Abstract
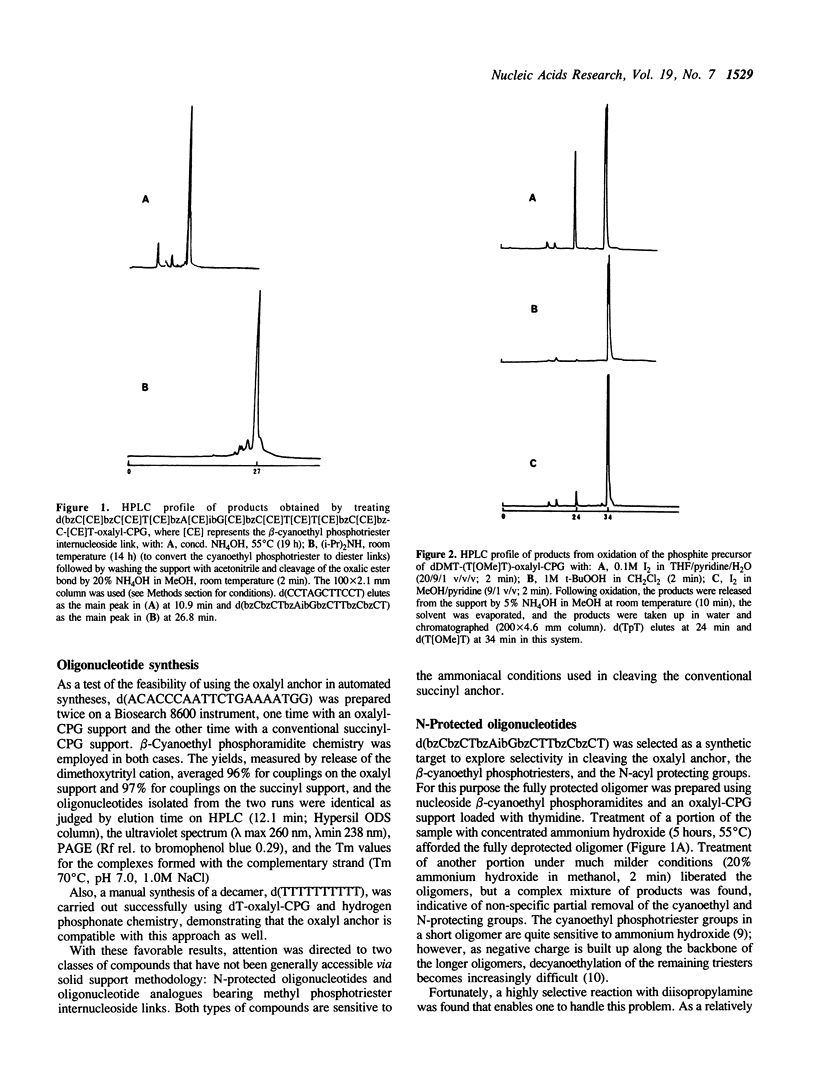
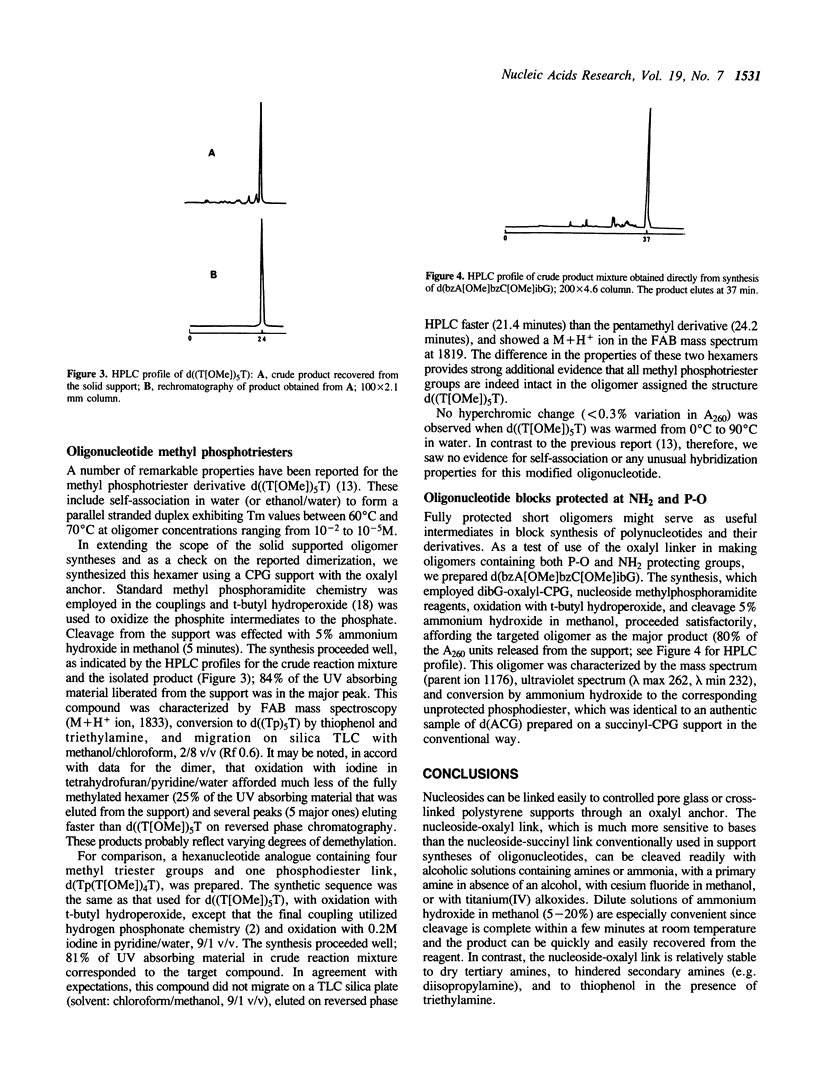
A procedure is described for linking nucleosides covalently to controlled pore glass or cross-linked polystyrene supports by means of an oxalyl anchor. Though stable to triethylamine and diisopropylamine, the nucleoside-oxalyl link can be cleaved within a few minutes at room temperature with ammonium hydroxide in methanol. This new anchor can be used in automated synthesis of conventional oligonucleotides. The primary value, however, is that it enables one to employ solid support methodology to synthesize a variety of base-sensitive oligonucleotide derivatives, as illustrated here by synthesis of oligomers with base protecting groups intact and with methyl phosphotriester groups at the internucleoside links.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Caruthers M. H., Barone A. D., Beaucage S. L., Dodds D. R., Fisher E. F., McBride L. J., Matteucci M., Stabinsky Z., Tang J. Y. Chemical synthesis of deoxyoligonucleotides by the phosphoramidite method. Methods Enzymol. 1987;154:287–313. doi: 10.1016/0076-6879(87)54081-2. [DOI] [PubMed] [Google Scholar]
- Froehler B. C., Ng P. G., Matteucci M. D. Synthesis of DNA via deoxynucleoside H-phosphonate intermediates. Nucleic Acids Res. 1986 Jul 11;14(13):5399–5407. doi: 10.1093/nar/14.13.5399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuijpers W. H., Huskens J., Koole L. H., van Boeckel C. A. Synthesis of well-defined phosphate-methylated DNA fragments: the application of potassium carbonate in methanol as deprotecting reagent. Nucleic Acids Res. 1990 Sep 11;18(17):5197–5205. doi: 10.1093/nar/18.17.5197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Letsinger R. L., Caruthers M. H., Miller P. S., Ogilvie K. K. Oligonucleotide syntheses utilizing beta-benzoylpropionyl, a blocking group with a trigger for selective cleavage. J Am Chem Soc. 1967 Dec 20;89(26):7146–7147. doi: 10.1021/ja01002a074. [DOI] [PubMed] [Google Scholar]
- Miller P. S., Barrett J. C., Ts'o P. O. Synthesis of oligodeoxyribonucleotide ethyl phosphotriesters and their specific complex formation with transfer ribonucleic acid. Biochemistry. 1974 Nov 19;13(24):4887–4896. doi: 10.1021/bi00721a003. [DOI] [PubMed] [Google Scholar]
- Miller P. S., Fang K. N., Kondo N. S., Ts'o P. O. Syntheses and properties of adenine and thymine nucleoside alkyl phosphotriesters, the neutral analogs of dinucleoside monophosphates. J Am Chem Soc. 1971 Dec;93(24):6657–6665. doi: 10.1021/ja00753a054. [DOI] [PubMed] [Google Scholar]
- Moody H. M., Quaedflieg P. J., Koole L. H., van Genderen M. H., Buck H. M., Smit L., Jurriaans S., Geelen J. L., Goudsmit J. Inhibition of HIV-1 infectivity by phosphate-methylated DNA: retraction. Science. 1990 Oct 5;250(4977):125–126. doi: 10.1126/science.2218505. [DOI] [PubMed] [Google Scholar]
- Reese C. B., Stewart J. C. Methoxyacetyl as a protecting group in ribonucleoside chemistry. Tetrahedron Lett. 1968 Aug;(40):4273–4276. doi: 10.1016/s0040-4039(00)76405-7. [DOI] [PubMed] [Google Scholar]


