Abstract
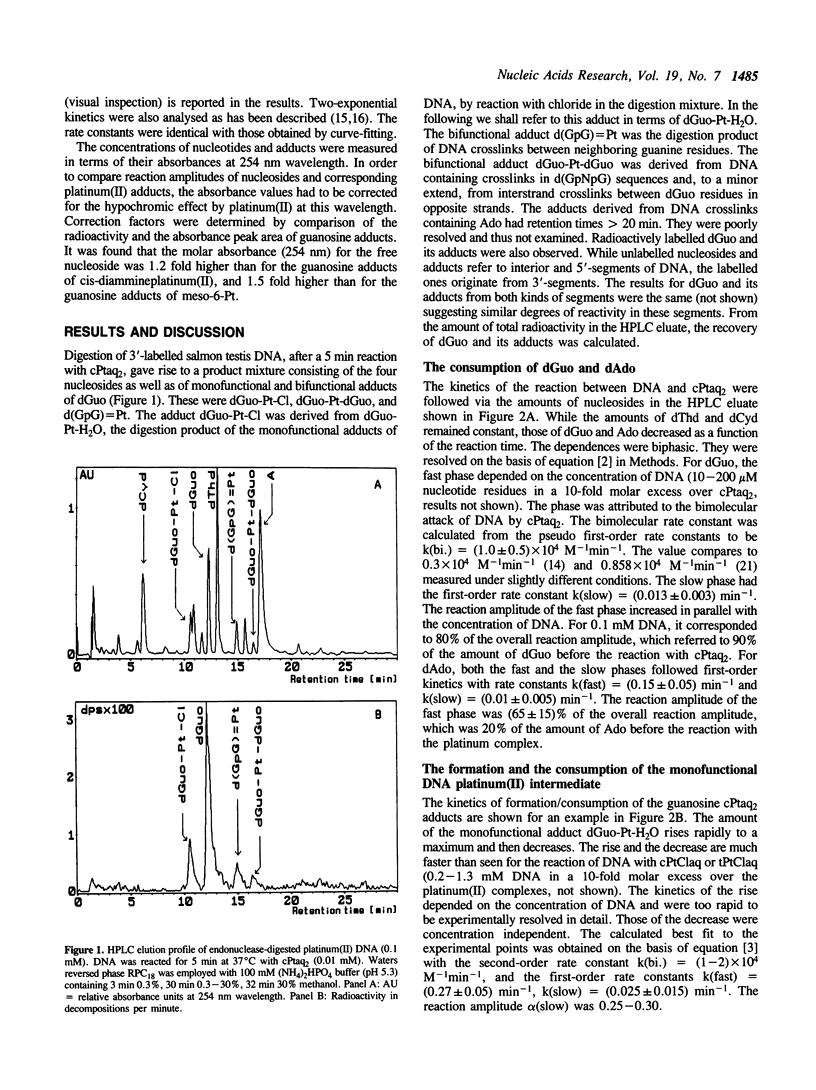
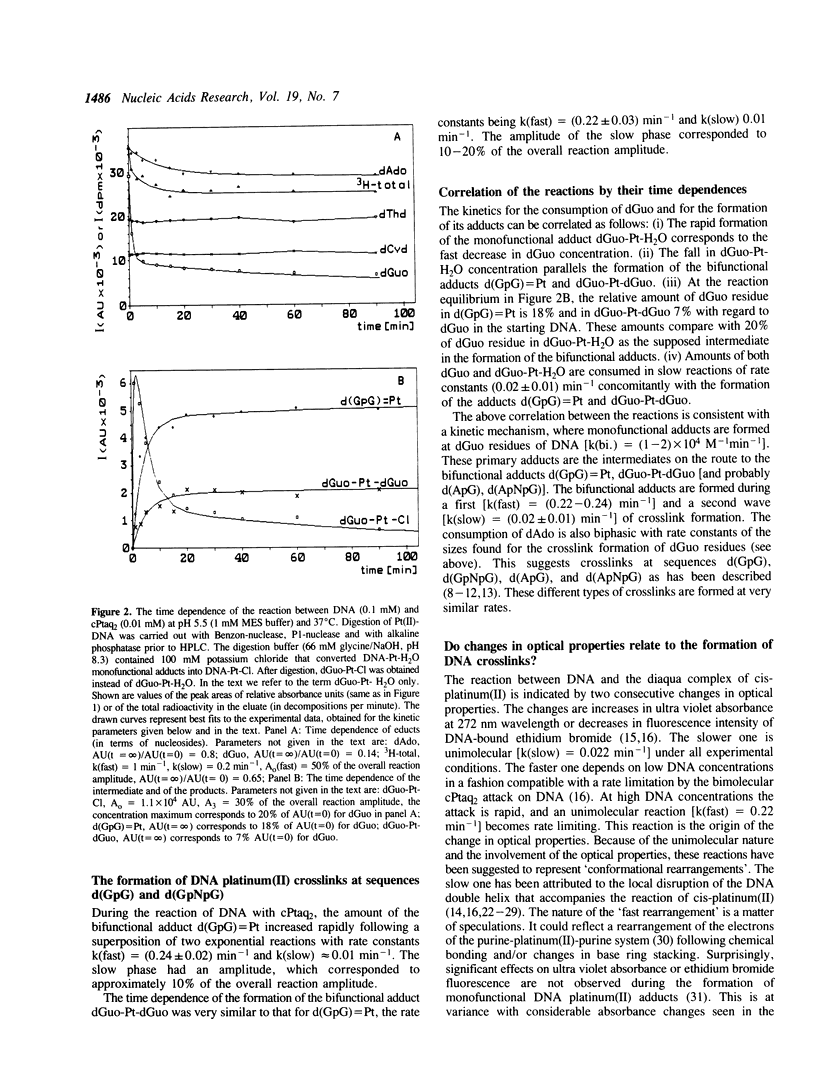
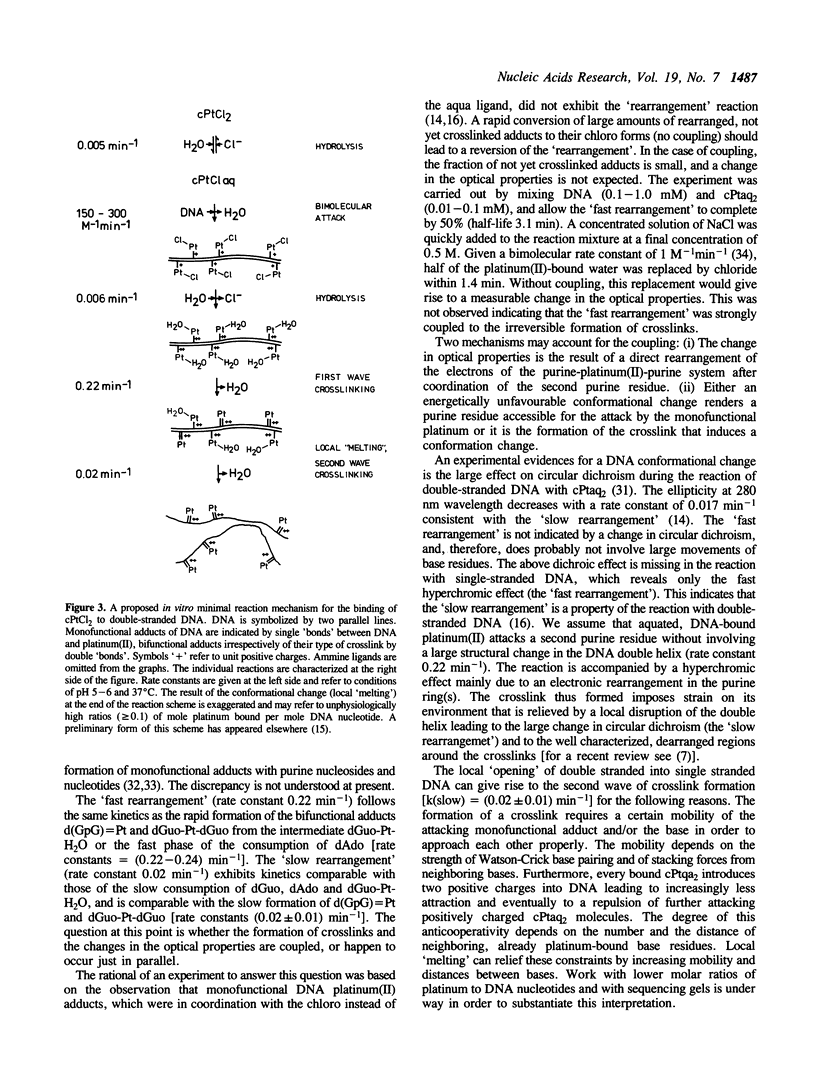
The kinetics of the formation of bifunctional DNA platinum(II) adducts (DNA-crosslinks) have been investigated by endonuclease digestion and subsequent HPLC analysis of the soluble nucleotides and nucleotide platinum(II) adducts. The results indicate two waves of crosslinking [rate constants (0.2-0.3) min-1 and (0.015-0.025) min-1] that correlate with changes in ultra violet absorbance and ethidium bromide dependent fluorescence intensity, previously interpreted in terms of two consecutive, local conformational rearrangements of platinum-DNA (Schaller, W., Reisner, H., and Holler, E. (1987) Biochemistry 26, 943-950). The formation of crosslinks at sequences d(GpG) and d(GpNpG) follows identical kinetics. A minimal reaction mechanism is proposed for the binding of cis-diamminedichloroplatinum(II) to DNA under in vitro conditions. The approximately 3-fold higher rate for meso-[1,2-bis(2,6-dichloro-4- hydroxyphenyl)ethylenediamine]diaquaplatinum(II) in comparison to the rate for cis-diamminediaquaplatinum(II) indicates that crosslink formation is affected by the nature of the non-leaving platinum ligand(s).
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bernges F., Dörner G., Holler E. Escherichia coli DNA polymerase I: inherent exonuclease activities differentiate between monofunctional and bifunctional adducts of DNA and cis- or trans-diamminedichloroplatinum(II). An exonuclease investigation of the kinetics of the adduct formation. Eur J Biochem. 1990 Aug 17;191(3):743–753. doi: 10.1111/j.1432-1033.1990.tb19183.x. [DOI] [PubMed] [Google Scholar]
- Eastman A. Characterization of the adducts produced in DNA by cis-diamminedichloroplatinum(II) and cis-dichloro(ethylenediamine)platinum(II). Biochemistry. 1983 Aug 2;22(16):3927–3933. doi: 10.1021/bi00285a031. [DOI] [PubMed] [Google Scholar]
- Eastman A. Reevaluation of interaction of cis-dichloro(ethylenediamine)platinum(II) with DNA. Biochemistry. 1986 Jul 1;25(13):3912–3915. doi: 10.1021/bi00361a026. [DOI] [PubMed] [Google Scholar]
- Eastman A. Separation and characterization of products resulting from the reaction of cis-diamminedichloroplatinum (II) with deoxyribonucleosides. Biochemistry. 1982 Dec 21;21(26):6732–6736. doi: 10.1021/bi00269a018. [DOI] [PubMed] [Google Scholar]
- Fichtinger-Schepman A. M., van der Veer J. L., den Hartog J. H., Lohman P. H., Reedijk J. Adducts of the antitumor drug cis-diamminedichloroplatinum(II) with DNA: formation, identification, and quantitation. Biochemistry. 1985 Jan 29;24(3):707–713. doi: 10.1021/bi00324a025. [DOI] [PubMed] [Google Scholar]
- Johnson N. P., Hoeschele J. D., Rahn R. O. Kinetic analysis of the in vitro binding of radioactive cis- and trans-dichlorodiammineplatinum(II) to DNA. Chem Biol Interact. 1980 May;30(2):151–169. doi: 10.1016/0009-2797(80)90122-2. [DOI] [PubMed] [Google Scholar]
- Kozelka J., Archer S., Petsko G. A., Lippard S. J., Quigley G. J. Molecular mechanics modeling of oligonucleotide adducts of the antitumor drug cis-diamminedichloroplatinum(II). Biopolymers. 1987 Aug;26(8):1245–1271. doi: 10.1002/bip.360260804. [DOI] [PubMed] [Google Scholar]
- Kozelka J., Chottard J. C. How does cisplatin alter DNA structure? A molecular mechanics study on double-stranded oligonucleotides. Biophys Chem. 1990 Apr;35(2-3):165–178. doi: 10.1016/0301-4622(90)80006-s. [DOI] [PubMed] [Google Scholar]
- Lepre C. A., Chassot L., Costello C. E., Lippard S. J. Synthesis and characterization of trans-[Pt(NH3)2Cl2] adducts of d(CCTCGAGTCTCC).d(GGAGACTCGAGG). Biochemistry. 1990 Jan 23;29(3):811–823. doi: 10.1021/bi00455a031. [DOI] [PubMed] [Google Scholar]
- Loehrer P. J., Einhorn L. H. Drugs five years later. Cisplatin. Ann Intern Med. 1984 May;100(5):704–713. doi: 10.7326/0003-4819-100-5-704. [DOI] [PubMed] [Google Scholar]
- Malinge J. M., Leng M. Reactivity of monofunctional cis-platinum adducts as a function of DNA sequence. Nucleic Acids Res. 1988 Aug 11;16(15):7663–7672. [PMC free article] [PubMed] [Google Scholar]
- Marrot L., Leng M. Chemical probes of the conformation of DNA modified by cis-diamminedichloroplatinum(II). Biochemistry. 1989 Feb 21;28(4):1454–1461. doi: 10.1021/bi00430a005. [DOI] [PubMed] [Google Scholar]
- Miller K. J., Taylor E. R., Basch H., Krauss M., Stevens W. J. A theoretical model for the binding of cis-Pt(NH3)2(+2) to DNA. J Biomol Struct Dyn. 1985 Jun;2(6):1157–1171. doi: 10.1080/07391102.1985.10507630. [DOI] [PubMed] [Google Scholar]
- Mong S., Daskal Y., Prestayko A. W., Crooke S. T. DNA supercoiling, shortening, and induction of single-strand regions by cis-diamminedichloroplatinum(II). Cancer Res. 1981 Oct;41(10):4020–4026. [PubMed] [Google Scholar]
- Pinto A. L., Lippard S. J. Binding of the antitumor drug cis-diamminedichloroplatinum(II) (cisplatin) to DNA. Biochim Biophys Acta. 1985;780(3):167–180. doi: 10.1016/0304-419x(85)90001-0. [DOI] [PubMed] [Google Scholar]
- Pinto A. L., Lippard S. J. Sequence-dependent termination of in vitro DNA synthesis by cis- and trans-diamminedichloroplatinum (II). Proc Natl Acad Sci U S A. 1985 Jul;82(14):4616–4619. doi: 10.1073/pnas.82.14.4616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts J. J., Thomson A. J. The mechanism of action of antitumor platinum compounds. Prog Nucleic Acid Res Mol Biol. 1979;22:71–133. doi: 10.1016/s0079-6603(08)60799-0. [DOI] [PubMed] [Google Scholar]
- Rosenberg B., VanCamp L., Trosko J. E., Mansour V. H. Platinum compounds: a new class of potent antitumour agents. Nature. 1969 Apr 26;222(5191):385–386. doi: 10.1038/222385a0. [DOI] [PubMed] [Google Scholar]
- Schaller W., Reisner H., Holler E. Kinetic investigation of the DNA platination reaction: evidence for a transient adduct between deoxyribonucleic acid and cis-platinum(II). Biochemistry. 1987 Feb 10;26(3):943–950. doi: 10.1021/bi00377a039. [DOI] [PubMed] [Google Scholar]
- Schwartz A., Marrot L., Leng M. Conformation of DNA modified at a d(GG) or a d(AG) site by the antitumor drug cis-diamminedichloroplatinum(II). Biochemistry. 1989 Oct 3;28(20):7975–7979. doi: 10.1021/bi00446a001. [DOI] [PubMed] [Google Scholar]
- Scovell W. M., Capponi V. J. Cis-diamminedichloroplatinum(II) modified DNA stimulates far greater levels of S1 nuclease sensitive regions than does the modification produced by the trans- isomer. Biochem Biophys Res Commun. 1982 Aug;107(3):1138–1143. doi: 10.1016/0006-291x(82)90640-4. [DOI] [PubMed] [Google Scholar]
- Scovell W. M., O'Connor T. Interaction of aquated cis-[NH3)2Ptii]with nucleic acid constituents. 1. Ribonucleosides. J Am Chem Soc. 1977 Jan 5;99(1):120–126. doi: 10.1021/ja00443a023. [DOI] [PubMed] [Google Scholar]


