Abstract
Background:
Hyperviscosity syndrome has been suggested as not simply an acute reaction. Yet, erythrocyte sedimentation rate is associated with whole blood viscosity and it is an indirect acute phase inflammation marker.
Aims:
This work investigates the prevalence of hyperviscosity in acute phase inflammation.
Materials and Methods:
Archived clinical pathology data for the period of 1999 to 2008 were utilized. 40,632-cases tested for C-reactive protein and/or erythrocyte sedimentation rate from five alternate years, which were concomitantly tested for haematocrit and total proteins, were extracted. The prevalence of abnormal viscosity associated with positive results of C-reactive protein and erythrocyte sedimentation rate were evaluated.
Results:
Hyperviscosity is infrequently associated with positive C-reactive protein (2.9%) and erythrocyte sedimentation rate (2.7%) sub-populations, and are not statistically different from their respective negative sub-populations. Normoviscosity is significantly more prevalent in the positive sub-populations (p < 0.01). Further analyses indicate that prevalence of acute phase inflammation is statistically significantly less in hyperviscosity compared to normoviscosity sub-population (p < 0.00001). Actual blood viscosity level increases with level of inflammation though.
Conclusion:
The study demonstrates that although blood viscosity level may increase with inflammation, hyperviscosity is not frequent in, or sensitive to acute phase inflammation. It portends that whole blood viscosity is not unspecific as acute phase inflammation markers. It calls for clinicians to consider utilizing whole blood viscosity in disease conditions where stasis is implicated, in which it is specific and valuable. It would also benefit to establish whether hyperviscosity is a chronic phase inflammation marker.
Keywords: Acute phase inflammation, cardiovascular complications, clinical laboratory evaluation, C-reactive protein, erythrocyte sedimentation rate, whole blood viscosity
Introduction
As a clinical management strategy, chronic disease patients in crisis are sent to the pathology for acute phase inflammation assessment. Especially, C-reactive protein (CRP) and erythrocyte sedimentation rate (ESR) are the routinely requested tests[1–4]. Others include acute phase proteins such as fibrinogen, which together with ESR are factors that are strongly associated with whole blood viscosity (WBV).
WBV is one of Virchow's triad, which has been an established concept of three phenomena that ultimately lead to, and/or result from cardiovascular complications[5,6]. Although, predicating factors (ESR and fibrinogen) are regarded as acute phase inflammation indices, WBV is merely considered as an inflammatory marker[7]. Whether abnormal WBV is prevalent in acute phase has yet to be evaluated with a robust data size. Further, WBV is considered a specific pathology index for stasis[8,9], while acute phase inflammation markers are considered not specific. However, the specificity of WBV has yet to be employed for evaluation of vasculopathy in chronic diseases where stasis in implicated.
Thus, it is unknown whether increased WBV vis-à-vis hyperviscosity is significantly prevalent in conditions where there is laboratory evidence of acute inflammation compared to where laboratory test is negative. Further, one of the issues being addressed is whether WBV is unduly sensitive like the acute phase inflammatory markers. The findings will contribute to formulate a statement of WBV's specificity and usefulness in chronic inflammatory disease management.
Considering the fact that more sensitive tests are often less specific and vice versa[10–12], one of the interests in the series of “WBV issues” is to determine whether WBV is unduly highly sensitive and less specific. The hypothesis here is that if WBV is highly sensitive to (and unspecific as) acute phase inflammation reaction, hyperviscosity would be highly prevalent in positive sub-populations of CRP and ESR compared to negative sub-populations.
The objective of this work is to investigate the prevalence of hyperviscosity in CRP and ESR including (i) whether hyperviscosity is significantly more associated with positive results of CRP and/or ESR, compared to the negative results; and (ii) whether CRP and ESR levels are higher at hyperviscosity group compared to hypo- and normo-viscosity group. The findings from this study will lend credence to whether WBV test result showing increased level should be considered non-specific, or a clinically specific complication worthy of clinical laboratory evaluation.
Materials and Methods
This work is part of Translational Biomedical Science Research initiative of the author. It is supported materially by the Albury South West Pathology – a unit of Western Pathology Cluster of NSW Health Australia. The Ethics Committee of the Area Health Service granted request through the Operations Manager for the use of de-identified data.
Ten years de-identified archived clinical pathology data for the period of January 1999 to December 2008 constitutes the database. 40,632-cases tested for CRP and/or ESR including 9,929 cases tested for both parameters from five alternate years including 1999, 2001, 2003, 2005 and 2007 were extracted. Selection was limited to those that were concomitantly tested for haematocrit and total proteins from the same phlebotomy collection time.
WBV at high shear stress was determined from haematocrit and total proteins as previously published[13]. Results of WBV were categorized within the continuum into levels of ≤15.00, 15.01-19.01 and ≥19.02 (208 Sec-1) as indicative of low, normal and high WBV levels respectively. CRP and ESR levels were compared between groups of high, low and normal WBV. Levels of WBV were also compared between negative vs. positive sub-populations of CRP and ESR. Statistical analyses were performed first by multivariate analysis of variance (MANOVA), followed with separate univariate analyses using S-Plus version 6.1. In the first analysis, the prevalence of abnormal CRP and ESR associated with different levels WBV were determined. Multivariate (age, CRP and ESR) comparison between first and forth quartiles levels of WBV were also evaluated.
CRP was measured by quantitative analysis and reported in mg/L. Normal value was ≤12mg/L and any result greater than 12mg/L was considered to indicate possible acute phase inflammatory reaction. Some negative CRP results were reported in semi-quantitative format (<0.5, <1, <2 or <10). To enable statistical analysis, these results were corrected by deleting the “<”. In the second statistical analysis, the CRP only data-set (n = 13,570) was sorted by CRP results and sub-grouped into negative and positive. The difference between WBV categories in the negative and positive CRP sub-populations were evaluated by Two-Sample Kolmogorov-Smirnov Test.
ESR was performed by VES-MATIC EASY™ automated analyser method and reported in mm/Hr. The third statistical analysis of this study was similar to the second. ESR data-set (n = 36,991) were separately sorted by ESR results and sub-grouped into negative and positive. The difference between WBV categories in the negative and positive ESR sub-populations were evaluated by Two-Sample Kolmogorov-Smirnov Test.
Results
The demographics of the data are presented in table (Table 1). The yearly prevalence of the different categories of WBV observed among sub-populations of negative and positive CRP and ESR are presented in (Table 2).
Table 1.
Summary demographics of data
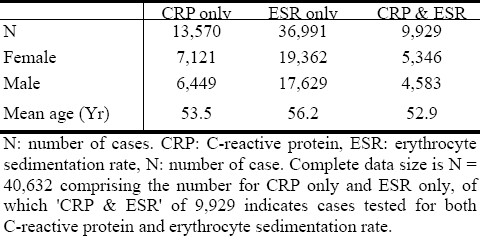
Table 2.
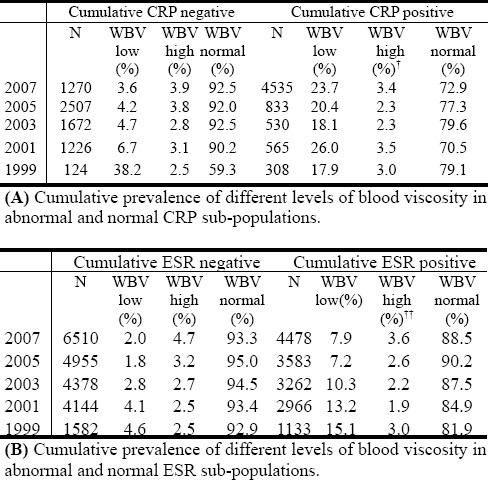
Cumulative prevalence of different levels of blood viscosity in abnormal and normal sub-populations of acute phase inflammation.
From the first statistical analysis, it is observed that laboratory indication of acute phase inflammation is less prevalent in the sub-population with high blood viscosity. The results show that on average, normal WBV is most prevalent in all sub-populations of negative and positive CRP/ESR (Fig. 1).
Fig. 1.
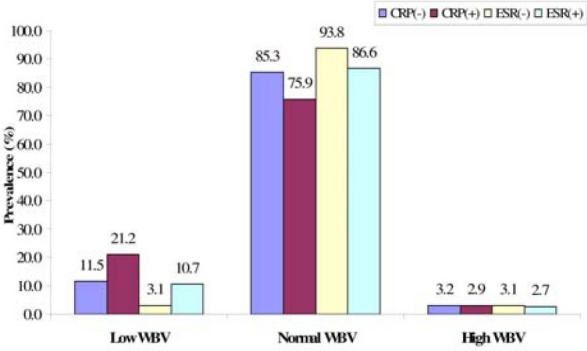
Prevalence of WBV categories in sub-populations of CRP and ESR.CRP: C-reactive protein (mg/L), ESR: erythrocyte sedimentation rate (mm/Hr), WBV: whole blood viscosity (208 Sec-1).CRP data-set (n = 13,570) and ESR data-set (n = 36,991) were sorted data by descending levels of CRP and ESR respectively, and categorized into negative and positive subgroups. The subgroups were further sorted by WBV and distributed into low, normal and high group. The fractions of in each group were then transformed as per cent prevalence of different levels of WBV associated with the separate indices of acute phase inflammation.
In the comparison of prevalence of the different WBV categories associated with negative and positive CRP and ESR, MANOVA presented a statistically significant difference (p < 0.0001). The statistical difference in prevalence of WBV levels was found to be significant between negative-ESR vs. positive-ESR (p < 0.0002), but not between negative-CRP vs. positive-CRP. In comparison of WBV levels between the first vs. forth quartiles of the acute phase inflammatory indices, MANOVA showed a statistically significant difference (p < 0.00001), including a difference between 1st quartile vs. 4th quartile CRP. The result further presented a clearer picture that WBV level increase with acute phase inflammation indices, as indicated by lower WBV levels in the 1st quartiles compared to the 4th quartiles of CRP and ESR (Fig. 2).
Fig. 2.
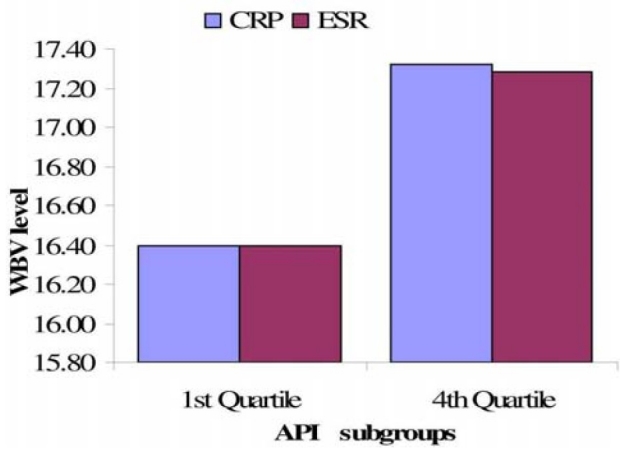
Comparison of WBV level with changes in CRP and ESR levels. API: acute phase inflammation, CRP: C-reactive protein (mg/L), ESR: erythrocyte sedimentation rate (mm/Hr), WBV: whole blood viscosity (208 Sec-1). The same trend of variation in WBV level is also observed in CRP and ESR.
From the univariate analyses, it is observed that low WBV is most associated with abnormal acute phase inflammation (Fig. 3). Further, Kolmogorov-Smirnov Test show that low WBV levels are statistically significantly less prevalent in the negative ESR sub-population than in the positive ESR sub-population (p < 0.01), but normal WBV levels are statistically significantly more prevalent in the negative ESR sub-population than in the positive ESR sub-population (p < 0.01). No statistically significant difference was observed in the prevalence of high WBV in the negative ESR sub-population compared to the positive ESR sub-population.
Fig. 3.
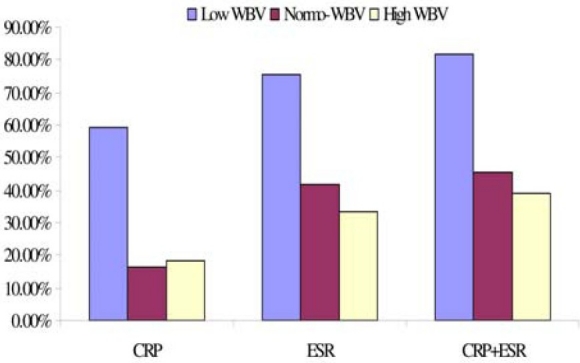
Prevalence of abnormal acute phase inflammation at different levels of WBV. CRP: C-reactive protein (mg/L), ESR: erythrocyte sedimentation rate (mm/Hr), WBV: whole blood viscosity (208 Sec-1). Data (n = 9,929) were sorted data by descending level of viscosity and grouped into low (n = 675), normal (n = 8,922) and high (n = 332) group. The fractions of abnormal CRP and ESR in each group were then critically evaluated as per cent prevalence. CRP+ESR indicate both acute phase inflammation markers concomitantly tested positive in a participant.
Discussion
This issue of the series is whether hyperviscosity is highly and significantly prevalent in acute phase inflammation. That is, the study investigated whether changes in blood viscosity level is a highly sensitive acute phase inflammation reaction like changes in CRP and ESR. This issue is addressed by phrasing the same question in alternate ways and the statistical analyses performed accordingly.
Is hyperviscosity frequently observable in acute phase inflammation, more than in subgroups (sub-populations) with negative CRP and ESR results?
Or
Are positive CRP and/or ESR more prevalent in hyperviscosity than in low and normal WBV groups? In other words, are there differences or similarities in stasis status among sub-populations of negative vs. positive CRP and negative vs. positive ESR?
Is hyperviscosity frequently observable in acute phase inflammation, more than in subgroups (sub-populations) with negative CRP and ESR results? The results from this study demonstrate a low prevalence of hyperviscosity among people who have laboratory evidence of possible acute phase inflammation reaction, as indicated by higher-than-normal CRP and ESR results. The low prevalence of hyperviscosity is not statistically significantly different between the sub-population who tested negative compared to the sub-population who tested positive for any of the routine laboratory markers. The cumulative prevalence of the different categories of WBV observed among sub-populations of negative and positive CRP (Table 2A), as well as those of ESR (Table 2B), indicate that normal WBV category is consistently the most prevalent in general population.
There is clearly a role for the measurement of other indices of the acute phase reaction beside CRP and ESR. Blood viscosity is one option. However, the choice of laboratory marker is recommended to depend on the diagnostic sensitivity and specificity of the measurement in the particular clinical situation[3,4]. The observation from this robust sample size study surmises, in part, that WBV is not significantly sensitive to acute phase inflammation. Therefore, laboratory test of WBV alone for indication of acute inflammation may be more likely to present false negative result.
Nevertheless, the observation of hypoviscosity being more prevalent than hyperviscosity in both negative and positive sub-populations of acute phase inflammation result has clinical management implication. It implies that beside the majority who fall within the normal WBV range, a considerable number of people do not have stasis requiring antiplatelet preventive therapy at the time of querying acute phase inflammation. It further provides information on those individuals who present with laboratory evidence of hyperviscosity syndrome and indication for antiplatelet therapy. The opinion here is that concomitant assessment of WBV will compliment to provide evidence-based clinical practice in the use of antiplatelet medication.
The averaged prevalence of the different WBV categories was found to be significant between negative and positive sub-populations (p < 0.0002; Fig. 1). A more critical evaluation of the WBV levels between first and fourth quartiles indicates that there is statistically significant difference in the actual level of WBV between high and low CRP/ESR. The result shows that blood viscosity increases with level of inflammation (Fig. 2). However, it is observed that the average WBV levels at the first and forth quartiles of CRP ranking are within the recommended normal range of 15.01-19.01. This observation makes the next question imperative.
Are positive CRP and/or ESR more prevalent in hyperviscosity than in low and normal WBV groups? The result shows that abnormal CRP and ESR are more prevalent at low WBV compared to hyper- and normo-viscosity (Fig. 3). This observation has two remarkable implications. First, it portends that acute phase inflammation may be a characteristic of hypoviscosity syndrome. Hypoviscosity has been mentioned in the literature but not followed up and not characterized with regards to inflammation[14,15]. Yet, it is believed that hyperviscosity is observed if there are changes in protein balance as seen in inflammation[15]. The belief is not in agreement with the observation presented in this report.
Second, the observation could be due to several factors that may require elucidation. For instance, oxidative changes to the protein cytoskeleton of erythrocyte membrane enhances oxidation of the haemoglobin molecule and/or peroxidation of the lipid membrane[16,17]. The oxidative changes literally cause erythrocyte oxidative stress, which in turn leads to anaemia on one hand[18], but also can make the blood more viscous on the other hand[19,20]. Whether the former effect (anaemia, which is associated with low blood viscosity) is capable of nullifying the latter effect (oxidative damage, which is associated with high viscosity) at acute phase inflammation has not been reported. Nevertheless, illustrated theory by this author is that increase in blood viscosity becomes a complication when oxidative stress is chronic/prolonged[21].
Further, atherogenesis is recognized as a two-phase process, of which the second phase is believed to be implicated with greater impact of inflammation including pro-coagulant, pro-inflammatory mediators and oxidative stress[22,23]. The theory holds that this second phase of atherogenesis is associated with a steadily maintained level of thrombin production and fibrin formation[22]. Two salient points need emphasis here viz: (1) the inflammation is implicated in the second phase of a pathophysiology. Therefore, it is thinkable that inflammation at this stage is not acute. (2) Oxidative stress, which is involved at this phase, complicates blood viscosity through opposing effects on haematocrit and red cell aggregation/deformability[18,21,24]. It is inferred that this may underlie hyperviscosity syndrome or stasis not being more associated with acute phase inflammation. That is, probably because of the complex pathology. Therefore, it may be worthy of consideration, or to establish, whether hyperviscosity is a chronic phase inflammation marker.
It is observed low WBV levels are statistically significantly less prevalent in the negative ESR sub-population than in the positive ESR sub-population. Also, normal WBV levels are statistically significantly more prevalent in the subpopulations with negative results of acute phase inflammation markers than in those with positive results. Given the strong association between ESR and WBV, this result of low prevalence was unexpected. However, one implication of this finding is evidence of low sensitivity of WBV to closely related haematological index. This may be indication that WBV is more specific than the routine acute phase inflammation markers. It corroborates with previous observations currently in press[25], to suggest putting into perspective the specificity of WBV to assess stasis in, not necessarily sensitivity to, any disease condition where cardiovascular risk is a potential complication.
Limitation
This study utilizing a comprehensive data has employed the author's suggested algorithm to determine WBV[13]. It is limited by the fact that the suggested reference ranges have yet to be validated. It is also limited by non-identification of the subjects who were on medications (e.g. antiplatelet) that could impact on laboratory result. Nevertheless, the report presents significant evidence that hyperviscosity is not frequent during acute phase inflammation, which means the speculated association between inflammation and stasis syndrome could be at the chronic phase.
Conclusion
This report presents evidence that stasis is not very prevalent in acute phase inflammation. The prevalence of hyperviscosity in acute phase inflammation is not different from when there is none. Low WBV is more associated with acute phase inflammation compared to people with no acute inflammatory reaction. In current clinical practice, CRP and ESR are sensitive but unspecific laboratory indices that are routinely used to assess acute phase inflammatory reaction. It is generally believed that WBV is also a marker of inflammation. The issue addressed in this study report is that WBV is neither highly prevalent/sensitive during acute phase inflammation, nor significantly different when compared to the sub-population with no evidence of acute inflammation. It corroborates with other reports to suggest putting into perspective the specificity of blood viscosity in the consideration of its usefulness.
Acknowledgement
Receipt of an approval from HREC committee of the Greater Southern Area Health Service, NSW Health was obtained, and hereby gratefully appreciated. The operations manager of the South West Pathology Service and Nathan Cann are appreciated for their contribution in data acquisition and collaboration in the research initiative. The Senior Haematologist of the Albury South West Pathology Service is also appreciated for her support at time of need.
References
- 1.Lippi G, Franchini M, Targher G, Poli G, Guidi GC. The significance of evaluating conventional inflammatory markers in Von Willebrand factor measurement. Clin Chim Acta. 2007;381:167–170. doi: 10.1016/j.cca.2007.03.007. [DOI] [PubMed] [Google Scholar]
- 2.Nielsen OH, Vainer B, Madsen SM, Seidelin JB, Heegaard NHH. Clinical Reviews. Am J Gastroenterol. 2000;95:359–367. doi: 10.1111/j.1572-0241.2000.t01-1-01790.x. [DOI] [PubMed] [Google Scholar]
- 3.Thompson D, Milford-Ward A, Whicher JT. The value of acute phase protein measurements in clinical practice. Ann Clin Biochem. 1992;29:123–131. doi: 10.1177/000456329202900201. [DOI] [PubMed] [Google Scholar]
- 4.Okafor B, MacLellan G. Postoperative changes of erythrocyte sedimentation rate, plasma viscosity and C-reactive protein levels after hip surgery. Acta Orthop Belg. 1998;64:52–56. [PubMed] [Google Scholar]
- 5.Mammen EF. Pathogenesis of venous thrombosis. Chest. 1992;102:640S–644S. doi: 10.1378/chest.102.6_supplement.640s. [DOI] [PubMed] [Google Scholar]
- 6.Smith BD, La Celle PL. Blood viscosity and thrombosis: clinical considerations. Prog Hemost Thromb. 1982;6:179–201. [PubMed] [Google Scholar]
- 7.Tzoulaki I, Murray GD, Lee AJ, Rumley A, Lowe GDO, Fowkes FGR. Relative value of inflammatory, hemostatic, and rheological factors for incident myocardial infarction and stroke: the Edinburgh Artery Study. Circulation. 2007;115:2119–2127. doi: 10.1161/CIRCULATIONAHA.106.635029. [DOI] [PubMed] [Google Scholar]
- 8.Yan B, Jiye A, Hao H, et al. Metabonomic phenotype and identification of “heart blood stasis obstruction pattern” and “qi and yin deficiency pattern” of myocardial ischemia rat models. Sci China C Life Sci. 2009;52:1081–1090. doi: 10.1007/s11427-009-0136-y. [DOI] [PubMed] [Google Scholar]
- 9.Lowe GD. Virchow's triad revisited: abnormal flow. Pathophysiol Haemost Thromb. 2003;33:455–457. doi: 10.1159/000083845. [DOI] [PubMed] [Google Scholar]
- 10.Anderson DR, Wells PS. D-dimer for the diagnosis of venous thromboembolism. Curr Opin Hematol. 2000;7:296–301. doi: 10.1097/00062752-200009000-00007. [DOI] [PubMed] [Google Scholar]
- 11.Karras DJ, Kane DL. Serum markers in the emergency department diagnosis of acute myocardial infarction. Emerg Med Clin North Am. 2001;19:321–337. doi: 10.1016/s0733-8627(05)70186-3. [DOI] [PubMed] [Google Scholar]
- 12.Palestro CJ, Love C. Nuclear medicine and diabetic foot infections. Semin Nucl Med. 2009;39:52–65. doi: 10.1053/j.semnuclmed.2008.08.006. [DOI] [PubMed] [Google Scholar]
- 13.Nwose EU. Whole blood viscosity assessment issues I: Extrapolation chart and reference values. North Am J Med Sci. 2010;2:165–169. doi: 10.4297/najms.2010.2165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Larcan A, Stoltz JF. Blood hyperviscosity syndromes. Classification and physiopathological understanding. Therapeutic deductions. Ann Med Interne (Paris) 1983;134:395–410. [PubMed] [Google Scholar]
- 15.Larcan A, Stoltz JF, Gaillard S. Blood viscosity. Measurement and applications (hyper--and hypoviscosity syndromes) (author's transl) Nouv Presse Med. 1981;10:1411–1415. [PubMed] [Google Scholar]
- 16.Boivin P. Molecular interactions of membrane proteins and erythrocyte deformability. Pathol Biol (Paris) 1984;32:717–735. [PubMed] [Google Scholar]
- 17.Suda T, Maeda N, Shiga T. Effect of cholesterol on human erythrocyte membrane. A spin label study. J Biochem (Tokyo) 1980;87:1703–1713. doi: 10.1093/oxfordjournals.jbchem.a132914. [DOI] [PubMed] [Google Scholar]
- 18.Richards RS, Roberts TK, McGregor NR, Dunstan RH, Butt HL. Erythrocyte antioxidant systems protect cultured endothelial cells against oxidant damage. Biochem Mol Biol Int. 1998;46:857–865. doi: 10.1080/15216549800204402. [DOI] [PubMed] [Google Scholar]
- 19.Solans R, Motta C, Solá R, La Ville AE, Lima J, Simeón P, et al. Abnormalities of erythrocyte membrane fluidity, lipid composition, and lipid peroxidation in systemic sclerosis: Evidence of free radical-mediated injury. Arthritis Rheum. 2000;43:894–900. doi: 10.1002/1529-0131(200004)43:4<894::AID-ANR22>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- 20.Yang ZC, Xia K, Wang L, et al. Asymmetric dimethylarginine reduced erythrocyte deformability in streptozotocin-induced diabetic rat. Microvasc Res. 2007;73:131–136. doi: 10.1016/j.mvr.2006.04.008. [DOI] [PubMed] [Google Scholar]
- 21.Nwose E, Richards R, Kerr P, Tinley R, Jelinek H. Oxidative damage indices for the assessment of subclinical diabetic macrovascular complications. Br J Biomed Sc. 2008;65:136–141. doi: 10.1080/09674845.2008.11732817. [DOI] [PubMed] [Google Scholar]
- 22.Spronk HM, van der Voort D, Cate H Ten. Blood coagulation and the risk of atherothrombosis: a complex relationship. Thromb J. 2004;2:12. doi: 10.1186/1477-9560-2-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ziegler D, Sohr CGH, Nourooz-Zadeh J. Oxidative stress and antioxidant defense in relation to severity of diabetic polyneuropathy and cardiovascular autonomic neuropathy. Diabetes Care. 2004;27:2178–2183. doi: 10.2337/diacare.27.9.2178. [DOI] [PubMed] [Google Scholar]
- 24.Lowe G. Can haematology laboratories predict thrombosis? Clin Lab Haematol. 2001;23:335–354. [Google Scholar]
- 25.Nwose EU. Whole blood viscosity assessment issues II: prevalence in endothelial dysfunction and hypercoagulation. North Am J Med Sci. 2010;2:252–257. doi: 10.4297/najms.2010.2252. [DOI] [PMC free article] [PubMed] [Google Scholar]


