Abstract
Although the number of mesenchymal stem cells (MSC) in the bone marrow is sufficient to maintain skeletal homeostasis, in osteopenic pathology, aggravated osteoclast activity or insufficient osteoblast numbers ensues, affecting normal bone remodeling. Most of the currently available therapies are anti-resorptive with limited osteogenic potential. Since mobilization of stem/progenitors from the BM is a prerequisite for their participation in tissue repair, amplification of endogenous stem cells may provide an alternative approach in these conditions. The present study determined the potential of MSC mobilization in vivo, using combinations of different growth factors with the CXCR4 antagonist, AMD3100, in a mouse model of segmental bone defect. Results indicated that among several factors tested IGF1 had maximum proliferative ability of MSC in vitro. Results of the in vivo studies indicated that the combination of IGF1 and AMD3100 provided significant augmentation of bone growth as determined by DXA, micro-CT and histomorphometry in mice bearing segmental fractures. Further, characterization of MSC isolated from mice treated with IGF1 and AMD3100 indicated Akt/PI3K, MEK1/2-Erk1/2 and smad2/3 as key signaling pathways mediating this effect. These data indicate the potential of in vivo stem cell mobilization as a novel alternative for bone healing.
Keywords: Fracture, stem cells, osteoinduction, mobilization
INTRODUCTION
Bone marrow (BM) is a source of stem and progenitor cells that participate in the regeneration of a variety of tissues following injury to organs, including the brain, liver, lung, kidney and heart. Generation of multilineage stem/progenitor cells from the bone marrow is a major response to such tissue repair [1-3]. Knowledge about the nature of signals released from the injured tissues to mobilize bone marrow-derived stem cells into the circulation is very limited, and precise molecular mechanisms governing stem cell fate, signals that control stem cell mobilization, their recruitment to the sites of engraftment and homing following release from their niches are extremely complex. Critical questions pertinent to studies in mesenchymal stem cell (MSC) trafficking include the following: a) Can host MSC be mobilized into peripheral blood? b) Can mobilized MSC home to sites of inflammation and target ischemic tissues? and c) Does mobilization of stem cells have therapeutic value for bone repair? Considerable evidence indicates that chemokines and/or cytokines that are upregulated during injury are released into the circulation from tissues, stimulating stem/progenitor cells to down-regulate adhesion molecules that retain them within their niches [4-6]. Cytokines and chemokines play critical roles in regulating mobilization, trafficking and homing of stem/progenitor cells. Many of these factors are chemo attractants. For example, stromal cell-derived factor (SDF) 1α and its receptor CXCR4 appear to play an important role in modulating MSC mobilization and over-expression of CXCR4 on MSC augmented myoangiogenesis in the infarcted myocardium [7]. Further, SDF1α/CXCR4 axis has been known to be critical for mobilization of progenitors/stem cells, such as the endothelial progenitor cells (EPC) [8,9], and inhibition of the SDF1α/CXCR4 interaction has been reported to partially block the homing of progenitors/stem cells to the ischemic myocardium [10].
Following stress, challenge or stimulation of the BM compartment, a portion of the stem/progenitor cells egress from the BM, circulate into peripheral blood and contribute to tissue repair. Recent studies have shown that chronic G-CSF therapy, combined with acute administration of a CXCR4 antagonist, synergistically enhances hematopoietic stem cells (HPC) mobilization from the bone marrow [11,12]. This combination therapy has been shown to be more effective when compared to G-CSF alone in phase III clinical trails of BM transplantation [13]. It has been shown that administration of a CXCR4 antagonist alone increases the number of circulating EPC and improves tissue perfusion following ischemia in animal models [14,15], and there is evidence that BM-derived MSC contribute to tissue regeneration, suggesting that these cells may also be mobilized in response to tissue injury. MSC have multilineage potential for differentiation hence may be used therapeutically to promote tissue regeneration. Thus, we hypothesized that systemic mobilization and proliferation of the stem/progenitor cells would be effective in bone healing and growth, especially in situations of ineffective bone formation such as in non-union fractures. In the present study, we tested the effectiveness of combinations of growth factors with AMD3100 in modulating stem cell peripheral mobilization and proliferation in a mouse tibial fracture model and determined that treatment with IGF1 and AMD3100 not only effectively mobilized progenitors into peripheral circulation but also resulted in improved bone growth. Further, characterization of possible mechanisms indicated that this effect was pronounced mainly through Akt, Erk and Smad pathways.
MATERIAL AND METHODS
Cells and Reagents
Mesenchymal stem cells were isolated from mouse bone marrow and maintained in Mesenchymal stem cell expansion medium (Sigma Aldrich, St. Louis, MO) supplemented with 10% newborn calf serum as described [16]. The Akt, total Akt, Phosho smad2/3, beta actin, P70, EGFR, Jak3, and cadherin antibodies were purchased from Cell signaling (Cell Signaling Inc, Danvers, MA). The CXCR4 antibody was purchased from e-biosciences (San Diego, CA). Purified recombinant IGF1, SCF, PDGF and VEGF were purchased from Prospec (Rehovot, Israel).
Creation of segmental defect in mouse tibia
All animal protocols were performed by approved guidelines of the Institutional Animal Care and Use Committee. Ten to twelve-week-old C57BL/6 mice were used for creating segmental defect. Briefly, mice were anesthetized with an isofluorane and oxygen mixture and transferred onto a heating pad (maintained at 37°C) in the operating field. Right tibiae of mice were fractured using a three point bending apparatus and a 2-3 mm-long segmental defect was created [17]. The fracture was stabilized with external pins and surgical sutures and tapes. Buprenorphine (0.05 mg/kg) was injected intraperitonially once before surgery and Carprofen (5 mg/kg) subcutaneously once before the surgery and repeated 24 hours later as a post-operative analgesia. Animals had free access to food and water and were monitored daily in the post operative period for any complications or abnormal behavior.
Growth factor treatments in vivo
In the initial experiment to test the potential of peripheral mobilization of various cytokines, mice were injected with PBS, IGF1 (2.5 μg/mouse, i.p.), SCF (2.5 μg/mouse, i.p), PDGF (2.5 μg/mouse, i.p) or VEGF (2.5 μg/mouse, i.p) for five consecutive days and on the 5th day, AMD3100 (5 mg/kg i.p.) was injected in a volume of 100 μl in PBS.
Colony Assay
Blood from all mice were collected in a heparin tube and cells were plated with mesencult medium supplemented with mesenchymal stem cell stimulatory factors (StemCell Technologies Inc, Vancuor, Canada), conditioned with mouse bone marrow cells for 48 hours and then filter sterilized (0.22 micron). The cells were allowed to grow for 10 days and numbers of adherent cell colonies were enumerated in a phase contrast microscope.
Faxitron imaging
Whole body X-ray imaging was performed in a Faxitron soft X-ray machine (Faxitron X-Ray LLC, 575 Bond St. Lincolnshire, IL 60069 USA) in UAB Small Animal Bone Phenotyping Core (SABPC). Briefly, mice were anesthetized with isofluorane and imaging was performed for 2-10 seconds to confirm the fractures in the tibia.
Dual-Energy X-ray Absorptiometry (DXA) analysis of bone
For DXA analysis, animals were briefly anesthetized with isoflurane (2%) and oxygen mixture and placed in a prostrate position on the imaging plate. Bone mineral density (BMD), bone mineral content (BMC) and other body composition were assessed using the GE-Lunar PIXImus, software version 1.45.
Micro-CT analysis
For determination of 3D architecture of the trabecular and cortical bones, mice were sacrificed, and tibiae were harvested and analyzed in an advanced micro-computed tomography instrument (μCT 40, Scanco Medical AG) at UAB SABPC. Two scans were obtained on each tibia around the fracture site. A three-dimensional reconstruction of the images was done with the region of interest consisting of trabecular and cortical areas. Histomorphometric parameters including bone volume, trabecular connectivity, trabecular thickness, trabecular separation and degree of anisotropy were evaluated.
Cell proliferation assay
Bone marrow from the fractured tibia was isolated from 5 animals from each group after 2 weeks of therapy with PBS, IGF1, AMD3100 or IGF1+AMD3100. Marrow contents from tibia were flushed and cultured for a week. MSC, obtained after 2 weeks of culture, were seeded in 96-well plates (Falcon 3072, Becton-Dickinson, Lincoln Park, NJ) at a density of 5000 cells per well and incubated at 37°C in MSC medium, supplemented with 10% FBS (Sigma, Mesenchymal stem cell expansion medium (Cat # 1569-1L, St. Louis, MO) for 4 days. Cell growth was measured by using a non-radioactive cell proliferation assay kit (CellTiter 96RAqueous from PROMEGA, Madison, WI). On days 2, 4 and 6, optical density was determined at 490 nm in a multi-well plate reader (BioTek Synergy 2, Vermont). Background absorbance of the medium in the absence of cells was subtracted. All samples were assayed in triplicate, and the mean for each experiment was calculated.
Cell migration assay
Transwell filters were coated on the underside of inserts with 20 μg/ml of fibronectin overnight at 4°C and air dried before the cells were seeded. Bone marrow mononuclear cells were isolated from PBS, IGF1, AMD3100 or IGF1+AMD3100 injected groups after 2 weeks of injections and cells were washed twice with FBS-free medium. Then, 0.5 ml cells in FBS free medium was added to the top of the insert and 1 ml of stem cell growth expansion medium with 10% FBS and 10 μg/ml collagen I was added to the lower chamber. The transwell filter inserts were placed into the lower chamber and incubated overnight at 37°C. Extra cells from the upper side of the filter were removed by scrubbing with a cotton-tipped swab, moistened with medium, and cells were stained with crystal violet followed by wash with distilled water.
Western blot
To identify possible major signaling pathways, MSC were isolated 24 hours after creation of fracture and cultured in serum-free stem cell medium in the absence or presence of IGF1 for 2 days. The cells were harvested and lysates containing equal amount of protein were separated in SDS-PAGE and transferred to PVDF membrane. Western blotting of the membrane was performed using antibodies for AKT, phosphor-AKT, SMAD, phosphor-SMAD, ERK, Phospho-ERK, CXCR4, p70, EGFR, cadherin and beta actin.
Histology
Formalin-fixed tissues were decalcified in EDTA solution for two weeks and embedded in paraffin. Longitudinal sections of 5 μm thicknesses were cut from paraffin embedded blocks of frontal sections of tibia, using a Leica 2265 microtome. Sections were then stained with hematoxylin and eosin for microscopic examination.
Lineage differentiation of cultured mouse MSC
Osteoblast differentiation was induced with culture medium containing 10% FBS, 0.1 μM dexamethazone, 2 mM β-glycerophosphate, and 150 μM ascorbate-2-phosphate.16 Cells were seeded at 10,000 cell/cm2 and incubated for 21 days at 37°C. Medium was changed every 3 days. Adipogenic differentiation was induced by culturing in medium with 20% FBS, 1 μM dexamethazone, 0.35 μM hydrocortisone, 0.5 mM isobutyl-methylxanthine (IBMX), 100 ng/ml insulin, and 60 μM indomethacin.16 Cells were seeded at 20,000 cells/cm2 and incubated for 21 days at 37°C. Medium was changed every alternate day. For evaluation of mineralized matrix, the cell layer was fixed in 10% buffered-formalin, then stained by von Kossa stain using 5% (w/v) silver nitrate (Sigma) under ultraviolet light for 30 min, followed by 5% (w/v) sodium thiosulphate (Sigma) for 2 min. For Oil red-O staining, cells were fixed in formalin and stained for 1 h with oil red-O (Sigma).
Statistical analysis
All data are reported as mean ± standard deviation (SD). Bone mineral density (BMD) and bone mineral contents (BMC) were analyzed using ANOVA. Comparison of differences between two variables was performed using the two-tailed, two-sample with equal variances, independent t-test. Results were con considered significant when p<0.05.
RESULTS
Progenitor cells egress from bone marrow in response to growth factor and CXCR4 antagonist (AMD3100)
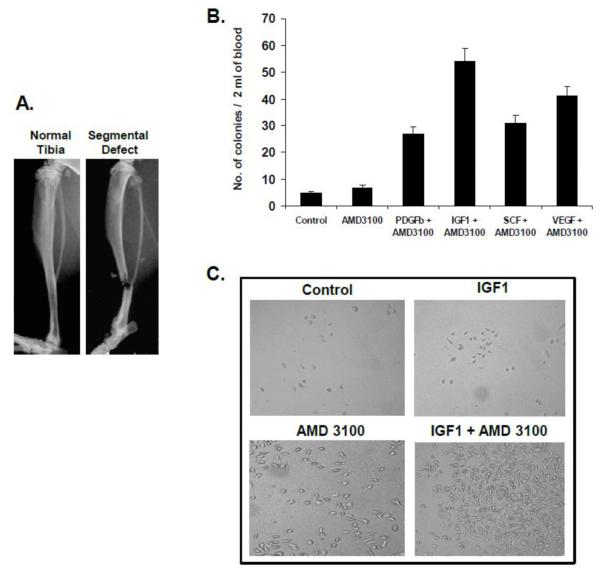
To examine the potential of growth factors in stem/progenitor cell mobilization with CXCR4 antagonist AMD3100, first, cohorts of mice were injected with PBS, IGF1, SCF, PDGF or VEGF for five consecutive days and on the fifth day ADM3100 was administered. Peripheral blood mononuclear cells were obtained for colony assay to enumerate MSC mobilization. Results of this preliminary screening for the mobilization efficiency performed indicated an increased number of colony-forming MSC in the peripheral blood after injection of all compounds in a tibia fracture mouse model (Figure 1A). However, the number and size of the colonies were highest in IGF1 plus AMD3100 injected mice compared to PDGF, SCF and VEGF treated groups, in combination with AMD3100 (Figure1B & 1C; p<0.05). Hence, subsequent studies were focused on the effectiveness of IGF1 plus AMD3100 in bone remodeling following segmental defect in the tibia.
Figure 1. Colony assay from the peripheral blood after treatment with growth factors and AMD3100.
Following the creation of segmental bone fracture (A), cohorts of mice were administered with PBS, IGF1, PDGF, SCF, or VEGF for five consecutive days and AMD3100 on the 5th day. Peripheral blood mononuclear cells were collected on day 7. The cells were cultured in mesencult medium supplemented with mesenchymal stem cell stimulatory factors (Stem Cell Technologies Inc. Vancouver, Canada) for 10 days and the number of colonies were enumerated (B). The size of the colonies was determined using an inverted microscope (C).
Treatment with IGF1 and AMD3100 results in augmented bone growth in a mouse segmental defect model
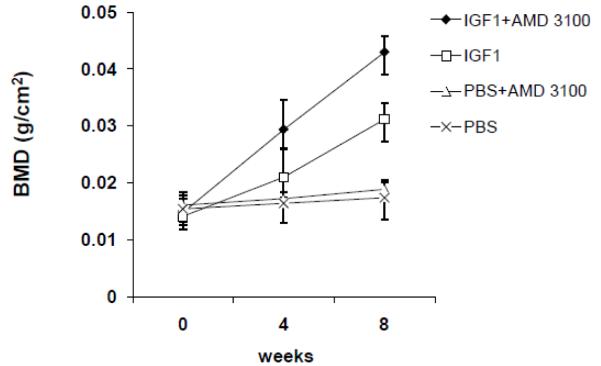
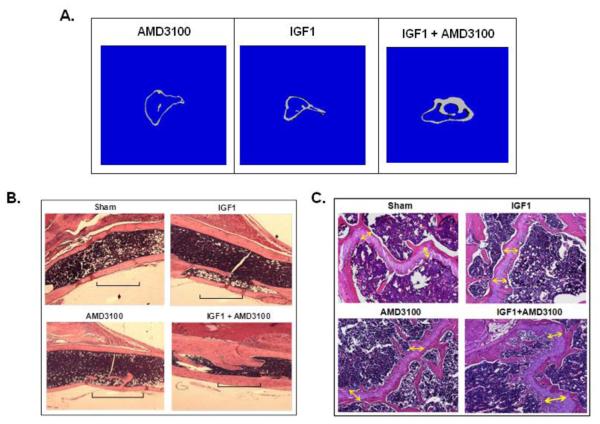
Therapeutic benefit of growth factor pretreatment and mobilization in vivo by injection of IGF1 and AMD3100 compounds were tested in a mouse segmental defect. Results indicated augmented bone growth in the IGF1+AMD3100 injected group. Data shown in Figure 2 on BMD, analyzed by DXA, around the fracture area indicated a significant enhancement in BMD in the IGF1+AMD3100 group compared to untreated or those treated with either IGF1 or AMD3100 (p<0.05). However, there was also a modest improvement in bone growth with IGF1 treatment alone compared to the AMD3100 or PBS treated group. The combination therapy with IGF1 and AMD3100 also resulted in an increase in the bone mineral content (BMC) as determined by DXA. The difference in total bone growth was also corroborated by micro-CT (Figure 3A), and hematoxylin and eosin (H&E) staining both around the fractured area (Figure 3B) and around the growth plate (Figure 3C). Morphologically, there were differences in the gross structure of bone marrow as treatment with IGF1 alone increased the number of adipocytes in the bone marrow. AMD3100 treatment did not show a significant change in bone marrow cellularity or overall bone marrow cell number but total BMC was highest in the IGF1+AMD3100 treated group (data not shown). Histological analysis of isolated bone sections revealed improved fracture healing and higher bone growth in IGF1+AMD3100 treated mice, as shown in Figures 3B and 3C, with increased cellular activity and involvement of bone forming cells around the fracture healing area (Figure 3B) as well as in the growth plate (Figure 3C).
Figure 2. Bone mineral density (BMD) in the fracture area following treatment with growth factors and AMD3100.
Segmental defect was created in the right tibia of eight-week-old mice and indicated compounds were injected for five consecutive days. Total bone mineral density (BMD) was determined around the fracture area by non-invasive dual energy X-ray absorptiometry (DXA) from the beginning of the experiment and thereafter on weeks 4 and 8. Briefly, mice were anesthetized with isofluorene for DXA analysis. DXA was done in GE Lunar PIXImus, Madison, WI, and data analysis was done with PIXImus software version 1.43.020.
Figure 3. Micro-CT and histomorphometry of fractured tibia following treatment with IGF1 and AMD3100.
Tibia from PBS, IGF1, AMD3100 and IGF1+AMD3100 injected group of mice were used for μCT analysis of cortical bone and trabecular bones. Representative images from indicated groups show three-dimensional images of tibia, extracted from reconstructed bone volume at the fracture site, 8 weeks after treatments. For histological analysis, bones were isolated and fixed in 10% buffered formalin for 48 hrs and decalcified in 2.5% EDTA solution for 3 weeks. Bones were mounted in a paraffin block and 5 μm sections were stained with hematoxylin and eosin (H&E) for microscopic analysis. Representative images from indicated groups shows hematoxylin and eosin (H&E) stained images of fractured tibia after the treatment (B), and bone growth around the growth plate of fractured tibia (C).
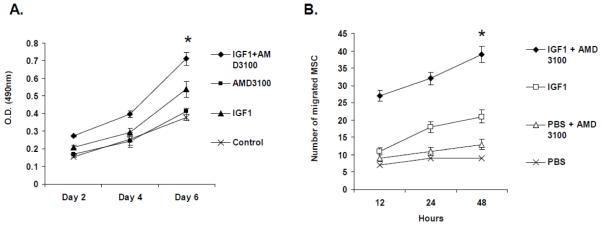
IGF1 and AMD3100 treatment increased proliferation and migration of isolated MSC
To investigate the direct effect of MSC mobilization following IGF1 treatment on fracture healing, fractures were created in the tibia of mice and peripheral blood was isolated from three mice in each of the groups following PBS, IGF1, AMD3100 and IGF1+AMD3100 treatments. Ten thousand cells were seeded and cultured in vitro using conditioned stem cell expansion media for up to six days and proliferation index of MSC was determined by MTT assay. Results of the study, shown in Figure 4A, indicated an increase in proliferative capacity of MSC after combined treatment with IGF1 and AMD3100 (p<0.05). Migration assay of cultured MSC in a Boyden chamber assay also corroborated the results of proliferation assay combination therapy with IGF1+AMD3100, as shown in Figure 4B.
Figure 4. Proliferation and migration assay to analyze the effects of IGF1 and AMD3100 on MSC.
Bone marrow mononuclear cells were isolated from cohorts of mice from each group (PBS, IGF1, AMD3100 and IGF1+AMD3100) and cultured using mesenchymal stem cell expansion medium. Cell proliferation assay was performed using a non-radioactive MTT assay kit (A). Replicates of MSC, treated similarly were further subjected to Boyden chamber assay to analyze their migratory response (B).
PI3K/Akt, MEK1/2-Erk1/2 and smad2/3 as possible, major signaling pathways involved in MSC-mediated bone growth after IGF1 and AMD3100 treatment
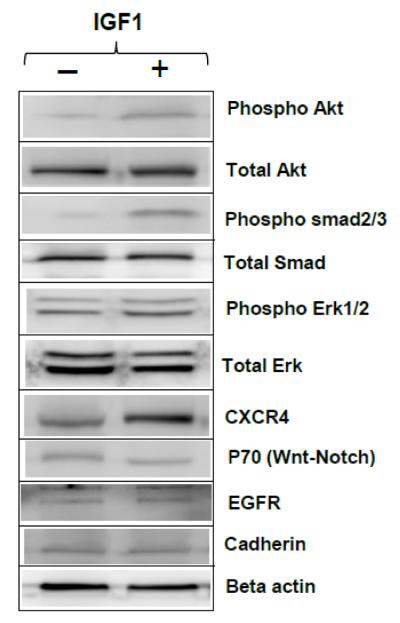
In order to identify the effect of IGF1 treatment on MSC and key signaling pathways through which the effects are mediated during fracture healing, MSC were isolated from the tibia 24 hrs after creating a fracture and cultured for 2 days in serum-free, stem cell expansion medium with or without IGF1 and cell lysates were subjected to Western blot analysis using specific antibodies. Results of this study, shown in Figure 5, indicated elevated phospho-Akt, phospho-Erk1/2 and phospho-smad2/3, signifying as likely pathways involved in the increased fracture healing response and augmented bone growth, while no significant changes were observed in p70, EGFR or cadherin levels.
Figure 5. Western blot analysis to identify key signaling events in MSC following IGF1 treatment.
Mesenchymal stem cells were isolated from fractured tibia and grown for 2 days with 1 ng/ml of recombinant IGF1 or without any additives in mesenchymal stem cell expansion medium and cell lysates were analyzed by Western blotting with indicated antibodies.
Effect of IGF1 and AMD3100 on the number of progenitor cells and their osteogenic, adipogenic differentiation
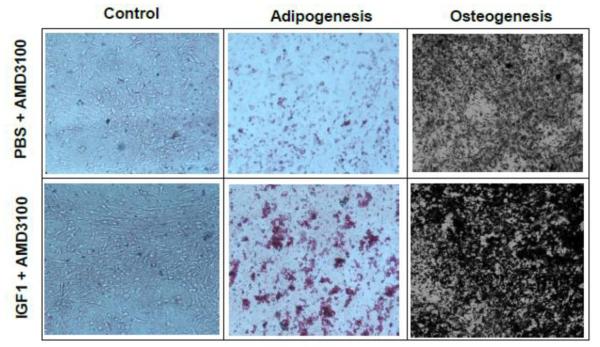
To test the possibility that IGF1 and AMD3100 might have altered the number of progenitor cells in the treated animals, isolated mononuclear cells from PBS+AMD3100 and IGF1+AMD3100 groups were subjected to colony assays and their differentiation potential was analyzed in osteoblast and adipocyte differentiation assays. Results of this study, shown in Figure 6, indicated a relatively higher number of oil red O-positive cells and significantly more mineralized von kossa positive cells in IGF1+AMD3100 group, combined with a greater number of colonies (data not shown) implying enhanced proliferative, osteogenic and adipogenic differentiation of MSC following mobilization.
Figure 6. Lineage differentiation of MSC following IGF1 and AMD3100 treatment.
MSC, isolated from bone marrow of sham or IGF1+AMD3100 treatment for 5 days were cultured in vitro in adipogenic or osteogenic media for 21 days to assess multilineage differentiation capability of isolated MSC from experimental mice. The cells were fixed and stained with von Kossa stain and oil red-O stain for osteoblast and adipocyte differentiation, respectively.
DISCUSSION
The widespread increase in life expectancy in the U.S. and an increase in the incidence of bone-related complications suggest the need for new skeletal reconstruction strategies. In the present study, we hypothesized that cytokines that have the potential to induce bone stem/progenitor cell proliferation may be used to augment bone healing through in vivo mobilization and expansion. Several reported evidences indicate that bone marrow harbors various primitive cells that possess the ability to repair non-hematopoietic organs [18,19]. Moreover, the identification of bone marrow-derived cells in various injured tissues, including brain, liver, kidney, lung, and heart, indicates that tissue injury can induce mobilization of cells from the bone marrow into the peripheral blood [20-25]. However, the mobilization of pluripotent stem cells to repair bone fracture has never been reported.
Despite the potential of MSC in regenerative medicine, in particular for bone remodeling, ex vivo manipulation and therapy using MSC have indicated serious limitations including the number of cells that could be infused without causing entrapment in the lungs, poor bone homing of transplanted cells and possible tumorigenesis [26-28].Thus, a major goal of this study was to enhance fracture repair through endogenous stem cell mobilization, whereby damaged or lost cells are replaced by cells with functional characteristic and organization similar to those represented before injury, alleviating the aforementioned limitations. Following initial analysis of IGF1, PDGF, SCF and VEGF for their influence on in vitro MSC proliferation, which indicated highest proliferative potential of IGF1, we chose to study the effect of IGF1 in detail.
Insulin-like growth factors are known mediators of skeletal growth and bone formation [29-31]. Studies have shown that IGF1 can promote the differentiation of bone cells in autocrine and paracrine fashion [32,33]. In an aged rat model, it was demonstrated that IGF1 can stimulate osteogenesis by regulating the marrow-derived osteoblasts [34-36], and also IGF1 has been shown to have similar osteogenic effects on human adipose-derived MSC [37]. Previous studies have demonstrated that expression of IGF1 decreases with age and senescence [37-39]. Given the complexity of bone formation and the involvement of several growth factors in the regulation of bone forming cells, it is reasonable to speculate that increasing the number of available progenitor cells for bone repair through endogenous mobilization and proliferation might increase the recruitment of progenitors, and the influence of IGF1 and/or other growth factors on these progenitors will have pro-osteoblastic microenvironment at the site of fracture site for augmented bone growth. In this study, we found that IGF1 treatment increased the bone marrow stem/progenitor cells in the bone marrow, which might have enabled more progenitors to home to the fracture site and participate in bone growth. This may be one of the possibilities because the results suggest that MSC isolated from experimental mice had higher migration capability and in the in vitro assay also showed higher CXCR4 receptor expression, which may translate into increased homing to required site. Further, an increase in osteogenesis in the growth plate may have resulted from an overall enhancement of the progenitor cells following treatment with AMD3100 and IGF1.
Observations of the present study also imply that enhancing the mobilization of endogenous progenitor cells via cytokine or growth factor administration may be utilized therapeutically to promote bone repair. The observed therapy effects of IGF1 and AMD3100 combination may have also been influenced through improved vasculogenesis/angiogenesis around the fracture site from mobilization of hematopoietic stem cells (HPC) and endothelial progenitor cells (EPC). Data from preclinical models have shown that inhibition of CXCR4/SDF1 interaction plays an important role in enhanced progenitor cell mobilization [40]. In the present study, IGF1 treatment was initiated prior to AMD3100 to ensue sufficient stem/progenitor cell proliferation before mobilization. This may have also altered the profile of progenitor cells and leukocytes in the blood, similar to the effects of GCSF treatment, which mobilizes both HPC and neutrophils by reducing their expression of CXCR4 and decreasing levels of SDF1α within the bone marrow [41,42]. HPC, mobilized by GCSF have been found to be exclusively in the G0/G1 phase of cell cycle, whereas HPC remaining in bone marrow are actively cycling [43-45]. This may be explained by the fact that proliferating HPC cannot migrate, a necessary step in their mobilization from bone marrow. Also, studies have demonstrated that mobilization of progenitor cell subset is differentially regulated by growth factors that affect their retention and cell cycle status [39]. Immediately following fracture, many cellular signals are released and IGF1 growth factor treatment at that time might help increase the pool of mobilizable progenitor cells in their niche.
IGF1 is among a number of well-characterized growth factors that promotes cell survival and regulation of bone biology. Characterization of MSC following treatment with IGF1 and AMD3100, which indicated elevated PI3k/Akt, suggests its effects on MSC proliferation [46,47]. PI3k-driven signaling, recruitment of GRB2/SOS by phosphorylated IRS1 or SHC leads to the recruitment of Ras and activation of MEK/ERK/RAF-1 pathway and downstream nuclear factors, resulting in the induction of cell proliferation [47]. Thus, the coordinated influence of IGF through PI3k appears to be highly effective with IGF1. The extent of SHC/GBR2 binding also appears to correlate with elevated levels of IGF1-activated ERK and c-fos transcription [48]. Although there appears to be a role for PI3 kinase/AKT in the observed effects, it remains to be studied further to precisely determine whether these two pathways alone are involved.
Collectively, the present study suggests the potential of combining MSC dissociation- and proliferation-inducing compounds in increasing the bone formation by enrichment of the stem and progenitor cells endogenously. Further development of this strategy should verify the long-term fate of mobilized MSC prior to clinical application.
Highlights.
The manuscript describes a novel method to enrich endogenous mesenchymal stem cells from the bone marrow for fracture healing
Results of this study demonstrate the therapeutic potential this approach in an immunocompetent mouse model of bone fracture
Molecular analysis signaling pathways indicated a key role for Akt/PI3K, MEK1/2-Erk1/2 and smad2/3 activation effecting the stem cell mobilization
Method described in this study has suggests its implication for non-union fractures in humans, which are difficult to treat
ACKNOWLEDGEMENTS
Financial support of the National Institutes of Health grants R01AR50251, R01CA133737, R01AR560948, P30 AR046031 and the U.S. Army Department of Defense grants BC101411, BC044440 and PC050949 is gratefully acknowledged.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
DISCLOSURE
All authors state that they have no conflict of interest.
REFERENCES
- [1].Nolta JA, editor. Science. Springer; 2006. Gene therapy for mesenchymal stem cells. [Google Scholar]
- [2].Orlic D, Kaistura J, Chimenti S, Bodine DM, Leri A, Anversa P. Bone marrow stem cells regenerate infarcted myocardium. Nature. 2001;410:710–705. doi: 10.1038/35070587. [DOI] [PubMed] [Google Scholar]
- [3].Takahashi T, Kalka C, Masuda H, Chen D, Silver M, Kearney M, et al. Ischemia- and cytokine-induced mobilization of bone marrow-derived endothelial progenitor cells for neurovascularization. Nat Med. 1999;5:434–438. doi: 10.1038/7434. [DOI] [PubMed] [Google Scholar]
- [4].Cottler-Fox MH, Lapidot T, Petit I, Kollet O, DiPersio JF, Link D, et al. Stem Cell Mobilization. Hematology. 2003;1:419–437. doi: 10.1182/asheducation-2003.1.419. [DOI] [PubMed] [Google Scholar]
- [5].Kucia M, Reca R, Miekus K, Wanzeck J, Woiakowski W, Janowska-Wieczorek A, et al. Trafficking of normal stem cells and metastasis of cancer stem cells involve similar mechanisms: pivotal role of the SDF-1-CXCR4 axis. Stem Cells. 2005;23:879–894. doi: 10.1634/stemcells.2004-0342. [DOI] [PubMed] [Google Scholar]
- [6].Chavakis E, Urbic C, Dimmeler S. Homing and engraftment of progenitor cells: A prerequisite for cell therapy. Journal of Molecular and Cellular Cardiology. 2008;45:514–522. doi: 10.1016/j.yjmcc.2008.01.004. [DOI] [PubMed] [Google Scholar]
- [7].Zhang D, Fan GC, Zhou X, Zhao T, Pasha Z, Xu M, et al. Over-expression of CXCR4 on Mesenchymal stem cells augments myoangiogenesis in the infarcted myocardium. J Mol Cell Cardiol. 2008;44:281–292. doi: 10.1016/j.yjmcc.2007.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Gallagher KA, Liu ZJ, Xiao M, Chen H, Goldstein LJ, Buerk DG, et al. Diabetic impairments in NO-mediated endothelial progenitor cell mobilization and homing are reversed by hyperoxia and SDF-1 alpha. J Clin Invest. 2007;117:1249–1259. doi: 10.1172/JCI29710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Liu ZJ, Velazquez OC. Hyperoxia, endothelial progenitor cell mobilization, and diabetic wound healing. Antioxid Redox Signal. 2008;10:1869–1882. doi: 10.1089/ars.2008.2121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Abbott JD, Huang Y, Liu D, Hickey R, Krause DS, Giordano FJ. Stromal cell-derived factor-1 alpha plays a critical role in stem cell recruitment to the heart after myocardial infarction but is not sufficient to induce homing in the absence of injury. Circulation. 2004;110:3300–3305. doi: 10.1161/01.CIR.0000147780.30124.CF. [DOI] [PubMed] [Google Scholar]
- [11].Broxmeyer HE, Orschell CM, Clapp DW, Hangoc G, Cooper S, Plett PA, et al. Rapid mobilization of murine and human hematopoietic stem and progenitor cells with AMD3100, a CXCR4 antagonist. J Exp Med. 2005;201:1307–1318. doi: 10.1084/jem.20041385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Martin C, Bridger GJ, Rankin SM. Structural analogues of AMD3100 mobilize haematopoietic progenitor cells from bone marrow in vivo according to their ability to inhibit CXCL12 binding to CXCR4 in vitro. Br J Haematol. 2006;134:326–329. doi: 10.1111/j.1365-2141.2006.06181.x. [DOI] [PubMed] [Google Scholar]
- [13].Calandra G, McCarty J, McGuirk J, Tricot G, Crocker SA, Badel K, et al. AMD3100 plus G-CSF can successfully mobilize CD34+ cells from non-Hodgkin’s lymphoma, Hodgkin’s disease and multiple myeloma patients previously failing mobilization with chemotherapy and/or cytokine treatment: compassionate use data. Bone Marrow Transplant. 2008;41:331–338. doi: 10.1038/sj.bmt.1705908. [DOI] [PubMed] [Google Scholar]
- [14].Capoccia BJ, Shepherd RM, Link DC. G-CSF and AMD3100 mobilize monocytes into the blood that stimulate angiogenesis in vivo through a paracrine fashion. Blood. 2006;108:2438–2445. doi: 10.1182/blood-2006-04-013755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Shepherd RM, Capoccia BJ, Devine SM, Dipersio J, Trinkaus KM, Ingram D, et al. Angiogenic cells can be rapidly mobilized and efficiently harvested from the blood flowing treatment with AMD3100. Blood. 2006;108:3662–3667. doi: 10.1182/blood-2006-06-030577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Kumar S, Ponnazhagan S. Bone homing of mesenchymal stem cells by ectopic alpha 4 integrin expression. FASEB J. 2007;21:3917–3927. doi: 10.1096/fj.07-8275com. [DOI] [PubMed] [Google Scholar]
- [17].Kumar S, Wan C, Ramaswamy G, Clemens TL, Ponnazhagan S. Mesenchymal stem cells expressing osteogenic and angiogenic factors synergistically enhance bone formation in a mouse model of segmental bone defect. Mol Ther. 2010;18:1026–1034. doi: 10.1038/mt.2009.315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Orlic D, Kajstura J, Chimenti S, Limana F, Jakoniuk I, Quaini F, et al. Mobilized bone marrow cellsrepair the infarcted heart, improving function and survival. Proc Natl Acad Sci U S A. 2001;98:10344–9. doi: 10.1073/pnas.181177898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Shyu WC, Lin SZ, Yang HI, Tzeng YS, Pang CY, Yen PS, et al. Functional recovery of stroke rats induced by granulocyte colony-stimulating factor-stimulated stem cells. Circulation. 2004;110:1847–54. doi: 10.1161/01.CIR.0000142616.07367.66. [DOI] [PubMed] [Google Scholar]
- [20].Kollet O, Shivtiel S, Chen YQ, Suriawinata J, Thung SN, Dabeva MD, et al. HGF, SDF-1, and MMP-9 are involved in stress-induced human CD34+ stem cell recruitment to the liver. J Clin Invest. 2003;112:160–9. doi: 10.1172/JCI17902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Machalinski B, Paczkowska E, Koziarska D, Ratajczak MZ. Mobilization of human hematopoietic stem/progenitor-enriched CD34+ cells into peripheral blood during stress related to ischemic stroke. Folia Histochem Cytobiol. 2006;44:97–101. [PubMed] [Google Scholar]
- [22].De Silvestro G, Vicarioto M, Donadel C, Menegazzo M, Marson P, Corsini A. Mobilization of peripheral blood hematopoietic stem cells following liver resection surgery. Hepatogastroenterology. 2004;51:805–10. [PubMed] [Google Scholar]
- [23].Kale S, Karihaloo A, Clark PR, Kashgarian M, Krause DS, Cantley LG. Bone marrow stem cells contribute to repair of the ischemically injured renal tubule. J Clin Invest. 2003;112:42–9. doi: 10.1172/JCI17856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Rojas M, Xu J, Woods CR, Mora AL, Spears W, Roman J, et al. Bone marrow-derived mesenchymal stem cells in repair of the injured lung. Am J Respir Cell Mol Biol. 2005;33:145–52. doi: 10.1165/rcmb.2004-0330OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Jackson KA, Majka SM, Wang H, Pocius J, Hartley CJ, Majesky MW, et al. Regeneration of ischemic cardiac muscle and vascular endothelium by adult stem cells. J Clin Invest. 2001;107:1395–402. doi: 10.1172/JCI12150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Gao J, Dennis JE, Muzic RF, Lundberg M, Caplan AI. The dynamic in vivo distribution of bone marrow-derived mesenchymal stem cells after infusion. Cells Tissue Organs. 2001;69:12–20. doi: 10.1159/000047856. [DOI] [PubMed] [Google Scholar]
- [27].Zhou YF, Bosch-Marce M, Okuyama H, Krishnamachary B, Kimura H, Zhang L, et al. Spontaneous transformation of cultured mouse bone marrow-derived stromal cells. Cancer Res. 2006;66:10849–10854. doi: 10.1158/0008-5472.CAN-06-2146. [DOI] [PubMed] [Google Scholar]
- [28].Isgaard J, Nilsson A, Lindahl A, Jansson JO, Isaksson OG. Effects of local administration of GH and IGF-1 on longitudinal bone growth in rats. American Journal of Physiology. 1986;250:E367–E372. doi: 10.1152/ajpendo.1986.250.4.E367. [DOI] [PubMed] [Google Scholar]
- [29].Schlechter NL, Russell SM, Spencer EM, Nicoll CS. Evidence suggesting that the direct growth-promoting effect of growth hormone on cartilage in vivo is mediated by local production of somatomedin. PNAS. 1986;83:7932–7934. doi: 10.1073/pnas.83.20.7932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Spencer EM, Liu CC, Si EC, Howard GA. In vivo actions of insulin-like growth factor -I (IGF-I) on bone formation and resorption in rats. Bone. 1991;12:21–26. doi: 10.1016/8756-3282(91)90050-s. [DOI] [PubMed] [Google Scholar]
- [31].Canalis E. Effect of insulinlike growth factor I on DNA and protein synthesis in cultured rat calvaria. Journal of Clinical Investigation. 1980;66:708–719. doi: 10.1172/JCI109908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Hock JM, Centrella M, Canalis E. Insulin-like growth factor I has independent effects on bone matrix formation and cell replication. Endocrinology. 1988;122:254–260. doi: 10.1210/endo-122-1-254. [DOI] [PubMed] [Google Scholar]
- [33].Mochizuki H, Hakeda Y, Wakatsuki N, Usui N, Akashi S, Sato T, et al. Insulin-like growth factor-I supports formation and activation of osteoclasts. Endocrinology. 1992;131:1075–1080. doi: 10.1210/endo.131.3.1505451. [DOI] [PubMed] [Google Scholar]
- [34].Zhao G, Monier-Faugere MC, Langub MC, Geng Z, Nakayama T, Pike JW, et al. Targeted overexpression of insulin-like growth factor I to osteoblasts of transgenic mice: increased trabecular bonen volume without increased osteoblast proliferation. Endocrinology. 2000;141:2674–2682. doi: 10.1210/endo.141.7.7585. [DOI] [PubMed] [Google Scholar]
- [35].Zhang M, Xuan S, Bouxsein ML, von Stechow D, Akeno N, Faugere MC, et al. Osteoblast-specific knockout of the insulin-like growth factor (IGF) receptor gene reveals an essential role of IGF signaling in bone matrix mineralization. Journal of Biological Chemistry. 2002;277:44005–44012. doi: 10.1074/jbc.M208265200. [DOI] [PubMed] [Google Scholar]
- [36].Levi B, James AW, Wan DC, Glotzbach JP, Commons GW, Longaker MT. Regulation of human adipose-derived stromal cell osteogenic differentiation by insulin-like growth factor-1 and platelet-derived growth factor-alpha. Skeletal Tissue Engineering. 2010;126:41–52. doi: 10.1097/PRS.0b013e3181da8858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Rudman D, Kutner MH, Rogers CM, Lubin MF, Fleming GA, Bain RP. Impaired growth hormone secretion in the adult population: relation to age and adiposity. Journal of Clinical Investigation. 1981;67:1361–1369. doi: 10.1172/JCI110164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Florini JR, Prinz PN, Vitiello MV, Hintz RL. Somatomedin-C levels in healthy young and old men: relationship to peak and 24-hour integrated levels of growth hormone. Journal of Gerontology. 1985;40:2–7. doi: 10.1093/geronj/40.1.2. [DOI] [PubMed] [Google Scholar]
- [39].Pitchford C, Furze RC, Jones CP, Wengner AM, Rankin SM. Differential mobilization of subsets of progenitor cells from bone marrow. Cell Stem Cell. 2009;4:62–72. doi: 10.1016/j.stem.2008.10.017. [DOI] [PubMed] [Google Scholar]
- [40].Wengner AM, Pitchford SC, Furze RC, Rankin SM. The coordinated action of G-CSF and ELR+ CXC chemokines in neutrophil mobilization during acute inflammation. Blood. 2008;111:42–49. doi: 10.1182/blood-2007-07-099648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Lévesque JP, Hendy J, Takamatsu Y, Simmons PJ, Bendall LJ. Disruption of the CXCR4/CXCL12 chemotactic interaction during hematopoietic stem cell mobilization induced by G-CSF or cyclophosphamide. J Clin Invest. 2003;111:187–196. doi: 10.1172/JCI15994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Bowie MB, McKnight KD, Kent DG, McCaffrey L, Hoodless PA, Eaves CJ. Hematopoietic stem cells proliferate until after birth and show a reversible phase-specific engraftment defect. J Clin Invest. 2006;116:2808–2816. doi: 10.1172/JCI28310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Roberts AW, Metcalf D. Noncycling status of peripheral blood progenitor cells mobilized by granulocyte colony-stimulating factor and other cytokines. Blood. 1995;86:1600–1605. [PubMed] [Google Scholar]
- [44].Uchida N, He D, Friera AM, Reitsma M, Sasaki D, Chen B, et al. The unexpected G0/G1 cell cycle status of mobilized hematopoietic stem cells from peripheral blood. Blood. 1997;89:465–472. [PubMed] [Google Scholar]
- [45].Yao R, Cooper GM. Growth factor-dependent survival of rodent fibroblasts requires PI3k but is independent of p70 activity. Oncogene. 1996;13:343–351. [PubMed] [Google Scholar]
- [46].Dudek H, Datta SR, Franke TF, Birnbaum MJ, Yao R, Cooper GM, et al. Regulation of neuronal survival by the serine-threonine protein kinase Akt. Science. 1997;275:661–665. doi: 10.1126/science.275.5300.661. [DOI] [PubMed] [Google Scholar]
- [47].Giorgetti S, Ballotti R, Kowalski-Chauvel A, Tartare S, Van Obberghen E. The insulin and IGF-I receptor substrate IRS-1 associates with and activates PI3k in vitro. J of Biological Chemistry. 1993;268:7358–7364. [PubMed] [Google Scholar]
- [48].Grey A, Chen Q, Xu X, Callon K, Cornish J. Parallel PI3k and P42/44 mitogen-activated protein kinase signaling pathways subserve the mitogenic and antiapoptotic actions of IGF I in osteoblastic cells. Endocrinology. 2003;144:4886–4893. doi: 10.1210/en.2003-0350. [DOI] [PubMed] [Google Scholar]