Abstract
Increasing evidence suggests that cytokine dysregulation in T-helper 1 and T-helper 2 (Th1/Th2) subsets contributes to the pathogenesis of Crohn’s disease (CD). This pilot study examines the hypothesis that cytokine profiles differ between pediatric and adult CD patients. Production of Th1 cytokines interferon-gamma (IFN-γ) and tumor necrosis factor-alpha (TNF-α) and of Th2 cytokines interleukin-4 (IL-4) and interleukin-6 (IL-6) were analyzed in peripheral blood of patients with CD and healthy controls (n=20) using flow cytometry after in vitro stimulation. In both pediatric and adult subjects, frequencies of TNF-α CD4+ T cells were higher in CD patients than in controls (p=0.009, p=0.047, respectively). Percentages of cells expressing IL-4 were slightly increased (p=0.036) while those for IFN-γ were decreased (p=0.009) in pediatric CD patients compared with controls. As expected, the overall production of Th1 cytokines was higher in adults compared with pediatric subjects. When memory CD4+CD45RO+ T cells were considered, lower IFN-γ expression was observed in pediatric CD subjects compared with controls (p=0.009), matching the trend seen in the general CD4+ T cell population. The percentage of CD4+CD45RO+ T cells were increased in adult CD patients compared with pediatric CD patients (p=0.016). This study describes a peripheral blood Th1/Th2 cytokine imbalance in CD and suggests different immunological mechanisms between children and adults in disease pathogenesis.
Keywords: Inflammatory Bowel Disease, Th1/Th2 cytokines, flow cytometry, children, adults
INTRODUCTION
Accumulating evidence suggests that a dysregulation in cytokines produced by T-helper (Th) cells is involved in the etiology and progression of inflammatory bowel disease (IBD).1 However, the underlying immunologic correlates of disease pathophysiology remain poorly understood. The two main subclasses of Th cells, T-helper 1 (Th1) and T-helper 2 (Th2) cells, secrete subsets of cytokines that mediate various immunological responses.2 Th1 cells secrete the cytokines IL-2, IFN-γ, and TNF-α to augment an immune response against intracellular pathogens such as viruses. In contrast, Th2 cells secrete IL-4, IL-5, IL-10, and IL-13,3 mainly to protect the host against extracellular pathogens and promote the synthesis of IgE antibodies in B-cells.4 IL-4 is a signature Th2 cytokine responsible for the development of naïve Th cells into a Th2 functional phenotype. IL-6 is a pleiotropic cytokine responsible for T cell stimulation and proliferation5 and is primarily secreted by macrophages and monocytes promoting granulocyte and macrophage colony formation.6
Traditionally, Crohn’s Disease (CD) has been considered a Th1-mediated disease. In many previous studies, cytokine profiles of CD patients displayed Th1 dominance characterized by higher than normal percentages of IFN-γ and/or lower or undetectable percentages of cells expressing IL-4 in peripheral blood mononuclear cells (PBMC) or intestinal mucosa.2,7–12 Higher TNF-α has also been reported in the intestinal mucosa and serum of CD patients compared with healthy controls.13–16 A shift towards the Th1 profile may contribute to the onset of CD by activating macrophages which produce pro-inflammatory cytokines thereby leading to the intestinal damage seen in CD.17 Recent therapies have targeted these cytokines for CD management, as exemplified by the administration of anti-TNF-α depleting antibodies.18
The role of CD4+CD45RO+ T cells in disease progression has been described in CD patients.19,20 In the mature human immune system, circulating CD4+ T cells can be separated into naïve and memory T cells by their expression of CD45. Naïve T cells express CD45RA and show little or no ability to produce effector cytokines while circulating between the peripheral blood and secondary lymphoid organs. In contrast, memory T cells express CD45RO and, when activated, proliferate in an antigen specific manner.19,21,22 CD4+CD45RO+ T cells are known for their ability to secrete large quantities of cytokines and their tendency to accumulate at sites of inflammation (e.g., luminal surfaces in IBD). Due to these characteristics, it is reasonable to propose that the activity of CD4+CD45RO+ T cells is implicated in the pathogenesis of CD. However, few studies have investigated the association between CD and cytokine production of CD4+CD45RO+ T cells. During childhood the immune system’s repertoire is expanded by exposure to antigens. This constant immune expansion may account for some of the differences in cytokine profiles observed between children and adults.23 Therefore, we explored the hypothesis that age- and disease-related cytokine profiles in children differ from adults with CD.
Our prior investigation of cytokine profiles in pediatric CD patients suggested that lower Th1 cytokine levels were present in newly-diagnosed pediatric children with CD compared with control subjects.24 In the current pilot study, we aimed to validate this finding and to expand the spectrum of cytokines and age of CD patients and controls to include adults. The intracellular production of IFN-γ, TNF-α, IL-4, and IL-6, cytokines that have been associated with various auto-inflammatory conditions including IBD, were analyzed in whole blood by flow cytometry.
MATERIALS AND METHODS
Study Population and Sample Collection
This study was conducted in collaboration between the School of Public Health, University of California, Berkeley (UCB), and the Departments of Pediatrics and Medicine, University of California, San Francisco (UCSF). Written informed consent was taken from the subjects of legal age or their parents/legal guardians, and assent was obtained from younger subjects in accordance with the protocol approved by the Committee on Human Research at UCSF, and the Committee for the Protection of Human Subjects at UCB.
Ten children and 10 adults were enrolled at UCSF from July 2008 to May 2009. Five subjects in each age group diagnosed with CD and an equal number of healthy controls were enrolled. All subjects were approached as they presented for endoscopic procedures, and pediatric subjects and controls were matched for age. Inclusion criteria for controls were no preexisting acute or chronic illnesses or inflammatory disorders. At the time of enrollment, most CD patients had some symptoms and others were asymptomatic. Body Mass Index (BMI; weight [kg]/height [m]2) was determined by medical record review or patient report. Laboratory analyses included measurement of red blood cell (RBC) folate levels and hematocrit. Samples were obtained from all subjects in a fasting state. Peripheral blood samples were kept at cool temperature with ice packs during transfer and delivered to the laboratory at UCB for processing within 4 hours of collection.
Cell Culture
Whole blood samples were diluted 1:1 with culture media (RPMI 1640) in a 12 x 75 mm fluorescence-activated cell sorting (FACS) tubes. Phorbol-12-myristate 13-acetate (PMA; 2.5 ng/mL; Sigma Chemical Co., St. Louis, MO) and ionomycin (1 μg/mL, Sigma Chemical) were added for lymphocyte activation. Cultures were incubated in the presence of brefeldin-A (10 ng/mL, Sigma Chemical Co.), a transport inhibitor that prevents cytokine release from cells, at 37°C and 5% CO2 for four hours.
Following the incubation, 200μL of whole blood was pipetted into a 12 x 75 mm FACS tube containing monoclonal antibodies specific for T-helper cell surface antigen CD4 (CD4-PerCP, Becton Dickinson, San Diego, CA). For CD4+CD45RO+ T-cells and their cytokine measurement, incubated blood samples were added to tubes containing monoclonal antibodies for CD4 (CD4-FITC, Becton Dickinson) and monoclonal antibodies for CD45RO (CD45RO-PE-Cy 5, Becton Dickinson). After a 10-minute incubation at room temperature in the dark, 1% paraformaldehyde (PFA) was added to stabilize the monoclonal antibody-surface antigen complex for five minutes. RBCs present in the mixture were then lysed using 3mL of 1x lysing solution (Becton Dickinson) for 8 minutes. Following centrifugation at 1,930 rpm for 5 minutes, the supernatant was aspirated, with 1x permeabilizing solution added to the pellet, and incubated for 10 minutes at room temperature in the dark. The mixture was then washed using 3mL of wash buffer (1% bovine serum albumin, 0.1% NaN3, 1x PBS) and centrifuged for five minutes at 2,370 rpm. After aspirating the supernatant, 20μL of Th1 and Th2 cytokine-specific antibodies (IFN-γ-FITC/ IL-4-PE, Becton Dickinson; TNF-α-FITC/IL-6-PE, Becton Dickinson) were added to the experimental tubes and the appropriate isotype control monoclonal antibodies (20μL, IgG2/IgG1, Becton Dickinson) were added to the control tubes and incubated for 30 minutes at room temperature in the dark. To identify cytokine-producing CD4+CD45RO+ cells, the samples were stained with 20μL of monoclonal antibodies specific for each cytokine (IFN-γ −PE ; IL-4- PE; TNF-α −PE; IL-6-PE, Becton Dickinson) in separate tubes and appropriate isotype control antibodies were used. Subsequently, the tubes were washed with 3ml of the buffer for three minutes and centrifuged at 2,520 rpm for five minutes. Finally, after aspirating the supernatant, the cells were resuspended in 500μL of 1% PFA and stored at 4°C until flow cytometry analysis.
Cytokine Level Detection by Flow Cytometry
The stained cells were detected by Becton-Coulter EPICS XL flow cytometer as previously described and illustrated24 to calculate the percentage of Th1 and Th2 positive cells from CD4+ and CD4+CD45RO+ cells.
CD4+ Cells
For each sample, control tubes containing fluorochrome-equivalent IgG2/IgG1 isotype controls were run to detect non-specific binding, followed by runs of the experimental tubes containing IFN-γ/IL-4 and TNF-α/IL-6 specific antibodies. During each run of the sample, 5,000 CD4+ cells were counted. The lymphocyte population was marked by placing a circular gate around the dense aggregation of lymphocytes on the forward-scatter/side-scatter (FS/SS) histogram based on the size and granularity of the cells. One-plot histogram was used to identify CD4+ cells by placing a linear gate on the right peak of the histogram. The percentage of CD4+ cells was calculated as the number of CD4+ cells over the total lymphocyte population. Th1 cells and Th2 cells were identified by using a two-plot histogram, where the IFN-γ or TNF- α positive cells were found in the lower right quadrant, and the IL-4 or IL-6 positive cells were found in the upper left quadrant. The percentage of Th1 positive cells were determined by dividing the number of IFN-γ or TNF- α positive cells by CD4 positive cells, and the percentage of Th2 positive cells were determined by dividing the number of IL-4 or IL-6 positive cells by CD4 positive cells.
CD4+CD45RO+ Cells
For each sample, isotype-matched FITC, PE-Cy5 and PE-labeled monoclonal antibodies were used as controls. Upon gating for the lymphocyte population in the FF/SS histogram, two-plot histogram was used to select 5,000 CD4+CD45RO+ cells in the upper-right quadrant. One-plot histogram was used to identify IFN-γ, IL-4, TNF-α, and IL-6 positive cells in separate tubes. The percentage of CD4+CD45RO+ cells was calculated as the number of CD4+CD45RO+ cells over the total lymphocyte population. The percentage of cytokine-producing cells was determined by dividing the number of IFN-γ, IL-4, TNF-α, or IL-6 cells by the number of CD4+CD45RO+ cells. Flow cytometry results are presented as percentages of cells.
Statistical Analysis
Statistical analyses were performed using STATA 11.0 (StataCorp. College Station, TX). Data were analyzed using Mann-Whitney and Kruskal Wallis tests to compare CD with non-CD subjects and adults versus children. Due to the small sample size, data are expressed as both mean with standard deviation and median with range. Values of p<0.05 were considered statistically significant.
RESULTS
Demographic characteristics and clinical parameters of the study participants are shown in Tables 1 and 2. The median age of adult CD patients was slightly lower compared with healthy controls, but the difference was not statistically significant (p=0.14). Children ranged from 9–15 years of age.
Table 1.
Characteristics of study population
| Characteristics | Total | Adult CD Patients | Adult Controls | Pediatric CD Patients | Pediatric Controls |
|---|---|---|---|---|---|
| Number of Subjects | 20 | 5 | 5 | 5 | 5 |
| Age, Median [Range] | 33 [29–52] | 46 [31–57] | 14 [9–15] | 14 [10–15] | |
| Gender | |||||
| Male | 11 | 3 | 2 | 4 | 2 |
| Female | 9 | 2 | 3 | 1 | 3 |
| Race | |||||
| Caucasian | 16 | 5 | 3 | 4 | 4 |
| Asian | 2 | 0 | 2 | 0 | 0 |
| Other* | 2 | 0 | 0 | 1 | 1 |
| BMI | 25.1 [17.6–29.8] | 21.7 [19.1–30.1] | 18.9 [14.9–24.6] | 18.1 [16.3–28.9] | |
| Folate (ug/L) | 398 [279–549] | 335 [246–546] | 431 [325–657] | 509 [355–1131] | |
| Hematocrit (%) | 42.1 [33.3–43.4] | 43.3 [35.2–52] | 34.6 [31.9–39.4] | 38.4 [31.0–38.9] |
Other includes two individuals who self-identified as Asian/White mixed race
Values expressed as median and [range].
(CD = Crohn’s Disease; BMI = Body Mass Index)
Table 2.
Clinical Features of Crohn’s Disease Patients
| Age | Sex | Age at Dx (years) | Time Since Dx (years) | Any Family History of IBD | Current Treatment | Surgical Resection | Symptomatic At Time of Study | Presence of Granuloma† | |
|---|---|---|---|---|---|---|---|---|---|
| Pediatric Patients | 9 | M | 9 | 0 | No | None | No | Yes | No |
| 9 | M | 10 | 0 | Yes | Abs | No | Yes* | Yes | |
| 14 | F | 10 | 5 | No | GH, MTX | No | Yes | No | |
| 14 | M | 9 | 6 | No | ASA, MTX | No | Yes | Yes | |
| 15 | M | 11 | 5 | No | Anti-TNF | No | Yes | Yes | |
| Adult Patients | 29 | M | 19 | 10 | No | Anti-TNF | No | No | No |
| 30 | M | 20 | 10 | Yes | Anti-TNF | No | Yes | No | |
| 33 | F | 13 | 20 | No | None | Yes | No | No | |
| 41 | M | 38 | 3 | No | 6MP | No | No | No | |
| 52 | F | 36 | 16 | Yes | Anti-TNF, 6MP | Yes | No | No |
Dx=diagnosis; Abs=antibiotics, GH=growth hormone, MTX=methotrexate, Anti-TNF=anti-tumor necrosis factor, 6MP=6-mercaptopurine
Symptoms included abdominal pain, bloody stools and/or diarrhea;
one patient had perianal disease only
Presence of granulomas on pathology at time of biopsy sample collection.
No differences were observed in BMI levels between healthy adults and children, and between adults and children with CD. All 4 of the pediatric subjects with BMI less than 18.5 were still within the normal range for their age (lowest Z-score was −1.6 and highest was 0.5). No differences were found between CD patients and healthy controls by age, gender, BMI, folate, or hematocrit.
Effects of Crohn’s disease on Cytokine Profiles
The distributions of cytokine profiles (CD4+, CD4+CD45RO+, IFN-γ, TNF-α, IL-4, IL-6) by age and disease status are shown in Table 3.
Table 3.
Immunological profiles of study population
| Adult Crohn’s Disease Patient | Adult Control | Pediatric Crohn’s Disease Patient | Pediatric Control | |
|---|---|---|---|---|
| Number of Subjects | 5 | 5 | 5 | 5 |
| CD4+ T Cells | ||||
| CD4 (%) | 37.0±10.9 | 29.7±9.9 | 31.2±7.6 | 31.5±8.4 |
| IFN-γ (%) | 15.1±3.8 ⋄ | 15.6±6.6 | 4.6±0.9 * | 9.1±4.4 |
| TNF-α (%) | 30.5±10.6 * | 16.5±4.9 | 23.3±7.6 * | 11.3±2.2 |
| IL-4(%) | 0.2±0.2 ⋄ | 0.2±0.1 | 0.4±0.2 * | 0.1±0.1 |
| IL-6 (%) | 0.2±0.2 | 0.3±0.2 | 0.33±0.3 | 0.4±0.4 |
| CD4+CD45RO+ T Cells | ||||
| CD4 CD45RO (%) | 14.1±3.5 ⋄ | 11.2±5.1 | 8.14±1.6 | 8.78±3.7 |
| IFN-γ (%) | 14.2±5.8 ⋄ | 13.0±7.2 | 7.1±0.9 * | 16.4±7.0 |
| TNF-α (%) | 24.3±6.4 | 21.5±9.8 | 22.8±8.2 | 22.6±3.7 |
| IL-4(%) | 0.7±0.3 | 1.0±0.9 | 0.8±0.6 | 0.9±0.5 |
| IL-6 (%) | 1.2±1.2 | 1.0±0.7 | 1.4±2.0 | 0.9±0.6 |
Values expressed as mean ± standard deviation
Difference between Crohn’s disease patients and controls in the same age group is statistically significant (p<0.05)
Difference between age groups within the same disease category is statistically significant (IFN-γ = Interferon-gamma; TNF-α = Tumor necrosis factor-alpha; IL-4 = Interleukin-4; IL-6 = Interleukin-6)
CD4+ T Cells
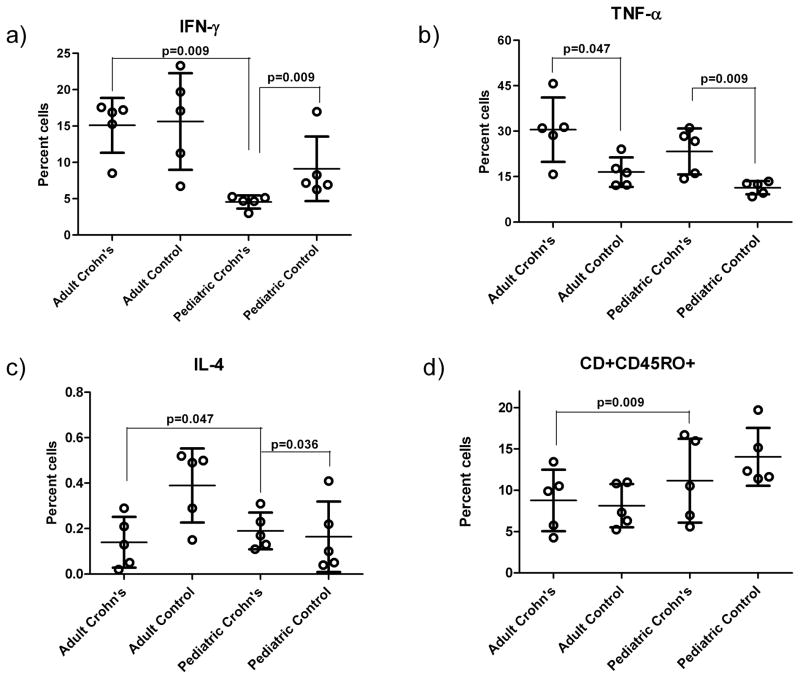
No differences were found in the frequency of CD4+ cells between CD patients and healthy controls in both adult and pediatric groups. TNF-α analyses revealed that pediatric CD patients had higher frequencies of TNF-α (23.3±7.6%) compared with the controls (11.3±2.2%; p=0.009) (Figure 1b). Similarly, the percentages of TNF-α were increased in adult CD patients (30.5±10.6%) compared with controls (16.5±4.9%; p=0.047). Percentages of cells expressing IL-4 were also higher in pediatric CD patients (0.4±0.2 %) than in pediatric controls (0.1±0.1%; p=0.036, while IFN-γ expressing cells were less common in pediatric CD patients (4.66±0.9%) compared with pediatric controls (9.1±4.4%; p=0.009) (Figure 1a and c)). In our previous study, newly diagnosed pediatric CD patients also possessed lower IFN-γ compared with healthy controls but the IL-4 did not differ significantly between the two groups.24 In the present study, IL-6 percentages did not differ between the two pediatric groups. No differences in IFN-γ, IL-4, and IL-6 percentages were found between adult CD patients and controls.
Figure 1. Cytokine expression in pediatric and adult CD patients and control subjects.
a) IFN-γ is significantly higher in adult CD patients than in pediatric CD patients (p=0.009). Pediatric CD patients have significantly lower percentage of cells expressing IFN-γ than pediatric controls (p=0.009).
b) Adult CD patients have increased TNF-α compared to adult controls (p=0.047). Pediatric CD patients have significantly higher TNF-α than pediatric controls (p=0.009).
c) IL-4 production is significantly higher in pediatric CD patients than in pediatric controls (p=0.036). Children with CD have higher IL-4 than adult patients (p=0.047).
d) Percentages of CD4+CD45RO+ T cells (memory T cells) among adult and pediatric CD patients and controls are higher in adult CD patients compared to pediatric CD patients (p=0.009).
Each circle represents one study subject.
Values are mean and standard deviation (CD = Crohn’s Disease; IL-4 = Interleukin-4; IFN-γ = Interferon-gamma; TNF-α = Tumor necrosis factor-alpha)
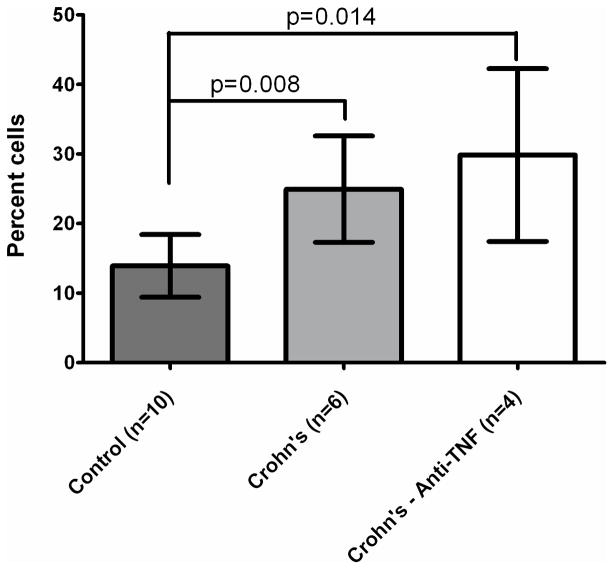
Noteworthy is that TNF-α expression was more prevalent in the patients whether or not they were being treated with anti-TNF medications compared with the control group subjects, independent of age (Figure 2).
Figure 2.
TNF-α Cell Percentages in Patients Treated or Untreated with anti-TNF-α Medications compared with Controls. Patients have higher TNF-α compared with controls, independent of use of anti-TNF-α medication (infliximab, adalimumab).
CD4+CD45RO+ T Cells
When only the memory T cell population cytokine profile was considered, no significant difference was found in CD4+CD45RO+ cells between CD patients and controls in both age groups. However, pediatric CD patients had decreased percentages of IFN-γ cells (7.1±0.9%) compared with controls (16.4±7.0%; p=0.009. No difference was found in TNF-α, IL-4, and IL-6 between CD patients and controls in both the adult and pediatric groups.
Effects of Age on Cytokine Profiles
The effect of age on cytokine profiles was investigated by comparing the CD4+, CD4+CD45RO+, Th1, and Th2 in children and adults who were either CD patients or controls.
CD4+ T cells
Among CD patients, the percentage of CD4+CD45RO+, IFN-γ+ cells was more than three-fold higher in adults (15.1±3.8%) than in pediatric subjects (4.6±0.9%; p=0.009) (Figure 1a). In contrast, IL-4 percentages were lower in adults (0.17±0.1%) compared with pediatric subjects (0.4±0.2%; p=0.047) (Figure 1c). No significant differences in the CD4+, TNF-α, and IL-6 were observed between the two age groups. Among healthy controls, the percentages Th1 cells (IFN-γ and TNF-α) were slightly higher in adults and no differences in CD4+, IL-4, and IL-6 were observed.
CD4+CD45RO+ T cells
The comparison of CD4+CD45RO+ T cell between pediatric (8.1±1.6%) and adult CD patients (14.1±3.5%) showed a notable increase with age (p=0.009) (Figure 1d). In addition, percentages of CD4+CD45RO+IFN-γ+ cells were significantly higher in adult CD patients (14.2±5.8%) than in pediatric CD patients (7.1±0.9%; p=0.016). No differences were observed for TNF-α, IL-4, and IL-6-expressing CD4+CD45RO+ cells between the two age groups.
DISCUSSION
We measured the intracellular production of IFN-γ, TNF-α, IL-4, and IL-6 from CD4+ and CD4+CD45RO+ T cells in the peripheral blood of adult and pediatric CD patients and age-matched controls. To distinguish between the effector capabilities between the CD4+CD45RO+ memory T cells and the broader class of CD4+ T cells, we obtained a separate set of flow cytometric data showing cytokine expression in the CD4+CD45RO+ memory T cell compartment and compared the profiles of CD patients to the controls in both age groups.
The mean IFN-γ levels from CD4+ T cells were significantly lower in pediatric CD patients than in controls. This supports our previous observation as well as other published data in treatment-naïve pediatric CD patients.24, 26 These results, however, are in contrast to findings from other studies that have evaluated the Th1/Th2 profiles in the intestinal mucosa and/or peripheral blood associated with CD. In adults, increased expression of IFN-γ was found in peripheral blood or intestinal tissue of adult CD patients compared with controls, in line with the accepted dogma of a Th1-mediated mechanism of disease.7,9,29 Similar to our study, IL-4 production has been reported to be inconsistent among CD patients.
We found that TNF-α expression was increased in pediatric CD patients, while levels of IFN-γ were decreased. Secretion of pro-inflammatory cytokines such as TNF-α in CD patients is triggered by the Th1 response originating from an increase in IFN-γ levels from lymphocytes and macrophages and leads to continual immune activation. Since this aberrant immune activation contributes to inflammation in IBD31, it is puzzling that the peripheral levels of TNF-α in our CD children were elevated despite an apparent down regulation of a Th1 response (i.e., decreased levels of IFN-γ and increased IL-4). This observation may be due to our relatively small number of subjects; however, the findings are consistent with previous reports.24,26
IL-6 has been associated with CD due to its capability to induce synthesis of acute phase proteins and inflammatory chemical pathways.6 In the present study, however, we did not find any significant differences in the intracellular production of IL-6 between CD patients and controls in both age groups. Previous studies suggest adult patients with CD have higher serum or plasma levels of IL-6 compared with controls.6,31 In our study, production of IL-6 was measured only from circulating CD4+ T cells and their subsets. The predominant sources of IL-6 in IBD patients during inflammation are activated monocytes, macrophages, and to a lesser extent epithelial cells.5 Our data may provide evidence that peripheral CD4+ T cell production of IL-6 is not a determining factor in the pathogenesis of pediatric and adult CD. It is necessary to evaluate IL-6 expression from other cell types to gain a better understanding of its role in CD pathogenesis.
Our analyses reveal that intracellular production of IFN-γ by CD4+CD45RO+ cells is significantly reduced in pediatric CD patients compared with healthy controls, reflecting the general trend seen in the overall CD4+ T cell population. Thus, it is likely that a down-regulation in the activity of circulating memory Th1 cells and their production of IFN-γ occur in pediatric CD patients. On the other hand, no differences were seen in the intracellular production of TNF-α, IL-4, and IL-6 from CD4+CD45RO+ T cells between the CD patients and controls in either age group. Previously, De Tena et al. reported that the percentages of CD4+CD45RO+ T cells producing TNF-α and IL-6 were higher in adults with active CD than in healthy controls. The intracellular production of IFN-γ was also greater in the active CD group compared with controls, though not statistically significant.19 These results are discordant with our findings in both age groups. However, the range and mean of ages of the study participants and the methods of cytokine analysis in the two studies were different. Furthermore, the clinical status of remission in most of our patients may have led to reduction in effector capabilities of circulating memory T cells to produce cytokines in our CD patients. An investigation of memory T cells in the intestinal mucosa of pediatric and adult CD patients may help elucidate the true function of CD4+CD45RO+ T cells in the immunodysregulation associated with CD.
Earlier studies have examined the relationship between age and cytokine profiles throughout development.23,33 However, with the exception of our previous assessment in newly diagnosed CD children, this is the first study to evaluate peripheral cytokine levels with age in CD patients. We previously reported that while CD4+ T cell frequencies remain constant among pediatric CD patients and controls with increasing age, the percentage of Th1 cells steadily increases with age in both groups.24 Similarly, in this study the overall production of Th1 cytokines from CD4+ T cells was generally higher in healthy adults compared with pediatric subjects. Our findings corroborate earlier reports of an increase in Th1 cells with age.23,33,34
We found that the percentages of CD4+CD45RO+ T cells increased with age in CD patients. In contrast, no difference was noted between the groups of healthy children and adults. Only a few studies have followed the changes in expression of circulating CD4+CD45RO+ population with age in healthy subjects.35,36
In summary, our study demonstrates an increased production of the pro-inflammatory cytokine TNF-α in CD patients of both pediatric and adult groups. Our clinical data also support differences in disease phenotype between adult and pediatric patients with inflammatory bowel diseases.37 The results suggest that fundamental differences exist between adults and children in the immunological mechanisms of CD pathogenesis. In addition, age can be an important determining factor in cytokine regulation in both healthy and CD-diagnosed subjects. Although our study was limited by sample size and subjects heterogeneity, we still detected several statistically significant differences in cytokine levels between groups. It should be noted that functional cytokine analyses were performed after in vitro stimulation with PMA and ionomycin which may over-stimulate a traditional in vivo process. Furthermore, not all of our CD patients were newly diagnosed with the disease, and some had been receiving medical therapy prior to sample collection, raising the possibility of cytokine profile alterations due to drug therapy. Nevertheless, the data obtained may help to elucidate immunological changes in CD patients of varying ages.
Although these investigations have shed light into the peripheral blood cytokine profiles of pediatric and adult CD patients, further research is necessary. Ex vivo analyses and in situ measurement of mucosal tissue must be performed to determine if peripheral cytokine profiles correlate with disease activity. Larger sample sizes with a broader age distribution would be ideal to determine if a correlation does exist – both in diseased and non-diseased patients – between age and percentage of Th1 or Th2 secreting CD4+ T cells. Additional analyses on the Th17 subset (e.g., IL-17, IL-23, IL-18, and TGF-β) should be included in future analyses.
Acknowledgments
We are grateful to the laboratory and clinical staff and participants of the study for all of their contributions. Helpful comments of Dr. Karen Huen and Vitaly Volberg are appreciated. Supported by grants from the Packard Foundation, the Crohn’s and Colitis Foundation of America, a Children’s Digestive Health and Nutrition Foundation/North American Society for Pediatric Gastroenterology, Hepatology, and Nutrition Career Development Award (BK), and NIH (R03 DK063187, K08 DK083334, M01 RR01271, T32 DK007762, K24 DK60617, and PO1 ES009605). This paper’s contents are solely the responsibility of the authors and do not necessarily represent official views of the Packard Foundation, the Crohn’s and Colitis Foundation of America, and the NIH.
References
- 1.Brown SJ, Mayer L. The Immune Response in Inflammatory Bowel Disease. Am J Gastroenterol. 2007;102(9):2058–2069. doi: 10.1111/j.1572-0241.2007.01343.x. [DOI] [PubMed] [Google Scholar]
- 2.Parronchi P, Romagnani P, Annunziato F, et al. Type 1 T-helper cell predominance and interleukin-12 in the gut of patients with Crohn’s disease. Am J Clin Pathol. 1997;150(3):823–832. [PMC free article] [PubMed] [Google Scholar]
- 3.Farrar JD, Asnagli H, Murphy KM. T-helper subset development: role of instruction, selection, and transcription. J Clin Invest. 2002;109:431–435. doi: 10.1172/JCI15093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kidd P. Th1/Th2 balance: the hypothesis, its limitations, and implications for health and disease. Altern Med Rev. 2003;8:223–246. [PubMed] [Google Scholar]
- 5.Brown KA, Back SJ, Ruchelli ED, et al. Lamina propria and circulating interleukin- 6 in newly diagnosed pediatric inflammatory bowel disease patients. Am J Gastroenterol. 2002;97(10):2603–2608. doi: 10.1111/j.1572-0241.2002.06030.x. [DOI] [PubMed] [Google Scholar]
- 6.Mahida YR, Kurlac L, Gallagher A, et al. High circulating concentrations of interleukin-6 in active Crohn’s disease but not ulcerative colitis. Gut. 1991;32:1531–1534. doi: 10.1136/gut.32.12.1531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Niessner M, Volk BA. Altered Th1/Th2 cytokine profiles in the intestinal mucosa of patients with inflammatory bowel disease as assessed by quantitative reversed transcribed polymerase chain reaction (RT-PCR) Clin Exp Immunol. 1995;101:428–435. doi: 10.1111/j.1365-2249.1995.tb03130.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Brandonisio O, Panaro MA, Sisto M, et al. Nitric oxide production by Leishmania-infected macrophages and modulation by cytokines and prostaglandins. Parassitologia. 2001;43 (Suppl ):1–6. [PubMed] [Google Scholar]
- 9.Camoglio L, Te Velde AA, Tigges AJ, et al. Altered expression of interferon-γ and interleukin-4 in inflammatory bowel disease. Inflamm Bowel Dis. 1998;4(4):285–290. doi: 10.1002/ibd.3780040406. [DOI] [PubMed] [Google Scholar]
- 10.West GA, Matsuura T, Levine AD, et al. Interleukin 4 in inflammatory bowel disease and mucosal immune reactivity. Gastroenterology. 1996;110:1683–1695. doi: 10.1053/gast.1996.v110.pm8964392. [DOI] [PubMed] [Google Scholar]
- 11.Mariani P, Bachetoni A, D’Alessandro M, et al. Effector Th-1 cells with cytotoxic function in the intestinal lamina propria of patients with Crohn’s disease. Dig Dis Sci. 2000;45(10):2029–2035. doi: 10.1023/a:1005516730754. [DOI] [PubMed] [Google Scholar]
- 12.Karttunnen R, Breese EJ, Walker-Smith JA, et al. Decreased mucosal interleukin-4 (IL-4) production in gut inflammation. J Clin Pathol. 1994;47:1015–1018. doi: 10.1136/jcp.47.11.1015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Reimund JM, Wittersheim C, Dumont S, et al. Increased production of tumour necrosis factor-a, interleukin-1B, and interleukin-6 by morphologically normal intestinal biopsies from patients with Crohn’s disease. Gut. 1996;39:684–689. doi: 10.1136/gut.39.5.684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.MacDonald TT, Hutchings P, Choy MY, et al. Tumour necrosis factor-alpha and interferon-gamma production measured at the single cell level in normal and inflamed human intestine. Clin Exp Immunol. 1990;81:301–305. doi: 10.1111/j.1365-2249.1990.tb03334.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Breese EJ, Michie CA, Nicholls SW, et al. Tumor necrosis factor-alpha producing cells in the intestinal mucosa of children with inflammatory bowel disease. Gastroenterology. 1994;106:1455–1466. doi: 10.1016/0016-5085(94)90398-0. [DOI] [PubMed] [Google Scholar]
- 16.Murch SH, Lamkin VA, Savage MO, et al. Serum concentrations of tumour necrosis factor a in childhood chronic inflammatory bowel disease. Gut. 1991;32:913–917. doi: 10.1136/gut.32.8.913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Romagnani P, Annunziato F, Baccari MC, et al. T cells and cytokines in Crohn’s disease. Immunology. 1997;9:793–799. doi: 10.1016/s0952-7915(97)80180-x. [DOI] [PubMed] [Google Scholar]
- 18.Diamanti A, Basso MS, Gambarara M, et al. Positive impact of blocking tumor necrosis factor alpha on the nutritional status in pediatric Crohn’s disease patients. Int J Colorectal Dis. 2009;24:19–25. doi: 10.1007/s00384-008-0578-x. [DOI] [PubMed] [Google Scholar]
- 19.De Tena JG, Manzano L, Leal JC, et al. Distinctive pattern of cytokine production and adhesion molecule expression in peripheral blood memory CD4+ T cells from patients with active Crohn’s disease. J Clin Immunol. 2006;26(3):233–242. doi: 10.1007/s10875-006-9016-4. [DOI] [PubMed] [Google Scholar]
- 20.Roman LI, Manzano L, De La Hera A, et al. Expanded CD4+CD45RO+ Phenotype and Defective proliferative response in T lymphocytes from patients with Crohn’s disease. Gastroenterology. 1996;110:1008–1019. doi: 10.1053/gast.1996.v110.pm8612987. [DOI] [PubMed] [Google Scholar]
- 21.De Tena JG, Manzano L, Leal JC, et al. Active Crohn’s disease patients show a distinctive expansion of circulating memory CD4+CD45RO+CD28null T Cells. J Clin Immunol. 2004;24 (2):185–196. doi: 10.1023/B:JOCI.0000019784.20191.7f. [DOI] [PubMed] [Google Scholar]
- 22.Wysocka J, Hassmann E, Lipska A, et al. Naïve and memory T cells in hypertrophied adenoids in children according to age. Int J Pediatr Otorhinolaryngol. 2003;67:237–241. doi: 10.1016/s0165-5876(02)00374-9. [DOI] [PubMed] [Google Scholar]
- 23.Hartel C, Adam N, Strunk T, et al. Cytokine responses correlate differentially with age in infancy and early childhood. Clin Exp Immunol. 2005;142:446–453. doi: 10.1111/j.1365-2249.2005.02928.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Holland N, Dong J, Garnett EA, et al. Reduced Intracellular T-helper 1 interferon gamma in blood of newly diagnosed children with crohn’s disease and age-related changes in th1/th2 cytokine profiles. Pediatr Res. 2008;63(3):257–262. doi: 10.1203/PDR.0b013e318163a897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Heyman MB, Garnett EA, Shaikh N, et al. Folate concentrations in pediatric patients with newly diagnosed inflammatory bowel disease. Am J Clin Nutr. 2009;89:545–550. doi: 10.3945/ajcn.2008.26576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mack DR, Beedle S, Warren J, et al. Peripheral blood intracellular cytokine analysis in children newly diagnosed with inflammatory bowel disease. Pediatr Res. 2002;51(3):328–332. doi: 10.1203/00006450-200203000-00011. [DOI] [PubMed] [Google Scholar]
- 27.Breese E, Braegger CP, Corrigan CJ, et al. Interleukin-2- and interferon-gamma-secreting T cells in normal and diseased human intestinal mucosa. Immunology. 1993;78(1):127–131. [PMC free article] [PubMed] [Google Scholar]
- 28.Akagi S, Hiyama E, Imamura Y, et al. Interleukin-10 expression in intestine of Crohn’s disease. Int J Mol Med. 2000;5(4):389–395. doi: 10.3892/ijmm.5.4.389. [DOI] [PubMed] [Google Scholar]
- 29.Autschbach F, Giese T, Gassler N, et al. Cytokine/chemokine messenger-RNA expression profiles in ulcerative colitis and Crohn’s disease. Virchows Arch. 2002;441:500–513. doi: 10.1007/s00428-002-0684-z. [DOI] [PubMed] [Google Scholar]
- 30.Akiho H, Lovato P, Deng Y, et al. Interleukin-4- and -13-induced hypercontractility of human intestinal muscle cells-implication for motility changes in Crohn’s disease. Am J Physiol Gastrointest Liver Physiol. 2005;288:G609–G625. doi: 10.1152/ajpgi.00273.2004. [DOI] [PubMed] [Google Scholar]
- 31.Szkaradkiewicz A, Marciniak R, Chudzicka-Strugala I, et al. Proinflammatory cytokines and IL-10 in inflammatory bowel disease and colorectal cancer patients. Arch Immunol Ther Exp. 2009;57:291–294. doi: 10.1007/s00005-009-0031-z. [DOI] [PubMed] [Google Scholar]
- 32.Sventoraityte J, Zvirbliene A, Kiudelis G. Immune System alterations in patients with inflammatory bowel disease during remission. Medicina. 2008;44(1):27–33. [PubMed] [Google Scholar]
- 33.Kawamoto N, Kaneko H, Takemura M, et al. Age-related changes in intracellular cytokine profiles and Th2 dominance in allergic children. Pediatr Allergy Immunol. 2006;17(2):125–133. doi: 10.1111/j.1399-3038.2005.00363.x. [DOI] [PubMed] [Google Scholar]
- 34.Duramad P, McMahon CW, Hubbard A, et al. Flow cytometric detection of intracellular TH1/Th2 cytokines using whole blood: validation of immunologic biomarker for use in epidemiologic studies. Cancer Epidemiol Biomarkers Prev. 2004;13:1452–1458. [PubMed] [Google Scholar]
- 35.Falcao RP, De-Santis GC. Age-associated changes of memory (CD45RO+) and naïve (CD45R+) T cells. Braz J Biol Res. 1991;24:275–279. [PubMed] [Google Scholar]
- 36.Aldhous MC, Raab GM, Doherty KV, et al. Age-related ranges of memory, activation, and cytotoxic markers on CD4 and CD8 cells in children. J Clin Immunol. 1994;14(5):289–299. doi: 10.1007/BF01540982. [DOI] [PubMed] [Google Scholar]
- 37.Heyman MB, Kirschner BS, Gold BD, et al. Children with early-onset inflammatory bowel disease (IBD): analysis of a pediatric IBD consortium registry. J Pediatr. 2005;146(1):35–40. doi: 10.1016/j.jpeds.2004.08.043. [DOI] [PubMed] [Google Scholar]