Abstract
Background:
Pneumonia is the biggest single cause of childhood death under the age of 5 years, and anemia affects approximately 30% of infants and children all over the world.
Aim:
Determination of the relationship between anemia and lower respiratory tract infection as a risk factor in Lebanese children.
Patients and Methods:
A total number of two hundred infants and children aged nine months to twelve years were included; One hundred cases were hospitalized for lower respiratory tract infection in Department of Pediatrics, Makassed General Hospital, and one hundred healthy, age and sex matched controls, were selected from outpatient department. Complete blood count, iron level, ferritin level, and total iron binding capacity were taken if hemoglobin level less than eleven gram per deci-liter. In addition peripheral blood smear, chest radiograph and C-reactive protein were done to hospitalized cases. Definition of iron deficiency anemia and normal laboratory values were predetermined.
Results:
Anemia was found in 32% of hospitalized cases and 16% of healthy controls. Mean hemoglobin level was 9.99 ± 0.62 gram per deci-liter and 11.99 ± 0.92 gram per deci-liter in anemic and non-anemic group respectively with a significant P-value of 0.001. C-reactive protein levels and number hospitalization days were similar among the anemic and non-anemic group. History of recurrent chest infections was significantly higher in both anemic group and hospitalized cases compared to non-anemic group and healthy controls. Low hemoglobin level was a risk factor for lower respiratory tract infection with a P-value of 0.008.
Conclusion:
Anemic children were two times more susceptible to lower respiratory tract infection compared to the control group, and iron deficiency anemia was predominating. Accurate diagnosis and prevention of anemia, whatever its etiology, is essential.
Keywords: Hemoglobin level, lower respiratory tract infection, iron deficiency anemia
Introduction
Lower respiratory tract infection (LRTI) includes all infections of the lungs and the large airways below the larynx. On average, children below 5 years of age suffer about 5 to 6 episodes of LRTI per year, and still a burden until 12 years of age and more[1].
Pneumonia is the biggest single cause of childhood death under the age of 5 years in developing countries[2]. Globally there are about three million deaths, less than 5 years of age, each year due to pneumonia. Of these deaths, 90 to 95% are in the developing countries[3].
LRTI associated with anemia occurs more commonly in children than in adults, with anemia affecting approximately 30% of children all over the world[4,5]. Iron deficiency anemia in children occurs most frequently between the age of 6 months and 3 years, the same period of age when repeated infections occur[6].
Whatever the etiology of anemia, the relation between low hemoglobin level and LRTI has not been fully evaluated, and only few reports are available evaluating this subject[7].
The goal of this prospective study is to determine the relationship between anemia as a risk factor and LRTI, in Lebanese children aged 9 months to 12 years.
Patients and Methods
This prospective comparative study, approved by the Institutional Review Board committee, was conducted in Department of Pediatrics in Makassed General Hospital (MGH) between September 2009 and April 2010. A total of 200 children aging between 9 months and 12 years were selected; 100 cases hospitalized for lower respiratory tract infection (LRTI), and 100 healthy controls without any respiratory problems, age and sex matched, attending Out Patient Department.
Inclusion Criteria
We included in the study all hospitalized children aged between 9 months and 12 years with a diagnosis of LRTI; fever, cough, tachypnea, chest retractions, and ronchi or crackles up on chest auscultation, as per WHO criteria[1,8,9]. Weight and height were recorded to all children in order to assess the nutritional status. A written consent was taken from parents or guardians before they were subjected to investigations. The following laboratory tests were done in all children: complete blood count, iron level, ferritin level, tuberculosis skin test PPD test, and total iron binding capacity (TIBC) if hemoglobin level was below 11 g/dl. Hospitalized cases had in addition peripheral blood smear, C-reactive protein level (CRP), and chest radiograph.
Exclusion Criteria
Exclusion criteria included children with prematurity, congenital chest wall malformations, severe systemic illness (congenital heart disease, tuberculosis, etc), chronic diseases (diabetes, hepatitis, liver failure, etc), intake of iron supplements, and previous history of infection in the control group.
Blood Screening
A trained phlebotomist drew blood from the antecubital vein of each child. Sterile, disposable syringes and needles, and proper tubes were used. The blood samples were analyzed at MGH clinical laboratory for complete blood count, iron level, ferritin level and TIBC. Hemoglobin level was estimated in the blood samples using an automatic blood cell counter. The cutoff point for low hemoglobin (Hb) level was 11g/dl; meeting the definition of anemia as Hb level being -2 standard deviations (SD) below the mean for age, as fixed by WHO[10]. Iron level and TIBC were measured using the ferrozine method without deproteinization. Reference ranges were 22 to 184μg/dl for iron level, and 228 to 428 μg/dl for TIBC[11]. As for ferritin, the electrochemiluminescence “ECLIA” was used with a cutoff point of 20 μg/l. The transferrin was measured by immunoturbidimetry assay with 200-360 mg/dl reference ranges[11]. Mentzer index was calculated through the formula: Mean corpuscular volume MCV/RBC and the transferrin saturation through the formula: IRON level/TIBC X 100 (normal values: 20-45%). CRP was considered positive if > 0.3 mg/dl. The diagnosis of iron deficiency anemia (IDA) was diagnosed in the control group when a low ferritin level was found with high TIBC, as recommended by the centers of disease control CDC/WHO expert groups on May 2004, being the most valuable indicators of IDA[12].
Considering the fact that infection can affect iron panel studies by increasing ferritin level (usually by more than 50 μg/l if iron deficiency is absent)[13], and decreasing iron level and TIBC, the diagnosis of iron deficiency anemia was done when at least 3 of the below parameters were present: 1) low MCV level with specificity around 96% (not affected by infections)[14]; 2) smear showing hypochromic microcytic anemia[15–17]; 3) red cell distribution width RDW > 14.5 with a sensitivity of 92.1% and specificity of 90.9% in detecting IDA[18–20]; 4) Mentzer index > 13.5 (with around 85% specificity and sensitivity)[21,22]; and 5) transferrrin saturation TS < 10% (with a specificity of 85% if below 15% and sensitivity around 80%)[23–25].
Statistical Analysis
Data analysis was performed using statistical package of social science (SPSS) version 16.0 for windows. Numerical variables were reported in terms of mean and standard deviation. Categorical variables were reported in terms of numbers and percentages. Association of each of the categorical variable with response variable was assessed by Chi-square test. Variables showing statistically significant association in univariate analysis with the outcome variable were considered as risk factor. Only those variables were subjected to multivariate analysis. Logistic regression method was used to find the risk factor for LRTI. In multivariate analysis, variables showing P-value less than 0.05 were considered to be statistically significant. The sample size, 100 in each group, was found to be capable to detect a difference of 44% reduction in the percentage of anemia between cases and control group with α = 5% and power 80%.
Results
A total of 200 infants and children aged between 9 Months and 12 years were enrolled in the study. One hundred patients were admitted to the pediatric ward at MGH with LRTI according to the inclusion criteria. Fifty one percent were males and 49% females. Another 100 healthy controls were studied in the outpatient department, 52% were males and 48% females. Both groups age ranged from 9 months to 12 years (Table 1).
Table 1.
Distribution of the patients according to the sex

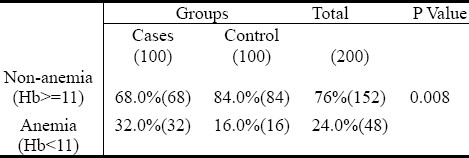
Height and weight were recorded to all patients, and body mass index (BMI) was calculated for children above 2 years. All infants and children studied were well nourished. Hemoglobin level was considered low when below 11g/dl, which is less than 2 standard deviation (SD), as per WHO definition. Out of the total 200 infants and children in the study, 48 were found to be anemic (24% with 54% boys and 46% girls), with hemoglobin level below 11 g/dl in 32% of cases admitted to pediatric ward with a diagnosis of LRTI and in 16% of healthy controls, which was significantly different with a P-value of 0.008 (Table 2).
Table 2.
Percentage of anemic and non anemic children in each group
The anemic group considered in both hospitalized cases and healthy controls has a mean age of 25 ± 17.8 months. When compared to non-anemic group in hospitalized cases and healthy controls with mean age of 56.9 ± 41.9 months was highly significant with a P-value of 0.001. P-value was highly significant (0.001) between anemic and non-anemic hospitalized cases with mean Hb level of 9.99 ± 0.62g/dl and 11.99 ± 0.92 g/dl, respectively. P-value was also very highly significant (P=0.0001) between anemic and non-anemic healthy controls with mean Hb level of 10.27 ± 0.52g/dl and 12.27 ± 0.75g/dl, respectively. Comparisons between both non-anemic groups was significant with P-value of 0.034, with mean Hb level of 11.99 ± 0.92g/dl of hospitalized cases and 12,27 ± 0, 75g/dl of healthy controls. Mild significance of P-value (0.125) was found between anemic hospitalized cases and healthy controls with mean Hb level of 9.99 ± 0.62g/dl and 10.27 ± 0.52g/dl respectively. As for comparison between all hospitalized cases and healthy controls P-value was highly significant with a P=0.0001.
Anemia was found to be a risk factor for LRTI with an Odds Ratio of 2.08 with 95% interval confidence of 1.03 - 4.20 and a significant P-value of 0.004 (Table 3). This means that children with Hb level below 11 g/dl were 2 times more susceptible to LRTI compared to the control group.
Table 3.
Multivariate logistic regression analysis showing the risk factor of LRTI

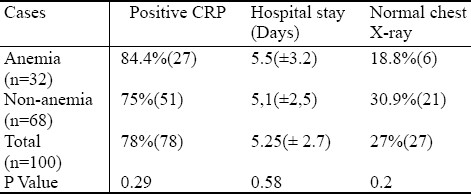
Concerning the cases hospitalized for LRTI, anemic group had a positive CRP in 84.4% (27), mean hospital stay of 5.5 (±3.2) days and normal chest X-ray in 18.8% (6) comparing to 75% (51), 5.1(± 2.5) days and 30.9% (21) of non-anemic group, respectively, with a non significant P-value between the 2 groups for positive CRP, hospitalization days, and no significance concerning normal chest radiograph finding, with 70% of all cases with a picture of pneumonia in chest radiograph (Table 4).
Table 4.
Comparison of positive CRP, hospitalization days and Normal chest X-ray between anemic and non-anemic groups of cases hospitalized for LRTI
From all 200 infants and children, 37.5% of anemic group had a history of recurrent chest infections compared to 14.5% of non-anemic group, with a highly significant P-value of 0.001 and even the difference between anemic hospitalized cases and healthy controls was significant with a P-value of 0.05. Family history of allergy was found in 33.3% of anemic group comparing to 37.5% of non-anemic group (P=0.83).
Difference between anemic and non-anemic groups concerning RDW, low MCV or both was significant with P-value 0.0001. Mean RDW was 15.1 ± 2.3 in anemic group and 13.1 ± 1.1 in non-anemic group. Low MCV was found in 48 % (23) of anemic group and 5.3% (8) of non-anemic group. However, we found a total of 48 infants and children in the anemic group, 32 from hospitalized cases and 16 from healthy controls. Twenty four of those met the criteria of IDA in anemic hospitalized children (75%) and 11 (68.75%) in anemic healthy controls (Fig. 1).
Fig. 1.
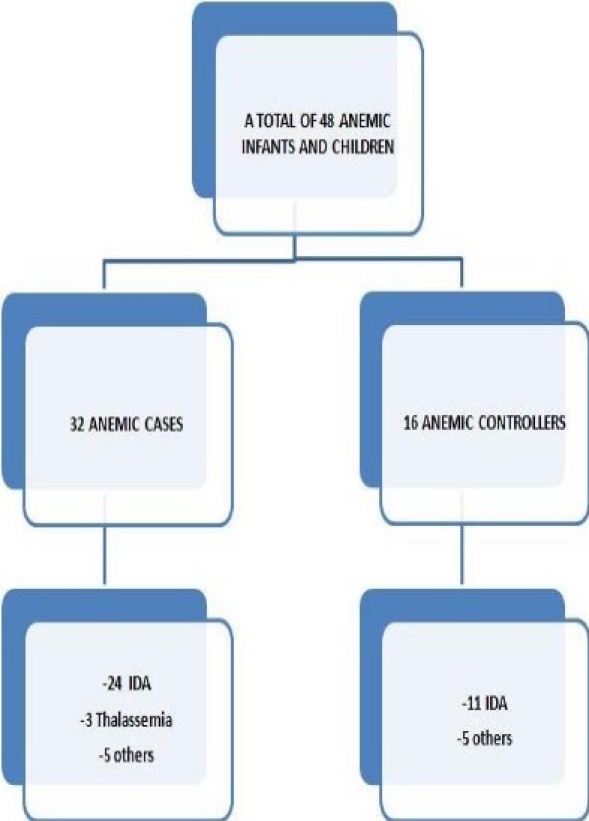
Repartition of anemic infants and children between cases
Discussion
The prevalence of anemia varies between developed and developing countries. Reaching up to 50% of preschool children in some developing countries, ranging from 20% to 67% across several Arab Gulf countries, and is principally caused by iron deficiency. As many as 20% of children in the United States and 80% of children in developing countries will be anemic at some point by the age of 18 years old[26–28]. In our study, 24% of the total number of patients had Hb level below 11g/dl, with 32 % of hospitalized cases and 16% of healthy controls. Mean Hb level was 11.35 ± 1.24g/dl in hospitalized cases and 11.95 ± 1.03g/dl in healthy controls. Anemic group was mainly below 5 years of age with no difference between boys and girls. WHO global database on anemia, the vitamin and mineral nutrition information system (VMNIS) 1998 in Lebanon found that Hb level below 11 g/dl was approximately 24%, and it varied in 614 preschool children according to region distribution, from a maximum percentage of 36.7% in South to 17 % in Beirut similar to our percentage findings for low Hb level.
Acute LRTI is the leading cause of death in children below 5 years. Identification of modifiable risk factors of LRTI may help in reducing the burden of disease[29]. Literature reveals few studies on anemia in patients with acute LRTI. Our study is one of the few studies evaluating Hb level in acute LRTI, and the first one to be done among Lebanese infants and children to our knowledge.
In comparison to the few other studies done on anemia and LRTI, Ramakrishnan et al in 2006 found, in a study of 200 infants and children between 9 months to 16 years, that 74% of cases and 33 % of controls were anemic (with 80% and 82 % IDA, respectively). Boys were more anemic than girls, and the anemic subjects were 5.7 times more susceptible to LRTI[7]. Malla et al, in 2010 in a study done on a total of 280 infants and children aged 1 Months to 5 years, recorded 68.6% of anemic cases and 21.4 % of anemic controls with mean Hb level of 9.8 g/dl and 12 g/dl, 82% and 60 % of IDA, respectively. Eighty three percent of the anemic group had a picture of pneumonia on chest radiograph. Anemia due to mainly IDA was a risk factor for LRTI with an Odds Ratio of 3.2[6]. Bhaskaram et al in 2003 reported anemic cases with 71% IDA and 25% of anemic controls with 46 IDA. Out of 159 children aged 3 to 5 years, the mean Hb level was 9.5 g/dl and 11.4g/dl in study and control group, respectively. Normal chest radiograph was found in 17 % of cases[30]. As for Broor et al, anemia was not found to be a risk factor for LRTI in 512 infants and children below 5 years of age; and normal radiograph was found in 21% of cases[29].
In our study, the percentages of overall anemia, and anemia in both hospitalized cases and healthy controls, were lower compared to the previous studies, unlike the mean Hb level that was higher than values reported in each group, and it is certainly due to the fact that these countries (India, Nepal, Sri Lanka) in which the studies were performed were of high prevalence of IDA compared to Lebanon. A picture of pneumonia in chest radiograph was significantly higher in the anemic group, and this was also observed by Malla et al and Bhaskaram et al[6,30]. The severity of illness assessed by the number of hospitalization days was similar in anemic and non-anemic groups. In our study, a history of recurrent chest infections was significantly related to both anemic group and hospitalized cases.
As for our main objective concerning Hb level as a risk factor for LRTI, results varied from a study to another, with no relation detected in one study[29], to a susceptibility of 3.2 and 5.7 of anemic children having LRTI in 2 other studies[6,7]. In our study, we reported an Odds Ratio of 2.07 with anemia being a risk factor for LRTI. The prevalence of IDA was 35% of our total population, which was approximately similar to the percentage found by other authors, with anemia found to be mainly IDA in both hospitalized cases and healthy controls. Among preschool children living in underprivileged communities in developing countries, infectious diseases such as LRTI and IDA are often coexistent[30]. Researchers have argued that any inadequate supply of iron to body tissues is detrimental to immunity[31]. The effects of IDA on immune function, and increase in susceptibility to infections are well established. Changes in iron status during commonly occurring acute infections in children are not well understood[30]. Because the number of the studies on the iron state of the body in the course of an acute infection is limited[14], and because infection or inflammation can influence iron status[32], it is always very challenging to exclude iron deficiency anemia in the context of concomitant inflammation, and that's why we tried to rely on at least 3 to 5 different available parameters for us to be as accurate as possible in diagnosing IDA alone or in association with anemia of inflammation in the hospitalized patients. Recent studies show that the sensitivity and specificity of diagnosing IDA can be improved by assessing transferrin iron saturation that does not get affected by infection or inflammatory process, age, sex or pregnancy[33–35].
WHO/CDC expert consultation[12] recommended addition of transferrin receptor to hemoglobin and serum ferritin for assessment of iron status in places where infection is common. Recent work suggests that measuring hepcidin levels (iron regulatory peptide) may have future utility in distinguishing between anemia of inflammation and IDA[36]. Iron concentrations have frequently been quantified in pulmonary cells and secretions and are observed to be increased; this supports a disruption in iron homeostasis of the lower respiratory tract. The usual source of iron in the lungs is serum iron which is derived from catabolised erythrocytes and absorbed iron[37]. Lower respiratory tract infection, exaggerates iron deficient erythropoiesis by blocking release of iron from the storage pools.
The sputum and lavage of patients with pneumonia will demonstrate sideromacrophages, which reflect elevated iron concentrations in the lower respiratory tract. With infection, the host iron status can be critical; that's why the issue of whether altered iron homeostasis functions as a primary pathogenic mechanism in lung injury is raised. However, decreasing available iron through either nutritional depletion or use of chelators directly impacts such injury[38–40]. The interplay between iron and infection has been the subject of enduring debate in nutritional immunology, primarily because iron deficiency impairs components of cell mediated immunity[41]. Subsequently, iron does appear to participate directly to immunity and lung injury which suggest several different approaches to prevention and treatment of lung disease[38].
Accordingly, our suggestions are:
-
1)
Screening for Hb or hematocrit level at the age of 9 or 12 months for all full terms infants and 6 months for premature and additional screening before the age of 5 years for patients at risk as American Academy of Paediatrics (AAP) recommends[42].
-
2)
The addition of transferrin receptor or other laboratory tools, in addition to Hb and ferritin, to assess iron status in hospitalized infected children, so we can diagnose properly and treat IDA in these patients, as recommended by CDC/WHO expert consultation[12].
-
3)
If screening anaemia or measuring transferrin receptor is not available for some reasons, then a therapeutic trial of iron should be given, because of the simplicity, low cost and relative safety for infants[43].
Whether to give iron supplementation during or after infection is still controversial, with conflicting results from previous studies, we mention that most studies that support the hypothesis that iron treatment contributes to increased risk of infection are based on data from populations living in impoverished conditions[44].
Conclusion
In summary, studies on anaemia related to lower respiratory tract infections, the role of iron supplementation in preventing the incidence and recurrence of LRTI in infants and children are still lacking in literature. Despite marked recent advances in understanding anaemia, the diagnosis of IDA in the presence of infection or inflammation is still very challenging, therefore, we recommend the use of new laboratory tools and anaemia screening, if not, therapeutical trial of iron is considered when IDA is highly suspected based on history and physical examination with the unavailability to make the diagnosis.
Acknowledgement
The authors would like to thank Dr. Tamima JISR, director of clinical laboratories in Makassed General Hospital, for her valuable contribution and support to this work.
References
- 1.Christi MJ, Tebruegge M, La Vincente S, Graham SM. Pneumonia in Severely Malnourished Children in Developing Countries-mortality risk, Etiology and Validity of WHO clinical signs: A systematic review. Trop Med Int Health. 2009;14(10):1173–1189. doi: 10.1111/j.1365-3156.2009.02364.x. [DOI] [PubMed] [Google Scholar]
- 2.Graham SM, English M, Hazir T, Enarson P. Challenges to improving case management of childhood pneumonia at health facilities in resource-limited settings. Bull WHO. 2008;86:349–355. doi: 10.2471/BLT.07.048512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bryce J, Boschi-Pinto C, Shibuya K. WHO Estimates of the Causes of Death in Children. Lancet. 2005;365:1147–1152. doi: 10.1016/S0140-6736(05)71877-8. [DOI] [PubMed] [Google Scholar]
- 4.Brotanek JM, Gosz J, Weitzman M. Iron Deficiency in Early Childhood in the United States: Risk Factors and Racial/Ethnic Disparities. Pediatrics. 2007;120:568–575. doi: 10.1542/peds.2007-0572. [DOI] [PubMed] [Google Scholar]
- 5.World Health Organization. Focusing on anemia: Towards an Integrated approach for effective anemia control. (Accessed December 12, 2006 at www.paho.org/English/AD/FCH/NU/WHO. )
- 6.Malla T, Pathak OK, Malla KK. Is Low Hemoglobin level a risk factor for acute lower respiratory tract infections? J Nepal Pediatric Soci. 2010;30:1–7. [Google Scholar]
- 7.Ramakrishnan K, Harish PS. Hemoglobin level as a risk factor for lower respiratory tract infections. Indian J Pediatr. 2006;73(10):881–883. doi: 10.1007/BF02859279. [DOI] [PubMed] [Google Scholar]
- 8.Hermiston LM, Mentzer W. A practical approach to the evaluation of the anemic child. Pediatr Clin N Am. 2002;49:877–891. doi: 10.1016/s0031-3955(02)00029-9. [DOI] [PubMed] [Google Scholar]
- 9.Beutler E, Waalen J. The definition of anemia: what is the lower limit for normal of the blood hemoglobin concentration. Blood. 2006;5:1747–1750. doi: 10.1182/blood-2005-07-3046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Iron Deficiency: Indicators for Assessment and Strategies for Prevention. Geneva, Switzerland: World Health Organization; 1997. World Health Organization. WHO/NUT Report No. 96.12. [Google Scholar]
- 11.Kliegman RM, Behrman RE, Jenson HB, Stanton B. Nelson Textbook of Pediatrics. 18th Edition. Saunders; [Google Scholar]
- 12.WHO/CDC. Best indicators to assess iron deficiency, a major cause of anemia. 2004 May 7th; [Google Scholar]
- 13.Cook JD, Skikne BS, Simpson KM, Baynes RD. Ttransferrin receptor distinguishes the anemia of chronic disease from iron deficiency anemia. J Lab Clin Med. 1992;119:385–390. [PubMed] [Google Scholar]
- 14.Tansu S, Tulin K, Betul T. Effects of acute Infection on Hematological Parameters. Pediatric Hematol Oncol. 2004;21:511–518. doi: 10.1080/08880010490477301. [DOI] [PubMed] [Google Scholar]
- 15.Hung OL, Kwon NS, Cole AE, et al. Evaluation of the physician's ability to recognize the presence or absence of anemia, fever and jaundice. Academ Emerg Med. 2007;7:146–156. doi: 10.1111/j.1553-2712.2000.tb00518.x. [DOI] [PubMed] [Google Scholar]
- 16.Patton WN, Cave RJ, Harris RI. A study of changes in red cell volume and hemoglobin concentration during phlebotomy induced iron deficiency and iron repletion using the technion H1. Clin Lab Hematol. 1991;131:153–161. doi: 10.1111/j.1365-2257.1991.tb00264.x. [DOI] [PubMed] [Google Scholar]
- 17.Bridges KR, Pearson HA. Anemias and other red cell disorders. New York, NY: Mc Graw Hill; 2008. pp. 99–100. [Google Scholar]
- 18.Ferrara M, Capozzi L, Russo R, et al. Reliability of red blood cell indices and formulas to discriminate between beta thalassemia trait and iron deficiency in children. Hematol. 2010;15(2):112–125. doi: 10.1179/102453310X12583347010098. [DOI] [PubMed] [Google Scholar]
- 19.Mateos ME, De la Cruz B, Lopez L. Review of hematology and biochemistry parameters to identify iron deficiency. An Pediatr. 2009;71(2):95–102. doi: 10.1016/j.anpedi.2009.04.004. [DOI] [PubMed] [Google Scholar]
- 20.Mentzer WC. Differentiation of iron deficiency from thalassemia trait. Lancet. 1973;1(7808):882. doi: 10.1016/s0140-6736(73)91446-3. [DOI] [PubMed] [Google Scholar]
- 21.Guyatt GH, Oxman AD, Ali M, Willan A, McIlroy W, Patterson C. Laboratory diagnosis of iron-deficiency anemia: an overview. J Gen Intern Med. 1992;7:145–153. doi: 10.1007/BF02598003. [DOI] [PubMed] [Google Scholar]
- 22.Cook JD. Diagnosis and management of iron deficiency anemia. Best Pract Res Clin Haematol. 2005;18:319–332. doi: 10.1016/j.beha.2004.08.022. [DOI] [PubMed] [Google Scholar]
- 23.McGee S. Simplifying likelihood ratios. J Gen Intern Med. 2002;17:646–649. doi: 10.1046/j.1525-1497.2002.10750.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wish Jay B. Assessing Iron Status: Beyond Serum Ferritin and Transferrin Saturation. Clin J Am Soc Nephrol. 2006;1:S4–S8. doi: 10.2215/CJN.01490506. [DOI] [PubMed] [Google Scholar]
- 25.Adaman JW, Dan L. Anemia and polycythemia. Section -10 Hematological Alterations. In: Kasper DL, Fauci AS, Longo DL, Braunwald E, Hauser SL, Jameson JL, editors. In Harrison's Principles of internal medicine. 16th edition. Vol. 1. NY: Mc Graw Hill; 2005. pp. 334–336. [Google Scholar]
- 26.Rasmussen Z, Pio A, Enarson P. Case management of childhood pneumonia in developing countries: Relevant Research and current Initiatives. Int J Tuber Lung Dis. 2000;4:807–827. [PubMed] [Google Scholar]
- 27.Muwakkit S, Nuwayhid I, Nabulsi M, Al Hajj R, et al. Iron deficiency in young Lebanese children: Association with elevated blood lead levels. J Pediatr Hematol Oncol. 2008;30:382–386. doi: 10.1097/MPH.0b013e318165b283. [DOI] [PubMed] [Google Scholar]
- 28.Martin PL, Pearson HA. The anemias. In: Oski FA, editor. Principles and practices of pediatrics. 2nd ed. Philadelphia: J. B. Lippincott; 1994. pp. 1657–1658. [Google Scholar]
- 29.Broor S, Pandey RM, Ghosh M, et al. Risk factors for severe acute lower respiratory tract infection in under–five children. Indian Pediatr. 2001;38(12):1361–1369. [PubMed] [Google Scholar]
- 30.Bhaskaran P, Nair K Madhavan, Balakrishnan N. Serum transferrin receptors in children with respiratory infections. Eur J Nutr. 2003;57:75–80. doi: 10.1038/sj.ejcn.1601496. [DOI] [PubMed] [Google Scholar]
- 31.Ryan AS. Year book of physical anthropology. 1997;40:25–62. [Google Scholar]
- 32.Rahman MA, Mannan A, Hamidur R. Influence of infection on iron profile in severely malnourished children. J Pediatr. 2009;76(9):907–911. doi: 10.1007/s12098-009-0098-x. [DOI] [PubMed] [Google Scholar]
- 33.Cook JD. The measurement of serum transferring receptor. Am J Med Sci. 1999;318:269–270. doi: 10.1097/00000441-199910000-00006. [DOI] [PubMed] [Google Scholar]
- 34.Skikne BS. Serum Transferrin receptor. Am J Hematol. 2008;83(11):872–875. doi: 10.1002/ajh.21279. [DOI] [PubMed] [Google Scholar]
- 35.White KC. Anemia is a poor predictor of iron deficiency among toddlers in the United States. J Pediatrics. 2005;115:315–320. doi: 10.1542/peds.2004-1488. [DOI] [PubMed] [Google Scholar]
- 36.Price EA, Schrier SL. Unexplained aspects of anemia of inflammation. Adv Hematol. 2010;2010:508739. doi: 10.1155/2010/508739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Fernando M, Brock J, Arellanoa JL. Iron metabolism in the lower respiratory tract. Thorax. 1998;53:594–600. doi: 10.1136/thx.53.7.594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ghio AJ. Disruption of iron homeostasis and lung disease. Biochim Biophys Acta. 2009;1790(7):731–739. doi: 10.1016/j.bbagen.2008.11.004. [DOI] [PubMed] [Google Scholar]
- 39.Ganong WF. Review of Medical Physiology. 22nd Ed. New York: Mc Graw-Hill; 2005. Gas transport between the lungs and the tissues; pp. 666–669. [Google Scholar]
- 40.Text Book of Medical Physiology. 11th ed. Philadelphia: Saunders; 2006. Guyton & Hall. Effect of hemoglobin to ‘Buffer’ the tissue PO 2; pp. 507–508. [Google Scholar]
- 41.Roth DE, Caulfield LE, Black RE. Acute lower respiratory infections in childhood opportunities for reducing global burden through nutritional interventions. WHO programmes and projects. Bull WHO. 2008;86(5):321–416. doi: 10.2471/BLT.07.049114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.AAP policy statements. Clinical practice guidelines. Iron Fortification of Infant Formulas. Pediatrics. 1999;104:119–123. [Google Scholar]
- 43.Scott S, Porter M. How should we follow up a positive screen for anaemia in a 1 year old? J Fam Pract. 2005;54(3):272–276. [PubMed] [Google Scholar]
- 44.De-Silva A, Weerasinghel AS. Iron supplementation improves iron status and reduces morbidity in children with or without URTI. Am J Clin Nutr. 2003;77:234–241. doi: 10.1093/ajcn/77.1.234. [DOI] [PubMed] [Google Scholar]


