberculin skin test reactions in exposed guinea pigs
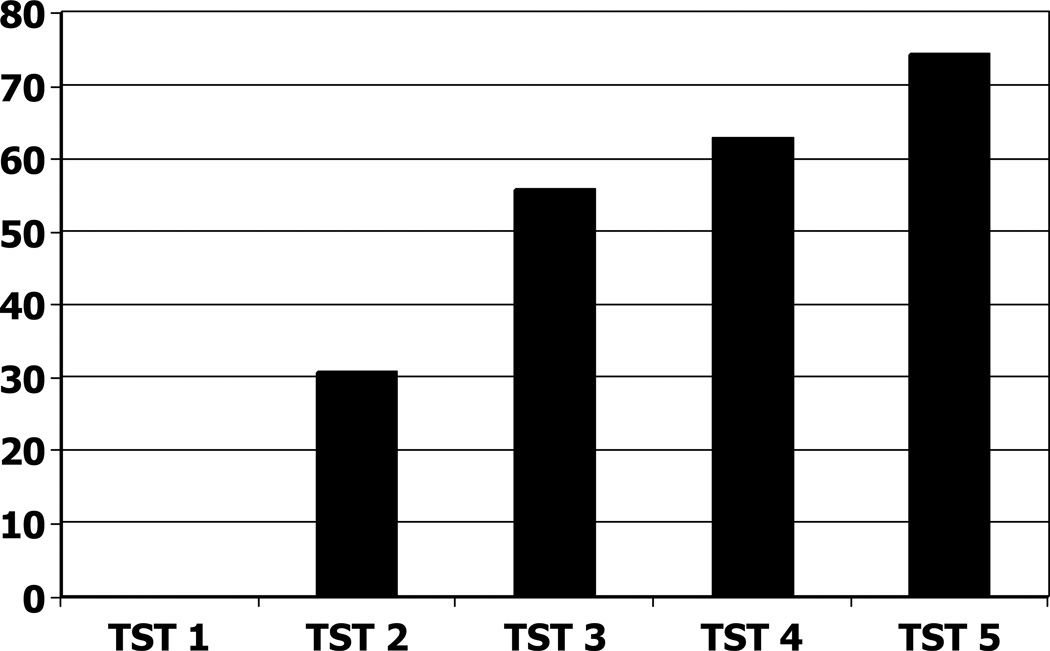
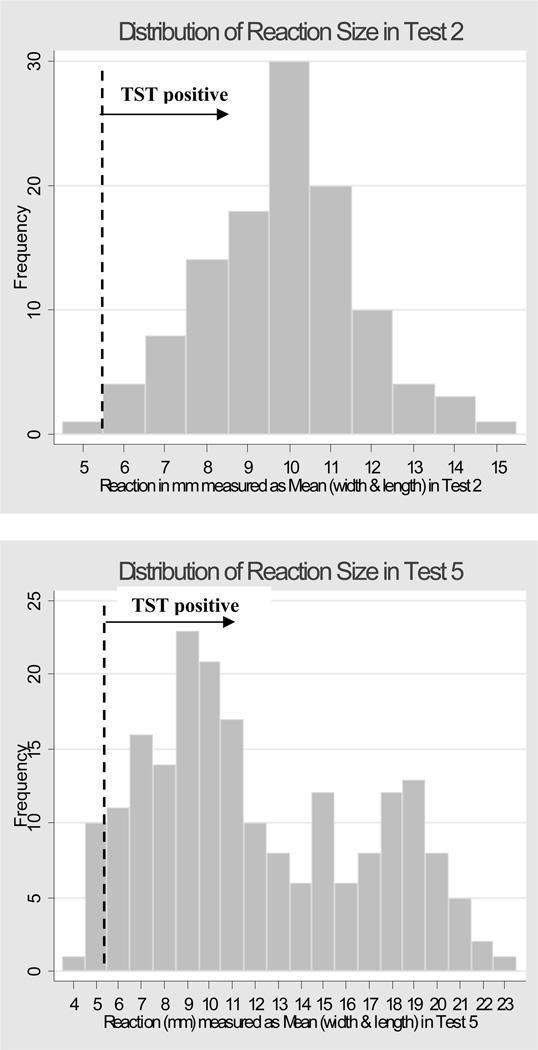
In this study 65.7% (n = 238/362) of the guinea pigs underwent all five TST measurements (the rest were tested only on the first two time points and the fifth time point due to their early development of reactions >10mm). No animals reacted to the first (baseline) TST before exposure, but after 1 month exposure (TST 2) 111/362 (30.6%) guinea pigs exhibited a TST ≥ 6 mm. For the third through the fifth TSTs the cumulative incidence of TST ≥ 6 mm was 55.5%, 62.4%, and 75.0% respectively (Figure 2). Given the occurrence of reversions at all time points after TST 2, attrition due to deaths (n = 19) or removal after TST 2 for routine animal health surveillance (n = 11), the point prevalence of positive tests was 43.7% for TST 3 (n=126/288), 27.9% for TST 4 (n=72/258), and 57.1% for TST 5 (n=194/340). The distribution of reaction sizes for TST 2 appeared normal, with a peak at 10 mm (Figure 3, panel A), but by TST 5 the distribution appeared bimodal, with one peak at 9 mm and a second peak at 19 mm (Figure 3, Panel B).
Figure 2.
Cumulative proportions of guinea pigs with positive (≥ 6 mm) TST results on each skin test.
Figure 3.
Panel A (top): Histogram showing the distribution of TST induration diameters among guinea pigs on TST 2. X-axis shows induration diameters and y-axis shows number of guinea pigs. Vertical dashed line illustrates cut-point for positive TST reaction (≥ 6 mm)
Panel B (bottom): Histogram showing the distribution of TST induration diameters among guinea pigs on TST 5. X-axis shows induration diameters and y-axis shows number of guinea pigs. Vertical dashed line illustrates cut-point for positive TST reaction (≥ 6 mm)
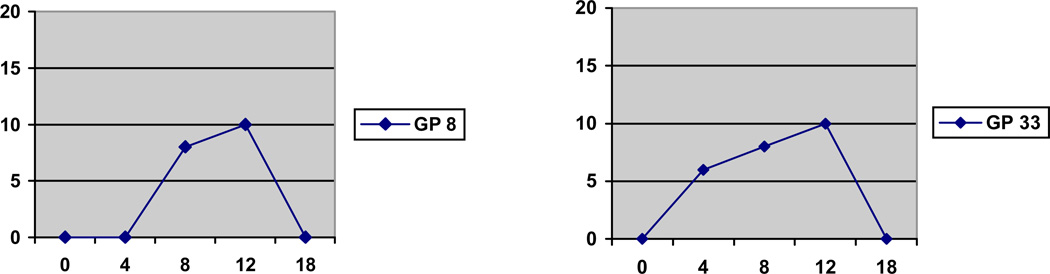
Reactions remaining consistently < 6 mm on all TSTs (e.g. negative) were observed in 25% of animals (n= 89/362,) despite 16 weeks of exposure to the patient ward air (Table 1). Just over half (n=196/362, 54%) of guinea pigs developed small (6–13 mm) TSTs, and approximately one fifth (n=77/362, 21%) of guinea pigs developed large (≥ 14 mm) TSTs (Table 1). TST reversion was observed in 81 (22%) of all guinea pigs (Table 1 and Figure 4) and nearly all reversions were to 0 mm. In 54 of these, TST reactions remained non-reactive on subsequent testing, while in the other 27 guinea pigs, reversions were followed by other positive TST reactions. All TST reversions occurred exclusively in guinea pigs that had small (6–13 mm) rather than large (≥ 14 mm) reactions (Table 1). Incident TST reactions were randomly distributed within the exposure chambers.
Table 1.
Proportions of different TST sizes and reaction patterns in the MDR-TB exposed guinea pig cohort.
| Maximize TST Size or Pattern of Reactions |
Number of Guinea Pigs |
Percent | |
|---|---|---|---|
| 0–5 mm reaction | 89/362 | 25% | |
| 6–13 mm reaction | 196/362 | 54% | |
| Among 6–13 mm reactors (n = 196) | |||
| Reversion – no further reaction | 54/196 | 28% | |
| Reversion – second reaction | 27/196 | 14% | |
| No – reversion | 90/196 | 46% | |
| Other – no opportunity to revert because first positive reaction occurred on TST 5 | 25/196 | 12% | |
| 14+ mm reaction | 77/362 | 21% | |
Figure 4.
Examples of TST reversion in 2 guinea pigs. X axis shows time in weeks and corresponds to timing of TST 1 through 5. Y axis shows TST induration size in millimeters.
Histopathology analysis
Of the initial 362 guinea pigs, 332 survived to the end of the 4-month exposure period. Surviving guinea pigs were generally healthy by clinical surveillance criteria, with few exceptions. As mentioned earlier, 11 animals were removed from the exposure chambers for routine health surveillance after being skin positive on TST 2 and 19 animals died from TB during the study. The tissues from these 30 guinea pigs were not part of the histology analyses described in this paper. However, organs from the 19 animals that died during the study were analyzed separately by a local veterinary pathologist and were determined to have histologic changes due to TB as well as cultures growing M. tuberculosis.
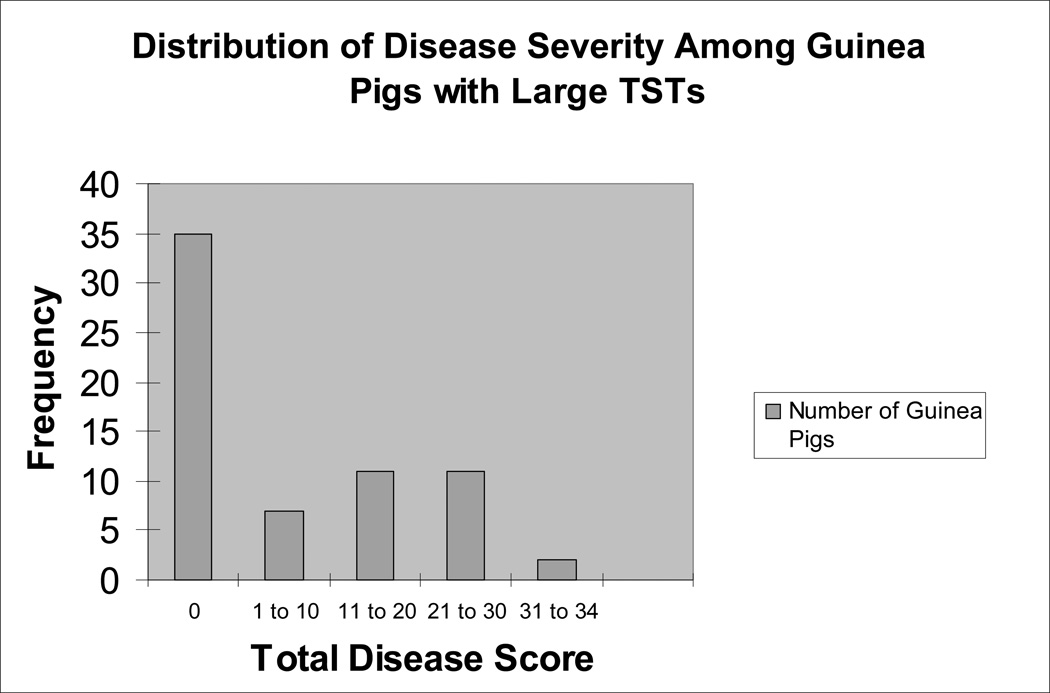
Of 332 animals, 40 (12%) had pathological evidence of tuberculosis in one or more of their organs (lung, hilar/mediastinal lymph node, spleen, or liver). Nearly half (n=31/66 or 47%) of guinea pigs with large TSTs had positive histology. The mean total disease score in these 31 animals was 17.8 (st. dev: 8.9; range 1 – 34). Figure 5 shows the distribution of disease severity in all 66 guinea pigs with large TSTs. In contrast, only 4/178 guinea pigs (2.2%) with small TSTs had positive histology. Two of these 4 animals had TST reversion and the other two had persistently small TSTs. Given the low prevalence of disease among guinea pigs with small TSTs, comparison of disease severity between TST reverter and TST non-reverter subgroups was not feasible. Additionally, we found that only 2/88 (2.2%) guinea pigs that never responded to PPD had positive histology. Both of these animals had 0 mm TSTs on all tests. The mean total disease score for all guinea pigs within each TST group is shown in Table 2.
Figure 5.
Disease Severity Among Guinea Pigs with Large TSTs.
Table 2.
Mean total disease score in guinea pigs of each major TST category.
| Guinea Pig Category |
n (with evaluable pathology) |
Mean (st. dev) Total Disease Score |
|---|---|---|
| Non reaction | 88 | 0.14 (0.91) |
| Small reaction (persistently positive) | 62 | 0.55 (3.03) |
| Small reaction (reverted) | 51 | 0.10 (0.70) |
| Large reaction (overall) | 66 | 8.35 (10.82) |
| Large reaction (received steroids) | 32 | 9.09 (10.89) |
| Large reaction (no steroids) | 34 | 7.65 (10.87) |
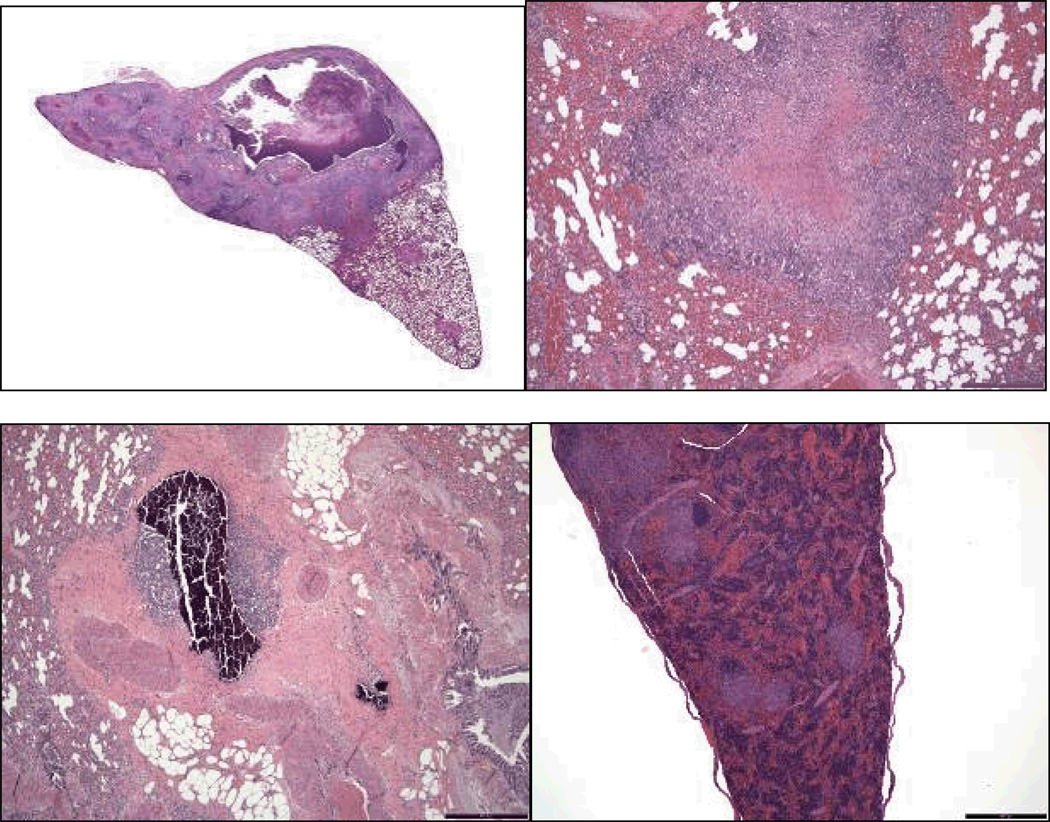
Representative photomicrographs of the range and types of lesions seen in the infected animals are shown in Figure 6. Panel A shows pulmonary cavitation in a guinea pig that had developed a 13 mm TST after 1 month exposure and showed a 20 mm TST on TST 5. This guinea pig did not receive steroids. Only 2 other guinea pigs exhibited cavities in the lungs. Panel B shows a well organized pulmonary granuloma with central caseous necrosis. This lesion was found in a guinea pig that first developed a 9 mm skin test reaction 2 months after the start of exposure (on TST 3), maintained skin test reactivity on all subsequent skin tests, and had a large final skin test reaction measuring 22 mm by TST 5. Despite a 28 week interval between initial skin test conversion and necropsy, this guinea pig remained clinically healthy and had limited progression of TB by histology, with disease confined to the lungs and a hilar lymph node. Panel C demonstrates evidence of lesion mineralization in an area occupied by a pulmonary granuloma in a guinea pig with large skin test reactions (15 and 17 mm) that first developed late in the exposure (TST 4). Like the guinea pig represented in Panel B, the animal represented in Panel C also had limited disease progression and no evidence of extrapulmonary dissemination, despite 24 weeks between TST conversion and necropsy. Panel D represents a guinea pig with evidence of disseminated disease found in multiple organs (lungs, lymph nodes, spleen, liver), with the photomicrograph showing multiple granulomata in the spleen at low magnification. This guinea pig remained skin test non-reactive until the last test (TST 5), at which point it had a 20 mm reaction. It received steroids before it was euthanized.
Figure 6.
Panel A (top left): Low magnification view of lung tissue from a guinea pig with cavity formation (1.3×, H&E). Panel B (top right): Low magnification view of lung tissue from a guinea pig with limited disease progression (4×, H&E). Panel C (bottom left): Low magnification view of mineralization within a granuloma from a guinea pig with minimal disease progression (4×, H&E). Panel D (bottom right): low magnification view of multiple granulomata seen in splenic section from a guinea pig (4×, H&E).
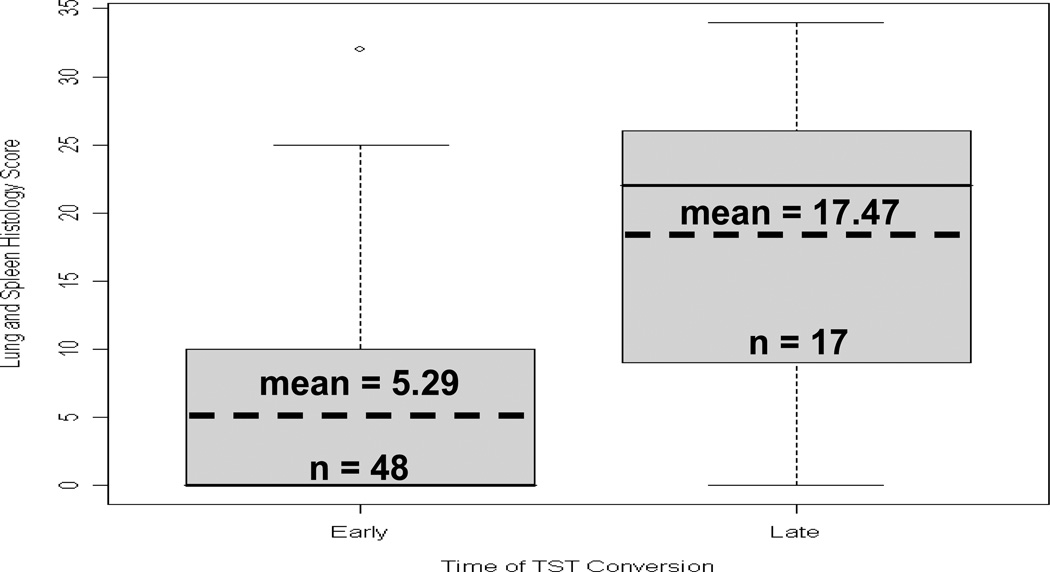
We further stratified guinea pigs that exhibited large reactions on TST 5 based on whether they had developed their first positive TST early (by TST 2 or 3) versus late (TST 4 or 5) in relation to the start of exposure period and compared the pathology scores between these two groups. As shown in Figure 8, mean disease severity was much lower in guinea pigs with early TST conversions (5.29 vs. 17.47, p<0.001).
Figure 8.
Box plot of Disease Score Among Large Reactors on TST 5 grouped by whether GP first developed its positive TST early or late in the study. Sample means are significantly different (p<0.001).
Effect of corticosteroid administration
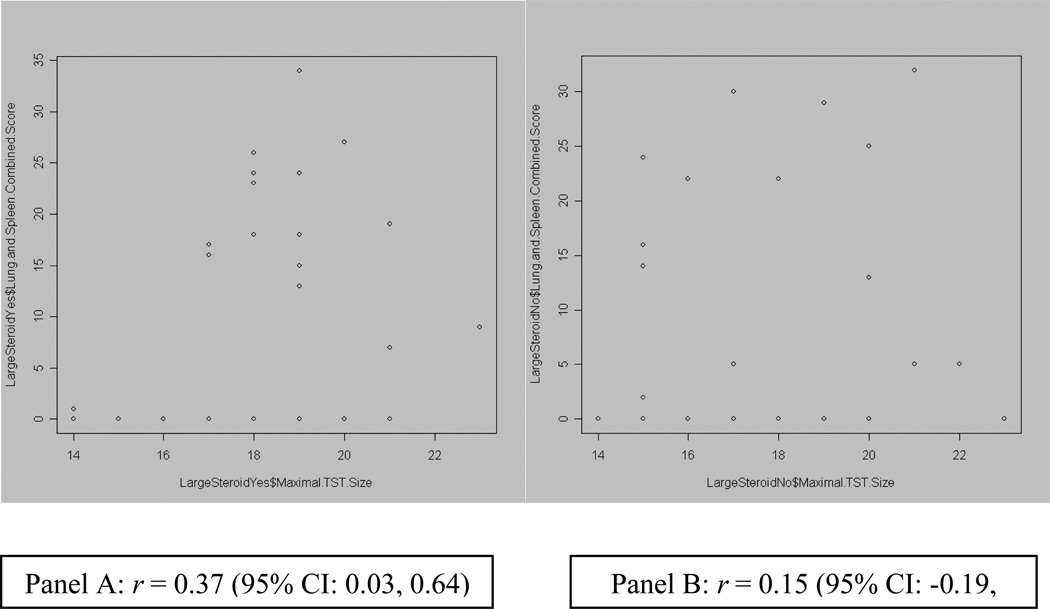
There was no correlation between the pathological score and the size of TST reactions when results were analyzed in aggregate for all guinea pigs. Moreover, correlation between TST reaction size and histologic disease severity in guinea pigs that had received steroids was very weak (r = 0.37, p = 0.03; Fig.7). Neither total disease scores nor the prevalence of any specific disease feature differed significantly between guinea pigs that had received steroids and those that had not (mean total disease score 18.2 vs. 13.8, respectively, p = 0.176; data on specific disease features not shown).
Figure 7.
Plots of correlation between TST diameter and Disease Score, among GPs with large TSTs. Panel A (left) shows relationship in GPs that received steroids. Panel B (right) shows relationship in GPs that did not receive steroids.
Harvesting and genotyping of isolates
Of the 26 human subjects and 275 (75%) guinea pigs infected in the study, only 13 patients’ M. tuberculosis isolates and only 18 guinea pigs’ M. tuberculosis isolates were available for second-line drug susceptibility testing and genotyping. Among the 13 human subject isolates, 8 different spoligotype patterns were identified. Only two spoligotypes from human sources identically matched types recovered from the 18 guinea pigs euthanized for signs of TB and immediately cultured. Five of 18 guinea pigs harbored both spoligotypes. The remaining 6 spoligotypes found among the 13 isolates from human subjects were either not transmitted to the guinea pigs or, if they were transmitted, failed to be isolated from the frozen guinea pig tissues. Environmental mycobacteria were not cultured from any guinea pig tissues. The culture yield from the guinea pigs that developed TB and had become ill and were immediately cultured was high (18/19; 95%), whereas the culture yield from the guinea pigs with histologic evidence of TB that had remained generally healthy during the study and whose organs were frozen and later cultured was low (8/34;24%).
DISCUSSION
The results of this study show that in the uniformly highly susceptible guinea pig model of TB, the large majority of animals exposed over 4 months to air exhausted from an inpatient ward housing patients with MDR/XDR-TB became skin test positive, indicating infection with M. tuberculosis. However, only a minority of these animals went on to develop active disease based on histopathology and/or clinical signs. TST responses were also diverse and included reversions and recurrence of positive reactions over the four month period of exposure and were not well correlated with histopathology or clinical signs of disease.
Strong evidence that TST reactions to comparable PPD formulations correlate well with M. tuberculosis infection in naturally exposed guinea pigs comes from the recent and older literature, and is further confirmed by the suppression of positive skin test responses in guinea pigs exposed to ultraviolet irradiated air from inpatient TB wards 20, 23–25. Skin test reactions after natural exposure require microbial replication in the host and are not caused by inhalation of non-viable organisms 23. Moreover, in our own and previous studies, repeat skin testing among control, uninfected guinea pigs does not in itself cause TST reactions.
Our observations on transmission of TB by our patient cohort to the guinea pigs are consistent with several potential hypotheses regarding natural transmission of M/XDR-TB. First, these strains were likely to have been sufficiently virulent to induce delayed-type hypersensitivity in a high proportion of exposed guinea pigs, but limited in their capacity to establish infections leading to progressive disease. These findings stand in contrast to studies of guinea pigs exposed to clinical MDR isolates in laboratory aerosol chamber studies, in which the exposure leads to progressive, disseminated, fatal disease that is typically non-cavitary 11. Second, when progression from infection to active disease occurred in the current study, it may have been the result of re-infection and/or the presence of particularly fit, virulent, or sensitizing strains within the cohort. As noted earlier, a few guinea pigs developed pulmonary cavities, which is unusual in this animal model. The wide range of disease severity in the guinea pig cohort, even after accounting for time since TST conversion, underscores the heterogeneity in virulence of strains passed from patients to guinea pigs and contradicts the notion that drug-resistant strains uniformly lose virulence 26, 27. Our data highlight the need to better understand the host, pathogen, and environmental factors driving MDR-TB propagation in the context of natural transmission.
Of note, guinea pigs also exhibited diverse TST kinetics despite continuous exposure. As seen in Figures 3A and 3B, the initial single mode around 10 mm for TST 2 evolved into a bimodal distribution by TST 5, with some TST reactions resolving and even reverting to zero mm while other reactions increased to give a second mode at 19 mm. About 21% of the exposed guinea pigs developed large TST reactions, indicating either sustained expansion of bacterial burden and/or infection with highly sensitizing strains. Of those animals with ≥ 14 mm TST induration on TST 5, 67% had developed their first positive TST (≥ 6 mm) reaction on TST 2 or 3, rather than on TST 4 or 5. However, despite earlier TST conversion, increase in TST induration diameter over time, and longer opportunity for disease progression from time of initial TST conversion, disease burden was lower in this group than in guinea pigs that developed their first positive skin test reaction on TST 4 or 5. This paradoxical observation may be evidence of a transient state of infection in this exposure model, but is also compatible with the transmission of less virulent strains by patients occupying the ward early in the study, transmission of strains capable of evoking immunity without disease progression, or possibly transmission of strains capable of immunizing some guinea pigs against subsequent disease progression after re-infection, resulting in less pathology but a durable TST response. Indeed, soon after the introduction of INH, Hobby demonstrated immunization due to non-progressive infection with an INH-resistant M. tuberculosis strain in the guinea pig model comparable to that produced by BCG 28. Histology from guinea pigs infected with MDR strains in the current study showed evidence of healed lesions, limited progression, and examples of spontaneous cure, and these observations were more frequent in the guinea pigs whose skin test reactions developed early and evolved into large sized reactions. Further research is needed to clarify whether there are any protective correlates to durable TST responses in our model.
Twenty-eight percent of the guinea pigs with 6–13 mm reactions reverted their skin tests and did not develop a subsequent positive skin test when tested. TST reversion may indicate contraction of immunity as the initial infection resolved, with no induction of a memory immune response needed to sustain TST reactivity 29. In Riley’s guinea pig exposure experiment, 6–13 mm TST reactions without necrosis also correlated poorly with findings on pathology 13. The older literature also suggests that some drug-resistant strains, especially catalase-negative INH resistant strains, might be of sufficiently low virulence to spontaneously cure with healing of early pathological lesions in the guinea pig model 30, 31. The observation that 28% of guinea pigs with small diameter TST reactions lost skin test reactivity on subsequent testing provides compelling evidence that at least some of the MDR/XDR strains transmitted by the patients were of limited virulence and may have led to only transient infection, and is further corroborated by the failure of these strains to either establish disease in a uniformly highly susceptible animal model of TB or lead to progressive disease even when the guinea pigs were immunosuppressed with steroids. As with TST reactivity, the pathogenic course in each guinea pig likely depended on the virulence of the initial infecting inoculum and the number and virulence of subsequent re-infecting organisms. Smith considered re-infection an essential pathogenic pathway under conditions in which either host populations had acquired immunity from prior exposure to TB, BCG, environmental mycobacteria, or where infecting strains had lost virulence 32.
In interpreting these findings, it is important to contrast this natural exposure model with the conventional laboratory guinea pig exposure model. In the conventional model, a precise aerosol with a uniform isolate is delivered under negative pressure within a Madison or Henderson chamber, and all animals become evenly infected and develop active disease. This is important for practical reasons, such as vaccine or drug testing 12, 33–35. In the alternative “natural” exposure model presented here, only a minority of infected animals developed active disease, a situation akin to natural infection in humans. It is likely that natural exposure as reported here has uncovered microbial defects in virulence and transmission obscured by conventional laboratory exposure models. If highly and uniformly susceptible animals like guinea pigs can spontaneously survive and clear infection by some M. tuberculosis strains, as suggested by our observations, it is also possible that protection by vaccines candidates might be apparent that are otherwise obscured by laboratory chamber exposures. In addition, the guinea pigs in this experimental model were infected by organisms transmitted directly from patients, not by organisms cultured under artificial conditions. Recent microarray data on M. tuberculosis organisms obtained from fresh sputum suggest that most are metabolically inactive and fat-laden, possibly an adaptation to the rigors of airborne transport and implantation in a new host 36. Both infectivity and virulence might be altered when cultured organisms are used.
The experiments here were based on the classical studies of Riley and his colleagues, who exposed guinea pigs to human source M. tuberculosis under similar natural exposure conditions 13, 25, 37, 38. In those studies larger TST sizes correlated well with the extent of disease, but only among guinea pigs with skin reactions associated with necrosis, probably a result of the cruder tuberculin reagents used at that time. A more recent study in Peru used a similar approach to ours, albeit with a substantially different patient mix 20. In that study, guinea pig exposure to patients lasted over 500 days and higher proportions of TST positive animals with active disease were noted, perhaps attributable to the virulence of strains transmitted. However, even in that study, examples of guinea pigs with evidence of infection but without progression to disease were seen. We sampled tissues from all guinea pigs with TST reversions to establish a more consistent relationship between reversion, transient infection, and absence of disease. We also immunosuppressed half the guinea pigs and were still unable to demonstrate recrudescence of disease, further reinforcing the notion that infections from some of the MDR/XDR strains were transient or of limited virulence.
We are aware of several important limitations regarding this experimental design. Unlike controlled chamber experiments, we cannot be sure of the exact time and duration of infection, the infecting dose, the exact frequency of re-infection, or how exposure varied over time 39. Moreover, lower rates of TST conversions over successive months could have been due to less infectious patients, less virulent or sensitizing strains, or a reduction in the number of fully susceptible guinea pigs from continuous exposure. In addition, our ability to isolate M. tuberculosis from guinea pigs with histologic evidence for TB was only possible in those animals whose organ homogenates were cultured at the time of death. The yield was substantially lower in histology positive guinea pigs whose organs had been frozen, suggesting loss of viability of the isolates responsible for those cases of TB, even in some guinea pigs with extensive histopathology.
Despite these limitations, under the exposure conditions described, which we believe closely resemble natural transmission, we observed that a very high percentage of guinea pigs developed specific tuberculin hypersensitivity--a rate higher than previously seen in earlier natural exposure experiments 13. Secondly, animals with 6–13 mm TST reactions were much more likely to revert, but some then became positive for a second time, suggesting re-infection. Additional evidence of re-infection was seen in the finding of mixed M. tuberculosis genotypes among several diseased guinea pigs. Thirdly, in contrast to chamber studies, the majority of sensitized animals failed to progress to active disease or yield cultivable organisms, even after high dose corticosteroid immunosuppression. This critical finding strongly suggests spontaneous sterilization of the infection by host immunity. A state of latency or low-grade persistent infection in some guinea pigs (not showing reversion) is also possible, but unlikely given the absence of significantly worse disease in guinea pigs that received high-dose steroids in this study. These observations not only highlight the complexity of TST reactivity and disease progression after MDR/XDR TB exposure, but also demonstrate that repeated exposure and re-infection may be an important factor driving the development of active disease. A natural infection model of MDR/XDR TB using a uniformly highly susceptible host like the guinea pig has great potential to provide new information on the propagation of TB in endemic settings
Funding and Acknowledgments
This AIR Facility was established through grants from USAID, US Centers for Disease Control and Prevention, National Institute of Occupational Safety and Health (R01 OH009050, EN), South African Medical Research Council, Center for Scientific and Industrial Research (South Africa), and Harvard University, with cooperation from the Mpumalanga Provincial Department of Health in South Africa and staff from the eMahlahleni (formerly known as Witbank) Hospital. Additional contributions were made by Brigham and Women’s Hospital. Research was also supported by grant AI070456 from the NIAID, NIH. The funders had no role in the study design, the collection, analysis, or interpretation of data, or in the writing of the manuscript. The establishment of the AIR Facility was largely due to early efforts of Dr. Bernard Fourie and Dr. Charles Wells. Dr. Lourens Roberts contributed importantly to the success of this experiment.
We would also like to thank and acknowledge Dr. E.M. Streicher and Dr. P. van Helden of Stellenbosch University, South Africa for performing genetic fingerprinting on samples in this study. We are grateful to Dr. R. Warren and Dr. P. van Helden for their suggestions on earlier versions of this manuscript. We would also like to acknowledge Dr. G. Palanisamy for his assistance in evaluating guinea pig histology specimens.
Contributor Information
Ashwin S. Dharmadhikari, Email: adharmadhikari@partners.org.
Randall J. Basaraba, Email: randall.basaraba@colostate.edu.
Martie L. Van Der Walt, Email: mvdwalt@mrc.ac.za.
Karin Weyer, Email: weyerk@who.int.
Melvin W. First, Email: mfirst@hsph.harvard.edu.
Sydney Parsons, Email: saparsons@csir.co.za.
David N. McMurray, Email: mcmurray@medicine.tamhsc.edu.
Ian M. Orme, Email: ian.orme@colostate.edu.
Edward A. Nardell, Email: enardell@pih.org.
REFERENCES
- 1.Gandhi NR, Moll A, Sturm AW, Pawinski R, Govender T, Lalloo U, Zeller K, Andrews J, Friedland G. Extensively drug-resistant tuberculosis as a cause of death in patients co-infected with tuberculosis and HIV in a rural area of South Africa. Lancet. 2006;368:1575–1580. doi: 10.1016/S0140-6736(06)69573-1. [DOI] [PubMed] [Google Scholar]
- 2.World Health Organization. WHO Report 2010: Multidrug and extensively drug-resistant TB (M/XDR-TB): 2010 Global Report on Surveillance and Response. (Accessed at http://www.who.int/tb/publications/2010/en/index.html.
- 3.Gelmanova IY, Keshavjee S, Golubchikova VT, Berezina VI, Strelis AK, Yanova GV, Atwood S, Murray M. Barriers to successful tuberculosis treatment in Tomsk, Russian Federation: non-adherence, default and the acquisition of multidrug resistance. Bull World Health Organ. 2007;85:703–711. doi: 10.2471/BLT.06.038331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nodieva A, Jansone I, Broka L, Pole I, Skenders G, Baumanis V. Recent nosocomial transmission and genotypes of multidrug-resistant Mycobacterium tuberculosis. Int J Tuberc Lung Dis. 2010;14:427–433. [PubMed] [Google Scholar]
- 5.Dye C, Williams BG, Espinal MA, Raviglione MC. Erasing the world's slow stain: strategies to beat multidrug-resistant tuberculosis. Science. 2002;295:2042–2046. doi: 10.1126/science.1063814. [DOI] [PubMed] [Google Scholar]
- 6.Cohen T, Sommers B, Murray M. The effect of drug resistance on the fitness of Mycobacterium tuberculosis. The Lancet infectious diseases. 2003;3:13–21. doi: 10.1016/s1473-3099(03)00483-3. [DOI] [PubMed] [Google Scholar]
- 7.Dye C, Williams BG. The population dynamics and control of tuberculosis. Science. 2010;328:856–861. doi: 10.1126/science.1185449. [DOI] [PubMed] [Google Scholar]
- 8.McMurray DN. Guinea Pig Model of Tuberculosis. In: Bloom BR, editor. Tuberculosis: Pathogenesis, Protection and Control. American Society for Microbiology; 1994. pp. 135–147. [Google Scholar]
- 9.Dharmadhikari AS, Nardell EA. What Animal Models Teach Humans about Tuberculosis. Am J Respir Cell Mol Biol. 2008 doi: 10.1165/rcmb.2008-0154TR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.McMurray DN. Hematogenous reseeding of the lung in low-dose, aerosol-infected guinea pigs: unique features of the host-pathogen interface in secondary tubercles. Tuberculosis (Edinb) 2003;83:131–134. doi: 10.1016/s1472-9792(02)00079-3. [DOI] [PubMed] [Google Scholar]
- 11.Palanisamy GS, DuTeau N, Eisenach KD, Cave DM, Theus SA, Kreiswirth BN, Basaraba RJ, Orme IM. Clinical strains of Mycobacterium tuberculosis display a wide range of virulence in guinea pigs. Tuberculosis (Edinb) 2009;89:203–209. doi: 10.1016/j.tube.2009.01.005. [DOI] [PubMed] [Google Scholar]
- 12.McMurray DN. Disease model: pulmonary tuberculosis. Trends Mol Med. 2001;7:135–137. doi: 10.1016/s1471-4914(00)01901-8. [DOI] [PubMed] [Google Scholar]
- 13.Mills CC, O'Grady F, Riley RL. Tuberculin conversion in the "naturally infected" guinea pig. Bull Johns Hopkins Hosp. 1960;106:36–45. [PubMed] [Google Scholar]
- 14.Riley RL, Mills CC, Nyka W, Weinstock N, Storey PB, Sultan LU, Riley MC, Wells WF. Aerial dissemination of pulmonary tuberculosis. A two-year study of contagion in a tuberculosis ward. American Journal of Hygiene. 1959;70:2. doi: 10.1093/oxfordjournals.aje.a117542. [DOI] [PubMed] [Google Scholar]
- 15.Rusch-Gerdes S, Pfyffer GE, Casal M, Chadwick M, Siddiqi S. Multicenter laboratory validation of the BACTEC MGIT 960 technique for testing susceptibilities of Mycobacterium tuberculosis to classical second-line drugs and newer antimicrobials. J Clin Microbiol. 2006;44:688–692. doi: 10.1128/JCM.44.3.688-692.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kruuner A, Yates MD, Drobniewski FA. Evaluation of MGIT 960-based antimicrobial testing and determination of critical concentrations of first- and second-line antimicrobial drugs with drug-resistant clinical strains of Mycobacterium tuberculosis. J Clin Microbiol. 2006;44:811–818. doi: 10.1128/JCM.44.3.811-818.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kamerbeek J, Schouls L, Kolk A, van Agterveld M, van Soolingen D, Kuijper S, Bunschoten A, Molhuizen H, Shaw R, Goyal M, van Embden J. Simultaneous detection and strain differentiation of Mycobacterium tuberculosis for diagnosis and epidemiology. J Clin Microbiol. 1997;35:907–914. doi: 10.1128/jcm.35.4.907-914.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Weyer K, Lancaster J, Van der Walt M and the DOTSPlus Study group of South Africa. DOTSPlus for multidrug-resistant (MDR) tuberculosis in South Africa: Results from the first cohort of patients treated with a standardised regimen under tuberculosis control programme conditions. 33rd World Conference on Lung Health of the International Union Against Tuberculosis and Lung Disease (IUATLD) Montreal, Canada, 6–10 October 2002, International Journal of Tuberculosis and Lung Disease. 6(10) Suppl 1:S138. [Google Scholar]
- 19.Perla D. Experimental Epidemiology of Tuberculosis : The Elimination of Tubercle Bacilli in the Feces, Bile, and Urine of Infected Guinea Pigs. J Exp Med. 1927;45:1025–1035. doi: 10.1084/jem.45.6.1025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Escombe AR, Oeser C, Gilman RH, Navincopa M, Ticona E, Martinez C, Caviedes L, Sheen P, Gonzalez A, Noakes C, Moore DA, Friedland JS, Evans CA. The detection of airborne transmission of tuberculosis from HIV-infected patients, using an in vivo air sampling model. Clin Infect Dis. 2007;44:1349–1357. doi: 10.1086/515397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Basaraba RJ, Dailey DD, McFarland CT, Shanley CA, Smith EE, McMurray DN, Orme IM. Lymphadenitis as a major element of disease in the guinea pig model of tuberculosis. Tuberculosis (Edinb) 2006;86:386–394. doi: 10.1016/j.tube.2005.11.003. [DOI] [PubMed] [Google Scholar]
- 22.Turner OC, Basaraba RJ, Orme IM. Immunopathogenesis of pulmonary granulomas in the guinea pig after infection with Mycobacterium tuberculosis. Infect Immun. 2003;71:864–871. doi: 10.1128/IAI.71.2.864-871.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Escombe AR, Moore DA, Gilman RH, Navincopa M, Ticona E, Mitchell B, Noakes C, Martinez C, Sheen P, Ramirez R, Quino W, Gonzalez A, Friedland JS, Evans CA. Upper-room ultraviolet light and negative air ionization to prevent tuberculosis transmission. PLoS Med. 2009;6:e43. doi: 10.1371/journal.pmed.1000043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Escombe AR, Moore DA, Gilman RH, Pan W, Navincopa M, Ticona E, Martinez C, Caviedes L, Sheen P, Gonzalez A, Noakes CJ, Friedland JS, Evans CA. The Infectiousness of Tuberculosis Patients Coinfected with HIV. PLoS Med. 2008;5:e188. doi: 10.1371/journal.pmed.0050188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Riley RL, Mills CC, O'Grady F, Sultan LU, Wittstadt F, Shivpuri DN. Infectiousness of air from a tuberculosis ward. Ultraviolet irradiation of infected air: comparative infectiousness of different patients. Am Rev Respir Dis. 1962;85:511–525. doi: 10.1164/arrd.1962.85.4.511. [DOI] [PubMed] [Google Scholar]
- 26.Borrell S, Gagneux S. Infectiousness, reproductive fitness and evolution of drug-resistant Mycobacterium tuberculosis. Int J Tuberc Lung Dis. 2009;13:1456–1466. [PubMed] [Google Scholar]
- 27.Gagneux S. Fitness cost of drug resistance in Mycobacterium tuberculosis. Clin Microbiol Infect. 2009;15(Suppl 1):66–68. doi: 10.1111/j.1469-0691.2008.02685.x. [DOI] [PubMed] [Google Scholar]
- 28.Hobby GL, Lenert TF, Auerbach O. The immunizing properties of an isoniazid-resistant mutant of the Vallee strain of M. tuberculosis as compared with BCG; observations in the mouse and guinea pig. Am Rev Tuberc. 1954;70:527–530. doi: 10.1164/art.1954.70.3.527. [DOI] [PubMed] [Google Scholar]
- 29.Barry CE, 3rd, Boshoff HI, Dartois V, Dick T, Ehrt S, Flynn J, Schnappinger D, Wilkinson RJ, Young D. The spectrum of latent tuberculosis: rethinking the biology and intervention strategies. Nat Rev Microbiol. 2009;7:845–855. doi: 10.1038/nrmicro2236. doi: nrmicro2236 [pii] 10.1038/nrmicro2236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Middlebrook G, Cohn ML. Some observations on the pathogenicity of isoniazid-resistant variants of tubercle bacilli. Science. 1953;118:297–299. doi: 10.1126/science.118.3063.297. [DOI] [PubMed] [Google Scholar]
- 31.Cohn ML, Kovitz C, Oda U, Middlebrook G. Studies on isoniazid and tubercle bacilli. II. The growth requirements, catalase activities, and pathogenic properties of isoniazid-resistant mutants. Am Rev Tuberc. 1954;70:641–664. doi: 10.1164/art.1954.70.4.641. [DOI] [PubMed] [Google Scholar]
- 32.Smith DW, Wiegeshaus EH. What animal models can teach us about the pathogenesis of tuberculosis in humans. Rev Infect Dis. 1989;11(Suppl 2):S385–S393. doi: 10.1093/clinids/11.supplement_2.s385. [DOI] [PubMed] [Google Scholar]
- 33.McMurray DN. Determinants of vaccine-induced resistance in animal models of pulmonary tuberculosis. Scandinavian journal of infectious diseases. 2001;33:175–178. doi: 10.1080/00365540151060743. [DOI] [PubMed] [Google Scholar]
- 34.Williams A, Hall Y, Orme IM. Evaluation of new vaccines for tuberculosis in the guinea pig model. Tuberculosis (Edinb) 2009;89:389–397. doi: 10.1016/j.tube.2009.08.004. [DOI] [PubMed] [Google Scholar]
- 35.Ordway DJ, Shanley CA, Caraway ML, Orme EA, Bucy DS, Hascall-Dove L, Henao-Tamayo M, Harton MR, Shang S, Ackart D, Kraft SL, Lenaerts AJ, Basaraba RJ, Orme IM. Evaluation of standard chemotherapy in the guinea pig model of tuberculosis. Antimicrobial agents and chemotherapy. 54:1820–1833. doi: 10.1128/AAC.01521-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Garton NJ, Waddell SJ, Sherratt AL, Lee SM, Smith RJ, Senner C, Hinds J, Rajakumar K, Adegbola RA, Besra GS, Butcher PD, Barer MR. Cytological and transcript analyses reveal fat and lazy persister-like bacilli in tuberculous sputum. PLoS Med. 2008;5:e75. doi: 10.1371/journal.pmed.0050075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Riley RL. Aerial dissemination of pulmonary tuberculosis. American review of tuberculosis. 1957;76:931–941. doi: 10.1164/artpd.1957.76.6.931. [DOI] [PubMed] [Google Scholar]
- 38.Sultan L, Nyka W, Mills C, O'Grady F, Wells W, Riley RL. Tuberculosis disseminators. A study of the variability of aerial infectivity of tuberculous patients. The American review of respiratory disease. 1960;82:358–369. doi: 10.1164/arrd.1960.82.3.358. [DOI] [PubMed] [Google Scholar]
- 39.Nardell EA. Catching droplet nuclei: toward a better understanding of tuberculosis transmission. American journal of respiratory and critical care medicine. 2004;169:553–554. doi: 10.1164/rccm.2401003. [DOI] [PubMed] [Google Scholar]