Abstract
Background:
Few articles have linked the consumption of green tea to certain liver diseases but several articles have disputed these assertions and the consumption of green tea has been on the increase.
Aims:
The effects of oral administration of green tea on the liver of Wistar rats were studied in order to compare biochemical findings with histological findings.
Materials and Methods:
36 male and female Wistar albino rats were grouped into 6, consisting of 6 rats in each group. They were given 1%, 2%, 3%, 4% and 5% concentration of green tea in tap water for 42 days. The 6th group was on normal diet and received 0% of the tea. Their blood samples were analyzed for total and conjugated bilirubin, total protein, albumin, globulin, alanine aminotransferase, aspartate aminotransferase and alkaline phosphatase. The liver tissues were also processed for histological examination.
Results:
The liver tissues were essentially normal and similar to the control tissues. The biochemical parameters studied were also normal and similar to the results obtained from the control animals.
Conclusion:
Sub chronic consumption of green tea has no injurious effects on the liver of Wistar rats.
Keywords: Green tea, histology, biochemical, sub-chronic
Introduction
The use of plant extracts by traditional medical practitioners for the treatment of liver disorders has been on for centuries[1]. Camellia sinensis (Tea) has a wide range of effects on animal and human health. It is anti-inflammatory[2] and has been reported to have beneficial effects in conditions such as collagen-induced arthritis[3], inflammatory bowel disease[4] and carrageenan-induced paw oedema[5]. An increased consumption of green tea may reduce the risk of liver disease[6]. Green tea polyphenol prevents oxygen free radical-induced hepatocyte lethality, prevent lipopolysaccharide-induced liver injury through inhibition of inducible nitric oxide synthase and tumor necrosis factor-α expression and inhibits carcinogen or toxin-induced liver oxidative DNA damage[7–9]. Epigallocatechin gallate, isolated from green tea, has antioxidant properties and is thought to act as an antioxidant in biological systems[10]. The protective effects of tea extracts or tea polyphenol against liver fibrosis and liver cirrhosis in rats have been reported[11,12], and confirmed[13] when a study on the hepatotoxicity of high concentration of the tea on Wistar rats was found to be safe. Green tea has also been found useful in the treatment of other body ailments. The polyphenols contained in the tea are antimutagenic and anticarcinogenic by inhibiting cancer cell proliferation and induction of apoptosis[14]. They have also been found to help reduce chromosomal damage during mutagen exposure[15]. Green tea catechin, act as an antioxidant scavenger of reactive oxygen species as superoxide, hydroxyl radicals, inhibition of lipid peroxide and inhibition of 2-deoxyuanosine oxidation in DNA to 8-hydroxy-2-deoxygnanosine[16]. Many xenobiotics are capable of causing some degree of liver injury[17]. The liver is prone to xenobiotic-induced injury because of its central role in xenobiotic metabolism, its portal location within the circulation, and its anatomic and physiologic structure[18].
The aim of this work was to compare biochemical results of liver function tests with the histological observations and establish the relationship between consumption of green tea and inflammatory reactions in the liver of Wistar rats.
Materials and Methods
Preparation of Plant Extracts
Six preparations of 0 g, 1 g, 2 g, 3 g, 4 g and 5 g of the dry green tea (Camellia cinensis) were put in 100 ml tap water each and boiled for 5 minutes. The decoction was filtered with a No 1 Whatman filter paper and allowed to cool at room temperature. They were labeled 0%, 1%, 2%, 3%, 4% and 5% respectively. The dry green tea used in this experiment was obtained from Unilever France and contained 12.75% (w/w) epigallocatechin-3-gallate, 9.21% epigallocatechin, 3.73% epicatechin gallate, 2.4% epicatechin, 5.94% caffeine, and 0.195% L-theanine.
Experimental animals
Thirty six Wistar albino rats 12 weeks old, weighing 180±10 g were obtained from the animal house of the Faculty of Basic Medical Sciences, Delta State University, Nigeria. They were housed in rat cages in a well ventilated house, temperature of 32±2°C during the day with 12 hr natural light and 24±2°C in the night with 12 hr darkness. The rats had free access to tap water and dry rat pellets obtained from Delta State University Nigeria. The rats were allowed to acclimatize for ten days before the experiment.
Oral Acute Toxicity Study
Thirty six rats were divided into six groups. Each group had 6 rats of 3 males and 3 females. 1 ml each of the 0% (tap water), 1%, 2%, 3%, 4% and 5% of the green tea decoction was given to the rats in the groups respectively. The animals given 0%, i.e. tap water served as controls. The drug was administered orally with a canula attached a graduated syringe. All the rats were placed under observation for 24 hours for possible deaths of the rats.
Oral Sub-Chronic Toxicity Study
None of the animals in the oral toxicity study died. Therefore, administration of the decoction on the animals continued for a further 42 days. At the end of 42 days, the rats were weighed and blood samples collected through cardiac puncture under chloroform anaesthesia into lithium heparin specimen bottles for liver function tests. The animals were subsequently sacrificed by cervical dislocation and liver tissues taken and immediately fixed in 10% formol saline for histological examination.
Biochemical analysis
The blood samples were centrifuged, plasma aspirated and analyzed for bilirubin, total protein, albumin, globulin, alanine aminotransferase, aspartate aminotransferase and alkaline phosphatase according to standard biochemical methods at the Obafemi Awolowo University Teaching Hospital, Ile-Ife Nigeria.
Alanine aminotransferase - Alanine aminotransferase catalysed the transfer of an amino group between the amino acids: L-alanine and L-glutamate. The ketoacids formed in this process were α-ketoglutarate and pyruvate. The pyruvate formed reacted with dinitrophenylhydrazine to produce a corresponding dinitrophenylhydrazone, which was measured with the spectrophotometer at 505nm[19].
Aspartate aminotransferase
Aspartate aminotransferase catalysed the interco version of the amino acids: L-aspartate and L-glutamate to produce oxaloacetate and L-glutamate. The oxaloacetate then coupled with 2,4-dinitrophenylhydrazine to produce a brownish color hydrazone which was measured with the spectrophotometer at 505 nm[19].
Alkaline phosphatase - Alkaline phosphatase catalyzed the hydrolysis of 4-nitrophenol phosphate forming phosphate and free 4-nitrophenol, which in dilute acid solution was colourless. Under alkaline conditions 4-nitrophenol was converted to 4-nitrophenoxide ion which had a very intense yellow color. The rate of formation of 4-nitrophenol by the addition of alkaline phosphatase on 4-nitrophenol at 37°C was then monitored at 405nm with a recording spectrophotometer[20].
Bilirubin
Plasma was added to a solution of sodium acetate and caffeine (sodium benzoate) which was then added to diazotized sulfanilic acid to form a purple azo bilirubin. The sodium acetate buffered the pH of the diazotized sulfanilic acid. The reaction was terminated by the addition of ascorbic acid which destroyed the excess diazo reagent A strongly alkaline tartrate solution was then added to convert the purple azobilirubin to blue azoblilrubin and the intensity of the color was measured at 600nm with a spectrophotometer[21].
Protein
The peptide bonds of plasma protein in plasma reacted with biuret reagent which contained copper in alkaline solution to form a violet colored chelate which was measured with a spectrophotometer at 540nm[22].
Albumin
Bromocresol green, a dye at an acid pH of 3.8 preferentially bound albumin to produce a shade of green color which was measured with a spectrophotometer at 630nm[23].
Histology
Sections measuring approximately 0.2 cm × 0.2 cm were taken from the liver of each rat. They were dehydrated through graded solutions of alcohol ending in two changes of absolute alcohol for 2 hours each. They were cleared in 2 changes of xylene, infiltrated in 2 changes of paraffin wax for 2 hours each using the automatic tissue processor obtained from Sakura fine tek, Netherlands and embedded in molten paraffin wax. Sections were cut at 4μ with the rotary microtome obtained from Sakura fine tek, Netherlands and stained with haematoxylin and eosin[24].
Results
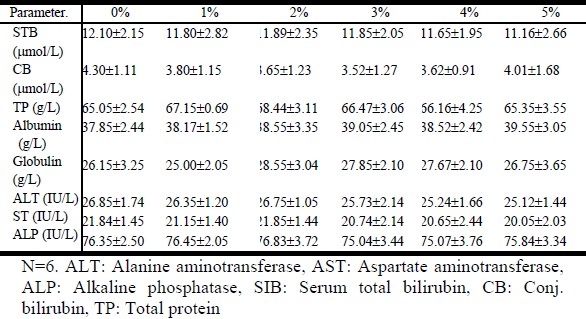
All the biochemical markers investigated were not significantly different from the control samples. The total bilirubin, conjugated bilirubin, total protein, albumin, globulin, alanine aminotransferase, aspartate aminotransferase and alkaline phosphatase were within normal range and were not elevated even at a concentration of 5% green tea extract (Table 1).
Table 1.
Effects of the oral administration of the decoction of green tea on liver markers
All the tissue sections obtained from the liver of experimental Wistar rats fed with the green tea extracts from 1% to 5% concentration were not different from tissues from the control animals. All the sections were essentially normal without any inflammatory lesion (Fig. 1).
Fig. 1.
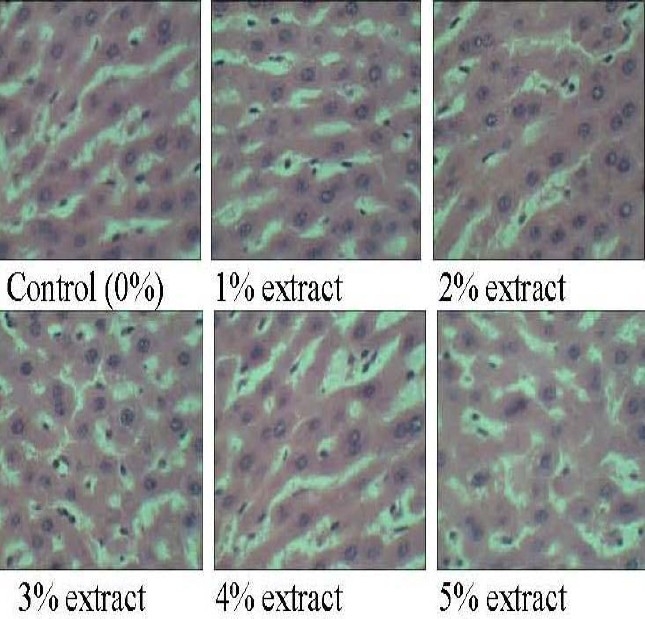
Photomicrographs (H&E ×400) of liver tissues from control animals on normal diet and experimental animals treated with green tea extract.
Discussion
The liver is prone to xenobiotic-induced injury because of its central role in xenobiotic metabolism and its portal location within the circulatory system[18]. Green tea is not an exemption and its constituents will be acted upon by the cells of the liver. Tea is the most consumed beverage in the world and there are numerous reports of the health benefits of tea. There are also few reports of its hazard to health. Some of the beneficial effects of tea are its anti-inflammatory property[25], and its anticancer property[26]. Green tea has been used for the treatment of diarrhea, and typhoid[27,28], influenza virus, Herpes simplex[29,30] and adenovirus[31]. Green tea catechins also have an inhibitory effect on Helicobacter pylori infection[32] and antifungal activity especially Candida albicans. Green tea has also been used in the preparation of a variety of foods, pharmaceutical preparations, dentifrices, and cosmetics[33].
Although intraperitoneal injection of 100 mg epigallocatechin gallate/kg bodyweight into mice was said to increase plasma concentrations of alanine transaminase (ALT) and concentrations above 150 mg/kg were found to be lethal[34], we have not found this in oral administration of the extract in Wistar rats. However, there are reports of abnormally high concentrations of liver injury markers in a few patients who took green tea. In these patients, cessation of green tea consumption normalized liver function and resumption of green tea drinking again elevated these biomarkers[35,36].
A herbal medicinal product (Exolise) named AR25 containing alcoholic extract of green tea is said to cause hepatic failures and had to the withdrawn from the market[13], this experiment could not establish the toxicity of green tea on the liver in Wistar rats as the histology and biochemical parameters were essentially normal. Perhaps the toxicity of AR25 was in the mode of preparation and extraction of the active ingredients in the tea or in the genetic makeup of such individuals.
Green tea contains enzymes, proteins, carbohydrates such as cellulose, pectins, glucose, fructose, and sucrose; amino acids such as theanine, glutamic acid, tryptophan, glycine, serine, aspartic acid, tyrosine, valine, leucine, threonine, arginine, and lysine. It also contains minerals and trace elements such as calcium, magnesium, chromium, manganese, iron, copper, zinc, molybdenum, selenium, sodium, phosphorus, cobalt, strontium, nickel, potassium, fluorine, and aluminum; and trace amounts of lipids, sterols, vitamins (B, C, E), caffeine, theophylline, pigments and volatile compounds[37]. These may account for its many protective effects on the tissues of the body. The various concentrations of green tea administered to the rats had no adverse effects in the histology of the liver (Fig. 1) and the biomarkers (Table 1).
Conclusion
We conclude that sub chronic oral administration of green tea up to 5% for 42 days has no effect on the liver biomarkers and the histology of the liver of Wistar rats. This study confirms previous biochemical experiments of green tea on hepatic cells[12,13], and we have corroborated these findings with normal histopathological observations.
References
- 1.Schuppan D, Jia J, Brikhaus B, Hahn EG. Herbal products for liver disease: A therapeutic challenge for the new millennium. Hematology. 1999;30:1099–1104. doi: 10.1002/hep.510300437. [DOI] [PubMed] [Google Scholar]
- 2.Dona M, Dell’Aica I, Calabrese F, Benelli R, Morini M, Albini A, Garbisa S. Neutrophil restraint by green tea: inhibition of inflammation, associated angiogenesis, and pulmonary fibrosis. J Immunol. 2003;170:4335–4341. doi: 10.4049/jimmunol.170.8.4335. [DOI] [PubMed] [Google Scholar]
- 3.Haqqi TM, Anthony DD, Gupta S, Ahmad N, Lee MS, Kumar GK, Mukhtar H. Prevention of collagen-induced arthritis in mice by a polyphenolic fraction from green tea. Proc Natl Acad Sci USA. 1999;96:4524–4529. doi: 10.1073/pnas.96.8.4524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Varilek GW, Yang F, Lee EY, deVilliers WJ, Zhong J, Oz HS, Westberry KF, McClain CJ. Green tea polyphenol extract attenuates inflammation in interleukin-2-deficient mice, a model of autoimmunity. J Nutr. 2001;131:2034–2039. doi: 10.1093/jn/131.7.2034. [DOI] [PubMed] [Google Scholar]
- 5.Das M, Sur P, Gomes A, Vedasiromoni JR, Ganguly DK. Inhibition of tumor growth and inflammation by consumption of tea. Phytother Res. 2002;16:S40–S44. doi: 10.1002/ptr.797. [DOI] [PubMed] [Google Scholar]
- 6.Jin X, Zheng RH, Li YM. Green tea consumption and liver disease: a systematic review. Liver International. 2008;28(7):990–996. doi: 10.1111/j.1478-3231.2008.01776.x. [DOI] [PubMed] [Google Scholar]
- 7.Hasegawa R, Chujo T, Sai-Kato K, Umemura T, Tanimura A, Kurokawa Y. Preventive effects of green tea against liver oxidative DNA damage and hepatotoxicity in rats treated with 2-nitropropane. Food Chem Toxicol. 1995;33:961–970. doi: 10.1016/0278-6915(95)00064-9. [DOI] [PubMed] [Google Scholar]
- 8.Cai YJ, Ma LP, Hou LF, Zhou B, Yang L, Liu ZL. Antioxidant effects of green tea polyphenols on free radical initiated peroxidation of rat liver microsomes. Chem Phys Lipids. 2002;120:109–117. doi: 10.1016/s0009-3084(02)00110-x. [DOI] [PubMed] [Google Scholar]
- 9.Klaunig JE. Chemopreventive effects of green tea components on hepatic carcinogenesis. Prev Med. 1992;21:510–519. doi: 10.1016/0091-7435(92)90058-p. [DOI] [PubMed] [Google Scholar]
- 10.Valcic S, Muders A, Neil EJ, Liebler DC, Timmermann BN. Antioxidant Chemistry of Green Tea Catechins. Identification of Products of the Reaction of (–)-Epigallocatechin Gallate with Peroxyl Radicals Chem Res Toxicol. 1999;12:382–386. doi: 10.1021/tx990003t. [DOI] [PubMed] [Google Scholar]
- 11.Xiao J, Lu R, Shen X, Wu M. Green tea extracts protected against carbon tetrachloride-induced chronic liver damage and cirrhosis. Zhonghua Yu Fang Yi Xue Za Zhi. 2002;36:243–6. [PubMed] [Google Scholar]
- 12.Li YM, Zhang XG, Zhou HL, Chen SH, Zhang Y, Yu CH. Effects of tea polyphenols on hepatic fibrosis in rats with alcoholic liver disease. Hepatobiliary Pancreat Dis Int. 2004;3:577–579. [PubMed] [Google Scholar]
- 13.Bun SS, Bun H, Guédon D, Rosier C, Ollivier E. Effect of green tea extracts on liver functions in Wistar rats. Food Chem Toxicol. 2006;44:1108–1113. doi: 10.1016/j.fct.2006.01.006. [DOI] [PubMed] [Google Scholar]
- 14.Fujiki H, Suganuma M, Okabe S. Mechanistic findings of green tea as cancer preventive for humans. Proc Soc Exp Biol Med. 1999;220:225–228. doi: 10.1046/j.1525-1373.1999.d01-38.x. [DOI] [PubMed] [Google Scholar]
- 15.Buschman JL. Green tea and cancer in humans: A review of the literature. Nutr Cancer. 1998;31:151–159. doi: 10.1080/01635589809514697. [DOI] [PubMed] [Google Scholar]
- 16.Esterbauer H, Cheeseman KH. Determination of aldehydic lipid peroxidation products: Malonaldehyde and 4-hydroxnonenal. Methods Enzymol. 1990;186:407–421. doi: 10.1016/0076-6879(90)86134-h. [DOI] [PubMed] [Google Scholar]
- 17.Bass NM, Ockner BA. Drug-induced liver disease. In: Zakin D, Boyer TD, editors. Hepatology: a textbook of liver disease. 3rd eds. Philadelphia: WB Saunders; 1996. pp. 962–1017. [Google Scholar]
- 18.Jones AL. Anatomy of the normal liver. In: Zakin D, Boyer TD, editors. Hepatology: a textbook of liver disease. 3rd ed. Philadelphia: WB Saunders; 1996. pp. 3–32. [Google Scholar]
- 19.Steven CK. Alanine and Aspartate aminotransferase, principle and usage. In: James JT, Jenifer R, editors. Liver function, In Clinical chemistry theory, analysis and correlation. 3rd ed. London: M. Mosby; 1996. pp. 504–527. [Google Scholar]
- 20.Juliet RH, John AL. Alkaline phosphatase, principle of analysis and current usage. In: James JT, Jenifer R, editors. Liver function, In Clinical chemistry, theory, analysis and correlation. 3rd ed. London: M. Mosby; 1996. pp. 504–527. [Google Scholar]
- 21.Steven CK. Total bilirubin.Principle of analysis and current usage. In: James JT, Jenifer R, editors. Liver function, In Clinical chemistry theory, analysis and correlation. 3rd ed. London: M. Mosby; 1996. pp. 504–527. [Google Scholar]
- 22.Doumas BT, Bayse D, Borner K, Cart RJ, Peters T, Schaffer R. A candidate reference method for the determination of total protein in serum: Development and validation. Clin Chem. 1981;27:1642–1650. [PubMed] [Google Scholar]
- 23.Gustafsson JEC. Improved specificity of serum albumin determination and estimation of acute phase reactant" by the use of bromocresol green reaction. Clin Chem. 1976;22:616–622. [PubMed] [Google Scholar]
- 24.Avwioro OG. 1st ed. Nigeria: Claverianum press; 2010. Histochemistry and tissue pathology: Principles and techniques. [Google Scholar]
- 25.Heping C, Meghan AK, Frank K, Dawson HD, Urban JF, Jr, Coves S, Roussel AM, Anderson RA. Green tea increases anti-inflammatory tristetraprolin and decreases pro-inflammatory tumor necrosis factor mRNA levels in rats. J Infl. 2007;4:1. doi: 10.1186/1476-9255-4-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Koo MWL, Cho CH. Pharmacological effects of green tea on the gastrointestinal system. Eur J Pharmacol. 2004;500:177–185. doi: 10.1016/j.ejphar.2004.07.023. [DOI] [PubMed] [Google Scholar]
- 27.McKay DL, Blumberg JB. The role of tea in human health: An update. J Am Coll Nutr. 2002;21:1–13. doi: 10.1080/07315724.2002.10719187. [DOI] [PubMed] [Google Scholar]
- 28.Lu H, Meng X, Li C, Sang S, Patten C, Sheng S, Hong J, Bai N, Winnik B, Ho CT, Yang CS. Glucuronides of tea catechins: enzymology of biosynthesis and biological activities. Drug Metab Dispos. 2003;31:452–461. doi: 10.1124/dmd.31.4.452. [DOI] [PubMed] [Google Scholar]
- 29.Mukoyama A, Ushijima H, Nishimura S, Koike H, Toda M, Hara Y, Shimamura T. Inhibition of rotavirus and enterovirus infections by tea extracts. Jpn J Med Sci Biol. 1991;44:181–186. doi: 10.7883/yoken1952.44.181. [DOI] [PubMed] [Google Scholar]
- 30.Yama TS, Shaha S, Hamilton-Millera JMT. Microbiological activity of whole and fractionated crude extracts of tea (Camellia sinensis), and of tea components. FEMS Microbiol Lett. 1997;152:169–174. doi: 10.1111/j.1574-6968.1997.tb10424.x. [DOI] [PubMed] [Google Scholar]
- 31.Weber JM, Ruzindana-Umunyana A, Imbeault L, Sircar S. Inhibition of adenovirus infection and adenain by green tea catechins. Antiviral Res. 2003;58:167–173. doi: 10.1016/s0166-3542(02)00212-7. [DOI] [PubMed] [Google Scholar]
- 32.Takabayashi F, Harada N, Yamada M, Murohisa B, Oguni I. Inhibitory effect of green tea catechins in combination with sucralfate on Helicobacter pylori infection in Mongolian gerbils. J Gastroenterol. 2004;39:61–63. doi: 10.1007/s00535-003-1246-0. [DOI] [PubMed] [Google Scholar]
- 33.Arburjai T, Natsheh FM. Plants used in cosmetics. Phytother Res. 2003;17:987–1000. doi: 10.1002/ptr.1363. [DOI] [PubMed] [Google Scholar]
- 34.Galati G, Lin A, Sultan AM, O’Brien PJ. Cellular and in vivo hepatotoxicity caused by green tea phenolic acids and catechins. Free Radic Biol Med. 2006;40:570–580. doi: 10.1016/j.freeradbiomed.2005.09.014. [DOI] [PubMed] [Google Scholar]
- 35.Federico A, Tiso A, Loguercio C. A case of hepatotoxicity caused by green tea. Free Radic Biol Med. 2007;43:474. doi: 10.1016/j.freeradbiomed.2007.05.010. [DOI] [PubMed] [Google Scholar]
- 36.Jimenez-Saenz M, Martinez-Sanchez Mdel C. Acute hepatitis associated with the use of green tea infusions. J Hepatol. 2006;44:616–617. doi: 10.1016/j.jhep.2005.11.041. [DOI] [PubMed] [Google Scholar]
- 37.Belitz DH, Grosch W. Acribia, Zaragoza Espania. 2nd ed 1997. Quı’mica de los Alimentos. [Google Scholar]


