Sir,
Actinic keratosis (AK) is cutaneous neoplasms resulting from proliferations of cytological aberrant epidermal keratinocytes that occur primarily on sun-exposed skin surfaces[1] usually of middle aged or older people. It presents as a skin-colored to reddish-brown or yellowish-black, well defined macule, papule or plaque varying from pinhead sized to several centimeters in diameter. The lesion usually has a hyperkeratotic surface that is often better recognized by palpation, as a roughening, rather than visualization.[1–6]
There are many therapeutic modalities used to treat patients with solar keratosis like curettage and cautry, topical therapy with several agents including 5 Flurouracil, 5% imiquimed cream, and 3% diclofenac gel.[1,2,4,5]
The main aim of treatment is to prevent the progression of the disease into invasive squamous cell carcinoma.
Zinc is one of essential trace elements required for physiological functions. It forms an integral part of several enzymes and cofactors and is an essential element in cell growth.[7–10]
In Iraq, topical zinc sulphate had been used successfully in the treatment of a wide variety of skin disorders such as: Cutaneous leishmaniasis,[11] plane warts,[12] basal cell carcinoma[13] xeroderma pigmentosa[14] and others.[15–20] So, the aim of the present work is to evaluate the effectiveness and safety of 25% topical zinc sulphate solution in the treatment of actinic keratosis especially in patients with multiple lesions.
This is a single-blind therapeutic trial conducted in the Department of Dermatology and Venereology, Baghdad Teaching Hospital during December 2006 to September 2008.
Twenty seven patients, 21 males and 6 females, were enrolled in this study. Nine patients, 6 females and 3 males were defaulted from the study for unsafe security reasons while18 male patients completed the study.
Full history and examinations were carried out for all patients especially for related relevant points like age, residence, occupation with an attempt to classify it as outdoors, indoors or combined, habitual use of sun protective clothes or sunscreen, and treatments used. Also, we questioned patients about the duration of lesions and any associated symptoms such as pain, local tenderness, itching or burning sensation.
Skin photo-type was established for all patients according to Fitzpatrick's classification and any signs of sun damage were recorded. AK lesions were assessed including their numbers, site, morphology, size, color and surface whether smooth, scaly, verrucous.
All the following conditions were excluded from this work: Lesions with erosion, oozing or crustation, size more than 1.5 cm in diameter unless proved by biopsy to be AK, the presence of regional lymphadenopathy. Associated predisposing medical conditions such as renal transplant, xeroderma pigmentosa, epidermodysplasia verruciformis were also excluded. All kinds of therapies were stopped 8 weeks before starting this work and no others treatment was given or allowed to use during therapy.
Diagnosis was clinical and confirmed by histopathological examination especially in patients with suspected lesions.Lesional biopsies from five treated patients were taken after stopping therapy for histopathological assessment.
Formal consent was taken before the start the therapy, after full explanation about the nature of the disease, course, the procedure of treatment, follow up, prognosis and the need for pre and post treatment photographs. Also, ethical approval was performed by the Scientific Council of Dermatology and Venereology- Iraqi Board for Medical Specializations.
Twenty five percent zinc sulphate solution was prepared by dissolving 25 grams of zinc sulphate crystals (ZnSO4 7H2O=287.54 from MERK, France) in 100 ml of distilled water, pH=5.5. Patients were instructed to use it twice daily by cotton tip applicator for 12 weeks and to be seen every 2 weeks for 12 weeks to assess the response to therapy and to report any side effects. Follow up for 8 weeks without treatment to see any sign of relapse.
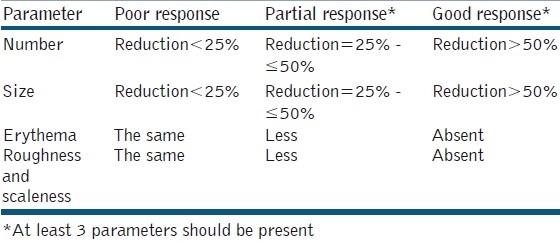
Response to treatment was determined by the following criteria: Number, size, erythema, roughness, and scaleness of lesions. Accordingly, the response was recorded as poor, partial or good response [Table 1]. Patient's therapy satisfaction was evaluated. All patients were photographed by a digital camera as a baseline and then every two weeks, in the same place with fixed illumination and distance by using a digital camera (Sony: Cyber shoot with resolution 7 mega pixels).
Table 1.
Criteria for response to therapy with 25% topical zinc sulphate solution in patients with actinic keratosis
For the determination of the statistical significance among different variables, descriptive statistics like mean and standard deviation were used together with analytic statistics like Chi-square, using EPI version 6.
Eighteen male patients with 100 lesions of AKs were included. Their ages ranged from 34-70 years with a mean+SD of 53.833±10.160 years. The duration of the disease ranged from 2 months to 10 years with a median of 2.37 years.
Regarding the occupation of patients, it was mainly outdoors in 11 (61.11%), indoors 3 (16.66%) and combined in 4 (22.22%) patients. While skin photo types were 5 (27.77%) patients type II and 13 (72.23%) patients with type III-IV. The number of lesions for each case was less than 10 AKs in 4 (22.22%) patients, between 10-40 AKs in 10 (55.56%), and more than 40 AKs in 4 (22.22%) patients, thus 100 lesions of actinic keratosis were treated.
The lesions were distributed mainly on the bald scalp, forehead, cheek, nose, ear, upper chest and dorsum of the hands [Table 2]. The lesions were asymptomatic in the majority of patients, apart from mild burning sensation seen in 5 patients. All lesions were erythematous in color. The clinical response of treated patients was illustrated in Table 3.
Table 2.
Sites affected in 18 patients with actinic keratosis
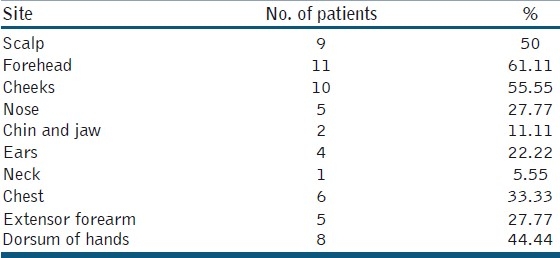
Table 3.
Response to treatment in 18 patients with actinic keratosis
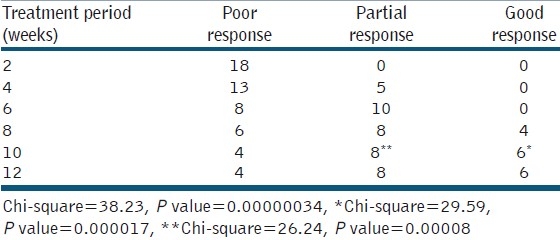
No patient showed signs of improvement during the first 2 weeks of treatment while partial response was seen in 5 patients after 4 weeks. Good response was seen in 4 patients after 8 weeks of treatment. After 10 weeks of treatment, 6 (33.33%) patients showed statistically significant good response (P value=0.000017), and 8(44.44%) patients showed statistically significant partial response (P value=0.00008). There was no difference in response between 10 and 12 weeks of treatment. Four (22.22%) patients failed to show any sign of improvement after 12 weeks of treatment.
Patients with partial and good response were satisfied with the final results of treatment. Mild transient burning sensation was recorded in 6 (33.33%) patients who did not need to discontinue therapy. Biopsies taken from 3 partially responding patients showed mild pathological changes, while it showed no abnormality in 2 patients with good response. No signs of recurrence were seen in responding patients during 8 weeks follow up and no pigmentary changes were noticed.
Actinic keratosis is cutaneous neoplasms that occur primarily on sun-exposed skin surfaces. It is a major problem among Europeans as a result of having fair skin. In Middle East countries, including Iraq, the problem is assumed to be less common due to melanin protection, but in recent studies, AKs seem to be not uncommon disease in middle age and elderly peoples.[1–6]
Chronic ultra violet light exposure is considered to be the most important factor in the development of actinic keratosis. Organ-transplant recipients, other immuno-compromised individuals, albinos, and persons with epidermodysplasia verruciformis and xeroderma pigmentosa have an increased propensity to develop actinic keratosis.[3]
Treatment of actinic keratosis is indicated to avoid any chance of progression to invasive squamous cell carcinoma. Treatment of this disease is also warranted to minimize symptoms in affected patients with painful and pruritic actinic keratosis and for cosmetic reasons.[2–6] Several topical agents are available for the treatment of actinic keratosis including 5 Flurouracil, 5% imiquimed cream, and 3% diclofenac gel. These topical agents are costly and treatment will be associated with significant side effects.[1,2,4,5]
Many Iraqi studies showed that intralesional zinc sulphate solution was an effective in clearance of viral warts, cutaneous leishmaniasis, and basal cell carcinoma.[11–13] In the last few years, we tried topical zinc sulphate solution in actinic keratosis in patients with xeroderma pigmentosa[14] and the results were encouraging, and for these reasons, we arranged the present work.
In comparison with 5-Fluorouracil, zinc sulphate solution achieved a response rate of 77.77% (partial plus good response), while treatment with 5 Flurouracil had a response rate of 75%.[2,4–6] Treatment with zinc sulfate solution was not associated with side effects apart from mild and transient burning sensation which was encountered in 33.33% of patients, while treatment with 5-Flurouracil is associated with severe inflammatory reaction in all treated patients. In addition zinc sulphate solution is less expensive than 5-Flurouracil.
Extending treatment period to 12 weeks showed no further changes in response to treatment. Hence treatment period of 10 weeks is optimum for the treatment of actinic keratosis by 25% zinc sulphate solution. Biopsy taken from 3 partially responding patients showed mild epidermal changes, while it showed no abnormality in 2 patients with good response. No patient showed signs of recurrence during the 8 weeks period of follow-up after completion of treatment. Mechanism of action of topical zinc sulphate solution is not fully understood, but we can speculate that it works through its multiple actions. Zinc in high concentration has a direct cytotoxic effect and is well known to induce apoptosis of malignant cells and to induce tissue necrosis.[11–20] Reactive oxygen species have been implicated in the pathogenesis of various hyper proliferative and inflammatory diseases including actinic keratosis, and as zinc sulphate has antioxidants function to protect the skin from the harmful effect of free radicals, so zinc sulphate has antineoplastic action. In addition zinc sulphate may actually cause suppression of unwanted immune reaction and accordingly it might affect the random migration of inflammatory cells. Also zinc sulphate solution is thought to inhibit prostaglandins synthesis.[11–20] Finally zinc sulphate solution has sun protection effect by blocking both ultra violet A and ultra violet B, and thus inhibiting the carcinogenic effect of sunlight.[11–20]
In conclusion, this study showed that topical zinc sulphate solution (25%) was an effective, safe and non-costly therapy, especially in patients with multiple actinic keratosis lesions. Further studies with a higher concentration are recommended.
REFERENCES
- 1.Duncan KO, Geisse JK, Jeffell DJ. Epidermal and appendageal tumours. In: Freedberg IM, Eison AZ, Wolff K, Austenk F, Goldsmith LA, Katz SI, editors. Fitzpatrick's’ Dermatology in General Medicine. 6th ed. Vol. 21. New York: McGraw Hill Book Company; 2008. pp. 1007–15. [Google Scholar]
- 2.Richard BO, William DJ, Timothy GR. Andrew's diseases of the skin. Clinical Dermatology. 10th ed. Philadelphia: WB Saunders Company; 2006. pp. 641–43. [Google Scholar]
- 3.Kadhim KA. Actinic keratosis among Iraqi patients. A thesis submitted to the Iraqi Board for Medical Specializations, Dermatology and Venereology. 2002 [Google Scholar]
- 4.Habif TP. Pre-malignant and malignant non-melanoma skin tumours. In: Clinical Dermatology. A Color Guide to Diagnosis and Therapy. 4th ed. Vol. 21. New-York: Mosby-year book Inc; 2004. pp. 736–43. [Google Scholar]
- 5.DeBerker D, McGregor JM, Hughest BR British Association of Dermatologists Therapy Guidelines and Audit Subcommittee. Guidelines for the management of actinic keratosis. Br J Dermatol. 2007;156:222–30. doi: 10.1111/j.1365-2133.2006.07692.x. [DOI] [PubMed] [Google Scholar]
- 6.Hunter JA, Savin JA, Dahl MV. Clinical Dermatology. 4th ed. Oxford: Blackwell Science Ltd; 2008. pp. 263–5. [Google Scholar]
- 7.Robert MR. Vitamins and trace elements deficiency and excess. In: Harrison's Principle of Internal Medicine. 16th ed. Vol. 61. New York: McGraw Hill Companies. Inc; 2005. pp. 403–11. [Google Scholar]
- 8.Reynold SJ, Parfitt K, Parsons A, Sweetmans . Martindale. The extrapharmacopoeia. 31st ed. London: Royal Pharmaceutical society, London; 1996. pp. 380–390. [Google Scholar]
- 9.Neldner KH. In: Acrodermatitis enteropathica and other zinc deficiency disorders. Fitzpatrick TB, Freedberg IM, Eison A, Wolf K, Austen K, Goldsmith LA, et al., editors. New York: McGraw Hill Book Company; 2008. pp. 1738–44. 148. [Google Scholar]
- 10.Buist N, Steiner R. In: Disorders of metal metabolism. Campbell AG, McIntosh N, editors. Philadelphia: Churchill Livingstone; 1998. [Google Scholar]
- 11.Sharquie KE, Al-Azzawi K. Intralesional therapy of cutaneous leishmaniasis with 2% zinc sulphate solution. J Pan Arab League Dermatol. 1996;7:41–6. [Google Scholar]
- 12.Sharquie KE, Khorsheed AA, Al-Nuaimy AA. Topical zinc sulphate solution for treatment of viral warts. Saudi Med J. 2007;28:1418–21. [PubMed] [Google Scholar]
- 13.Sharquie KE, Al-Nuaimy AA, Al-Shimary FA. New Intralesional therapy for basal cell carcinoma by 2% zinc sulphate solution. Saudi Med J. 2005;26:359–61. [PubMed] [Google Scholar]
- 14.Sharquie KE, Al-Mashhadani SA, Noaimi AA, Hayani RK, Subber SA. Thetherapeutic and prophylactic efficacy of 5% zinc sulphate mouth wash in the management of RAU. J Cosmet Dermatol Sci Appl. 2011 [In press] [Google Scholar]
- 15.Sharquie KE, Al-Mashhadani SA, Salman HA. Comparative clinical trial of topical 10% zinc sulphate solution for treatment of melasma. Dermatol Surg. 2008;34:1346–9. doi: 10.1111/j.1524-4725.2008.34287.x. [DOI] [PubMed] [Google Scholar]
- 16.Al-Tereihi IG. Topical 10% zinc sulphate solution in the treatment of superficial fungal infection. Thesis submitted to the Iraqi board for Medical Specializations, Dermatology and Venereology. 2006 [Google Scholar]
- 17.Sharquie KE, Al-Mashhadani SA, Hayani RK, Al-Noaimi AA, Subber SA. The therapeutic and prophylactic efficacy of 5% lactic acid mouth wash, 5% zinc sulphate oil in the management of RAU. Iraqi Postgrad Med J. 2011 [In press] [Google Scholar]
- 18.Al-Hamdi KI, Al-Waiz MM, Al-Kinani LC. Treatment of psoriasis with zinc sulphate cream 2.5% in comparison with clobetasol propionate cream. Int J Dermatol. 2007;6:1531–8. [Google Scholar]
- 19.Sharquie KE, Hayani RK, Al-Dori WS, Sharquie IK, Noaimi AA. Treatment of pityriasis versicolor with topical 15% zinc sulfate solution. In 15th Congress of EADV. 2006 Abstract no 950962. [Google Scholar]
- 20.Sharquie KE, Noaimi AA, Al-Salih MM. Topical therapy of acne vulgaris using 2% tea lotion in comparison with 5% zinc sulphate solution. Saudi Med J. 2008;29:1757–61. [PubMed] [Google Scholar]


