Abstract
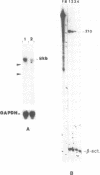
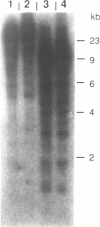
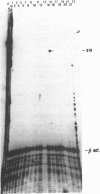
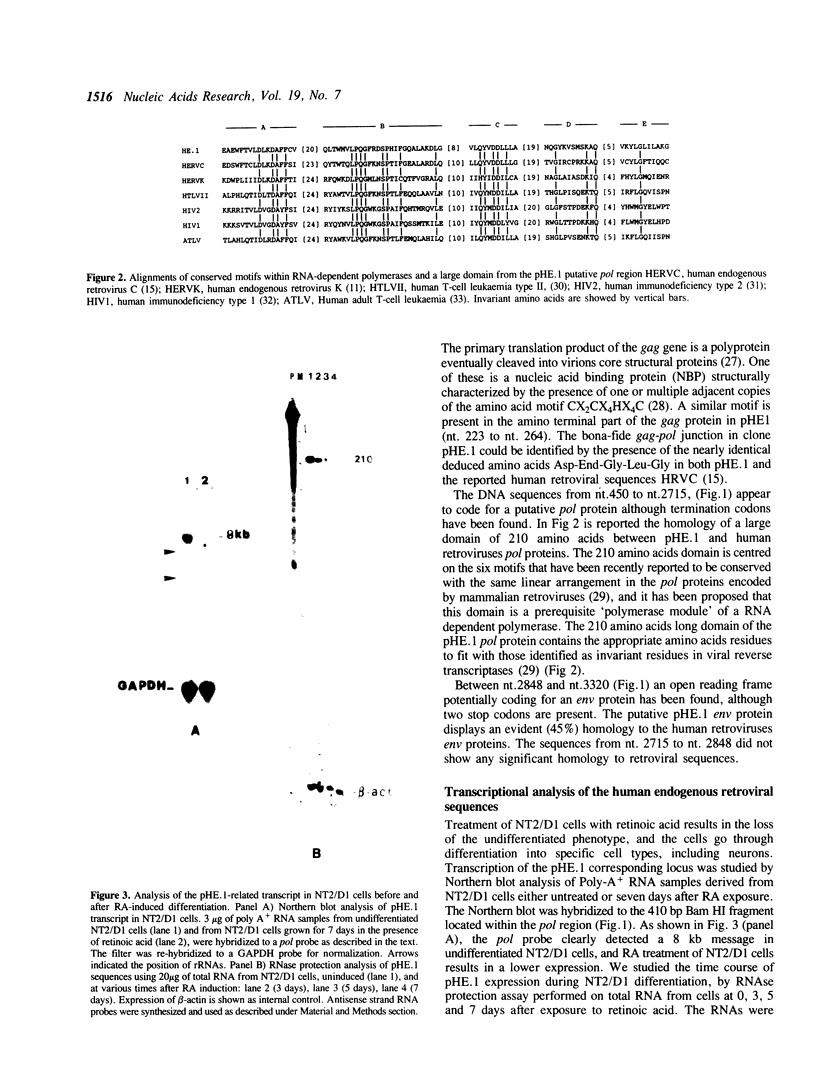
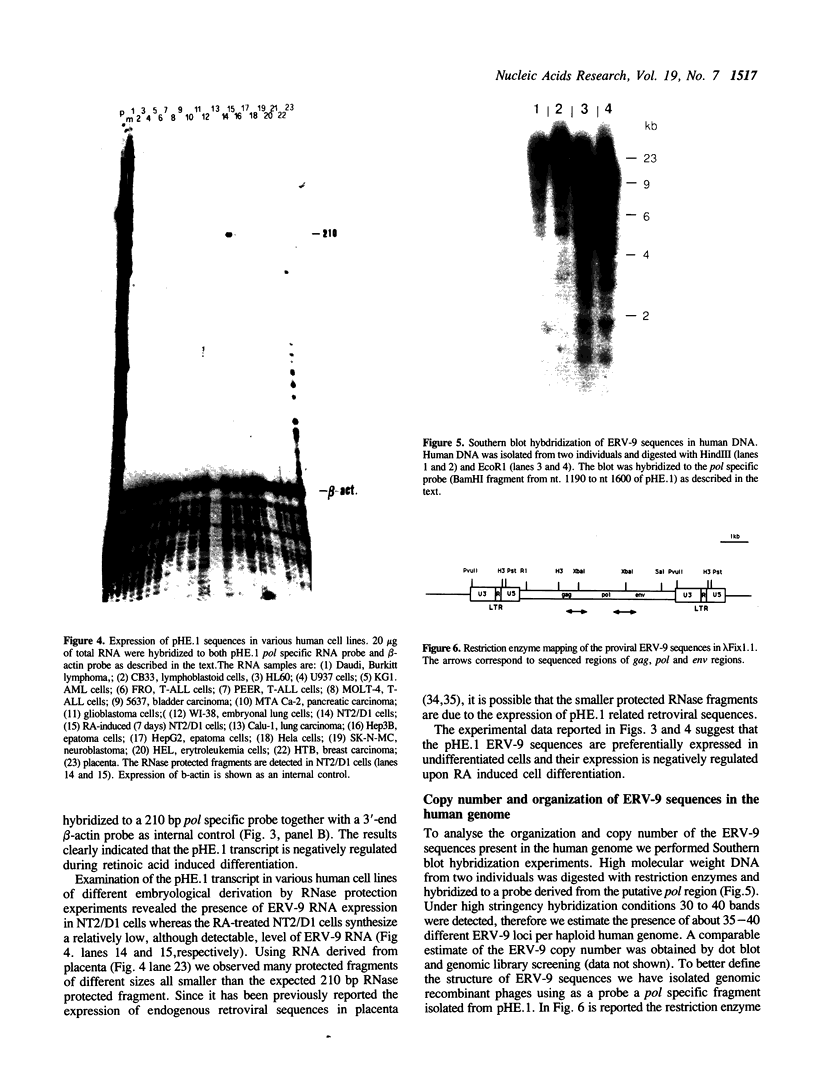
A novel endogenous retroviral sequence (ERV-9) has been isolated from a human embryonal carcinoma cDNA library by hybridization to a probe containing a recently described human repetitive element. DNA sequence analysis of the 4kb cDNA insert (pHE.1) revealed the presence of ORFs potentially coding for putative retrovirus-related gag, pol and env proteins. Northern blot and RNase protection experiments showed that RNA homologous to the pHE.1 insert is detected only in embryonal carcinoma cells as a 8 kb mRNA, and its expression is negatively regulated during retinoic acid induced differentiation of the human teratocarcinoma cell line NT2/D1. Using a pol specific probe we have isolated a genomic locus containing the ERV-9 sequences. Characterization by restriction enzyme analysis and DNA sequencing allowed us to define LTR-like sequences, that are composed by a complex array of subrepetitive elements. In addition we show that ERV-9 LTR sequences are capable to drive expression of linked CAT gene in a cell specific manner as LTR promoter activity has been detected only in NT2/D1 cells.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andrews P. W. Retinoic acid induces neuronal differentiation of a cloned human embryonal carcinoma cell line in vitro. Dev Biol. 1984 Jun;103(2):285–293. doi: 10.1016/0012-1606(84)90316-6. [DOI] [PubMed] [Google Scholar]
- Bonner T. I., O'Connell C., Cohen M. Cloned endogenous retroviral sequences from human DNA. Proc Natl Acad Sci U S A. 1982 Aug;79(15):4709–4713. doi: 10.1073/pnas.79.15.4709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Callahan R., Chiu I. M., Wong J. F., Tronick S. R., Roe B. A., Aaronson S. A., Schlom J. A new class of endogenous human retroviral genomes. Science. 1985 Jun 7;228(4704):1208–1211. doi: 10.1126/science.2408338. [DOI] [PubMed] [Google Scholar]
- Chen H. R., Barker W. C. Nucleotide sequences of the retroviral long terminal repeats and their adjacent regions. Nucleic Acids Res. 1984 Feb 24;12(4):1767–1778. doi: 10.1093/nar/12.4.1767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Covey S. N. Amino acid sequence homology in gag region of reverse transcribing elements and the coat protein gene of cauliflower mosaic virus. Nucleic Acids Res. 1986 Jan 24;14(2):623–633. doi: 10.1093/nar/14.2.623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curatola A. M., Basilico C. Expression of the K-fgf proto-oncogene is controlled by 3' regulatory elements which are specific for embryonal carcinoma cells. Mol Cell Biol. 1990 Jun;10(6):2475–2484. doi: 10.1128/mcb.10.6.2475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deragon J. M., Sinnett D., Labuda D. Reverse transcriptase activity from human embryonal carcinoma cells NTera2D1. EMBO J. 1990 Oct;9(10):3363–3368. doi: 10.1002/j.1460-2075.1990.tb07537.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Derks J. P., Hofmans L., Bruning H. W., von Rood J. J. Synthesis of a viral protein with molecular weight of 30,000 (p30) by leukemic cells and antibodies cross-reacting with Simian sarcoma virus p30 in serum of a chronic myeloid leukemia patient. Cancer Res. 1982 Feb;42(2):681–686. [PubMed] [Google Scholar]
- Gorman C. M., Moffat L. F., Howard B. H. Recombinant genomes which express chloramphenicol acetyltransferase in mammalian cells. Mol Cell Biol. 1982 Sep;2(9):1044–1051. doi: 10.1128/mcb.2.9.1044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guyader M., Emerman M., Sonigo P., Clavel F., Montagnier L., Alizon M. Genome organization and transactivation of the human immunodeficiency virus type 2. Nature. 1987 Apr 16;326(6114):662–669. doi: 10.1038/326662a0. [DOI] [PubMed] [Google Scholar]
- Hawley R. G., Shulman M. J., Murialdo H., Gibson D. M., Hozumi N. Mutant immunoglobulin genes have repetitive DNA elements inserted into their intervening sequences. Proc Natl Acad Sci U S A. 1982 Dec;79(23):7425–7429. doi: 10.1073/pnas.79.23.7425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jerabek L. B., Mellors R. C., Elkon K. B., Mellors J. W. Detection and immunochemical characterization of a primate type C retrovirus-related p30 protein in normal human placentas. Proc Natl Acad Sci U S A. 1984 Oct;81(20):6501–6505. doi: 10.1073/pnas.81.20.6501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kato N., Pfeifer-Ohlsson S., Kato M., Larsson E., Rydnert J., Ohlsson R., Cohen M. Tissue-specific expression of human provirus ERV3 mRNA in human placenta: two of the three ERV3 mRNAs contain human cellular sequences. J Virol. 1987 Jul;61(7):2182–2191. doi: 10.1128/jvi.61.7.2182-2191.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kongsuwan K., Allen J., Adams J. M. Expression of Hox-2.4 homeobox gene directed by proviral insertion in a myeloid leukemia. Nucleic Acids Res. 1989 Mar 11;17(5):1881–1892. doi: 10.1093/nar/17.5.1881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kröger B., Horak I. Isolation of novel human retrovirus-related sequences by hybridization to synthetic oligonucleotides complementary to the tRNA(Pro) primer-binding site. J Virol. 1987 Jul;61(7):2071–2075. doi: 10.1128/jvi.61.7.2071-2075.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- La Mantia G., Pengue G., Maglione D., Pannuti A., Pascucci A., Lania L. Identification of new human repetitive sequences: characterization of the corresponding cDNAs and their expression in embryonal carcinoma cells. Nucleic Acids Res. 1989 Aug 11;17(15):5913–5922. doi: 10.1093/nar/17.15.5913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leib-Mösch C., Brack R., Werner T., Erfle V., Hehlmann R. Isolation of an SSAV-related endogenous sequence from human DNA. Virology. 1986 Dec;155(2):666–677. doi: 10.1016/0042-6822(86)90226-6. [DOI] [PubMed] [Google Scholar]
- Löwer J., Wondrak E. M., Kurth R. Genome analysis and reverse transcriptase activity of human teratocarcinoma-derived retroviruses. J Gen Virol. 1987 Nov;68(Pt 11):2807–2815. doi: 10.1099/0022-1317-68-11-2807. [DOI] [PubMed] [Google Scholar]
- Lüscher B., Mitchell P. J., Williams T., Tjian R. Regulation of transcription factor AP-2 by the morphogen retinoic acid and by second messengers. Genes Dev. 1989 Oct;3(10):1507–1517. doi: 10.1101/gad.3.10.1507. [DOI] [PubMed] [Google Scholar]
- Mager D. L., Henthorn P. S. Identification of a retrovirus-like repetitive element in human DNA. Proc Natl Acad Sci U S A. 1984 Dec;81(23):7510–7514. doi: 10.1073/pnas.81.23.7510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Man Y. M., Delius H., Leader D. P. Molecular analysis of elements inserted into mouse gamma-actin processed pseudogenes. Nucleic Acids Res. 1987 Apr 24;15(8):3291–3304. doi: 10.1093/nar/15.8.3291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin M. A., Bryan T., Rasheed S., Khan A. S. Identification and cloning of endogenous retroviral sequences present in human DNA. Proc Natl Acad Sci U S A. 1981 Aug;78(8):4892–4896. doi: 10.1073/pnas.78.8.4892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mavilio F., Simeone A., Boncinelli E., Andrews P. W. Activation of four homeobox gene clusters in human embryonal carcinoma cells induced to differentiate by retinoic acid. Differentiation. 1988;37(1):73–79. doi: 10.1111/j.1432-0436.1988.tb00798.x. [DOI] [PubMed] [Google Scholar]
- O'Connell C. D., Cohen M. The long terminal repeat sequences of a novel human endogenous retrovirus. Science. 1984 Dec 7;226(4679):1204–1206. doi: 10.1126/science.6505687. [DOI] [PubMed] [Google Scholar]
- Okamoto K., Okazawa H., Okuda A., Sakai M., Muramatsu M., Hamada H. A novel octamer binding transcription factor is differentially expressed in mouse embryonic cells. Cell. 1990 Feb 9;60(3):461–472. doi: 10.1016/0092-8674(90)90597-8. [DOI] [PubMed] [Google Scholar]
- Ono M., Yasunaga T., Miyata T., Ushikubo H. Nucleotide sequence of human endogenous retrovirus genome related to the mouse mammary tumor virus genome. J Virol. 1986 Nov;60(2):589–598. doi: 10.1128/jvi.60.2.589-598.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pannuti A., La Mantia G., Lania L. Regulation of viral and cellular promoter activity by polyomavirus early proteins. Nucleic Acids Res. 1987 Feb 25;15(4):1595–1613. doi: 10.1093/nar/15.4.1595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perl A., Rosenblatt J. D., Chen I. S., DiVincenzo J. P., Bever R., Poiesz B. J., Abraham G. N. Detection and cloning of new HTLV-related endogenous sequences in man. Nucleic Acids Res. 1989 Sep 12;17(17):6841–6854. doi: 10.1093/nar/17.17.6841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poch O., Sauvaget I., Delarue M., Tordo N. Identification of four conserved motifs among the RNA-dependent polymerase encoding elements. EMBO J. 1989 Dec 1;8(12):3867–3874. doi: 10.1002/j.1460-2075.1989.tb08565.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ponte P., Gunning P., Blau H., Kedes L. Human actin genes are single copy for alpha-skeletal and alpha-cardiac actin but multicopy for beta- and gamma-cytoskeletal genes: 3' untranslated regions are isotype specific but are conserved in evolution. Mol Cell Biol. 1983 Oct;3(10):1783–1791. doi: 10.1128/mcb.3.10.1783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rabson A. B., Hamagishi Y., Steele P. E., Tykocinski M., Martin M. A. Characterization of human endogenous retroviral envelope RNA transcripts. J Virol. 1985 Oct;56(1):176–182. doi: 10.1128/jvi.56.1.176-182.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Repaske R., Steele P. E., O'Neill R. R., Rabson A. B., Martin M. A. Nucleotide sequence of a full-length human endogenous retroviral segment. J Virol. 1985 Jun;54(3):764–772. doi: 10.1128/jvi.54.3.764-772.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosner M. H., Vigano M. A., Ozato K., Timmons P. M., Poirier F., Rigby P. W., Staudt L. M. A POU-domain transcription factor in early stem cells and germ cells of the mammalian embryo. Nature. 1990 Jun 21;345(6277):686–692. doi: 10.1038/345686a0. [DOI] [PubMed] [Google Scholar]
- Seiki M., Hattori S., Hirayama Y., Yoshida M. Human adult T-cell leukemia virus: complete nucleotide sequence of the provirus genome integrated in leukemia cell DNA. Proc Natl Acad Sci U S A. 1983 Jun;80(12):3618–3622. doi: 10.1073/pnas.80.12.3618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimotohno K., Takahashi Y., Shimizu N., Gojobori T., Golde D. W., Chen I. S., Miwa M., Sugimura T. Complete nucleotide sequence of an infectious clone of human T-cell leukemia virus type II: an open reading frame for the protease gene. Proc Natl Acad Sci U S A. 1985 May;82(10):3101–3105. doi: 10.1073/pnas.82.10.3101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simeone A., Acampora D., Arcioni L., Andrews P. W., Boncinelli E., Mavilio F. Sequential activation of HOX2 homeobox genes by retinoic acid in human embryonal carcinoma cells. Nature. 1990 Aug 23;346(6286):763–766. doi: 10.1038/346763a0. [DOI] [PubMed] [Google Scholar]
- Skowronski J., Singer M. F. Expression of a cytoplasmic LINE-1 transcript is regulated in a human teratocarcinoma cell line. Proc Natl Acad Sci U S A. 1985 Sep;82(18):6050–6054. doi: 10.1073/pnas.82.18.6050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suni J., Närvänen A., Wahlström T., Aho M., Pakkanen R., Vaheri A., Copeland T., Cohen M., Oroszlan S. Human placental syncytiotrophoblastic Mr 75,000 polypeptide defined by antibodies to a synthetic peptide based on a cloned human endogenous retroviral DNA sequence. Proc Natl Acad Sci U S A. 1984 Oct;81(19):6197–6201. doi: 10.1073/pnas.81.19.6197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varmus H. E. Form and function of retroviral proviruses. Science. 1982 May 21;216(4548):812–820. doi: 10.1126/science.6177038. [DOI] [PubMed] [Google Scholar]
- Wain-Hobson S., Sonigo P., Danos O., Cole S., Alizon M. Nucleotide sequence of the AIDS virus, LAV. Cell. 1985 Jan;40(1):9–17. doi: 10.1016/0092-8674(85)90303-4. [DOI] [PubMed] [Google Scholar]
- White R. J., Stott D., Rigby P. W. Regulation of RNA polymerase III transcription in response to F9 embryonal carcinoma stem cell differentiation. Cell. 1989 Dec 22;59(6):1081–1092. doi: 10.1016/0092-8674(89)90764-2. [DOI] [PubMed] [Google Scholar]
- Ymer S., Tucker W. Q., Sanderson C. J., Hapel A. J., Campbell H. D., Young I. G. Constitutive synthesis of interleukin-3 by leukaemia cell line WEHI-3B is due to retroviral insertion near the gene. Nature. 1985 Sep 19;317(6034):255–258. doi: 10.1038/317255a0. [DOI] [PubMed] [Google Scholar]