Abstract
For routine quality assurance of helical tomotherapy plans, an alternative method, as opposed to the TomoTherapy suggested cylindrical solid water phantom with film and ionization chamber, is proposed using the PTW Seven29 2D-ARRAY inserted in a dedicated octagonal phantom, called Octavius. First, the sensitivity of the array to pitch was studied by varying the pitch during planning to 0.287, 0.433, 1.0, and 2.0. For each pitch selected, the dependence on field size was investigated by generating plans with field widths (FWs) of 1.06 cm, 2.49 cm, and 5.02 cm, for a total of 12 plans. Secondly, a total of 15 patient QA plans were delivered using helical tomotherapy with the Delta4 and Seven29/Octavius for comparison. Using the clinical gamma criteria, 3% and 3 mm, all FW and pitch plans had a passing percentage of >90%. For patient QA plans, the average gamma pass percentage was 97.0% (94.4–99.8%) for the Delta4 and 97.6% (92.5-100.0%) for the Seven29/Octavius. Both the Seven29/Octavius and Delta4 performed to a high standard of measurement accuracy and had a 90% or greater gamma percent for all plans and were considered clinically acceptable.
Keywords: Octavius phantom, patient QA, PTW Seven29, tomotherapy
Introduction
Advancements in the field of radiation therapy have brought about a gain in popularity in the use of Intensity Modulated Radiation Therapy (IMRT), helical tomotherapy delivery, and rotational, volumetrically modulated delivery methods such as RapidArc® (Varian, Palo Alto, CA, USA). IMRT methods are advantageous for patient treatments in that they allow for a more conformal dose to the target in all three spatial dimensions while better sparing normal tissues in the process.[1,2] IMRT is personalized to the specific patient and the specific treatment situation, thereby generally resulting in treatment field arrangements and multileaf collimator MLC patterns that are inherently more complex. Such personalized therapy requires rigorous commissioning of delivery systems and treatment planning systems. It is common practice that medical physicists conduct per-plan/per-patient quality assurance for IMRT techniques to ensure the prescribed treatment dose is accurate and achieved in present and clinically acceptable error tolerances.[3]
Traditionally, helical tomotherapy delivery quality assurance (DQA) plans and measurements are completed with film and a point measurement using the tomotherapy cylindrical solid water, i.e. cheese, phantom.[4–7] Many institutions have moved toward planar diode and ion chamber arrays in order to perform these DQA tests in a more efficient manner. These arrays have been shown to have good agreement with film and ion chamber measurements, and in some cases have proven to be more accurate.[8–11] Diode and ion chamber arrays in conjunction with selected phantoms have also been shown to significantly reduce the time necessary to complete a patient QA when compared to film and ion chamber measurement.[8,9] One such diode array phantom is the ScandiDos Delta4 which has been proven to be an accurate means of validating patient plans.[9]
With the constant need for a reliable and accurate means of patient plan verification, this study was performed to characterize and evaluate the Seven29 in conjunction with the Octavius phantom (PTW, Freiburg, Germany) for helical tomotherapy DQA. Characterization of this ion chamber array for use with tomotherapy is essential for many reasons. Due to couch translation through the bore, ionization chambers are often partially irradiated. Small field width (FW) and the known angular dependence, although compensated for with the insertion of the hemi-cylindrical air cavity, may need to be further corrected as a result of the partial irradiation of all the ion chambers involved in the measurement. The complexity of the treatment plan is reflected in an increase in the modulation factor quantity which leads, in general, to smaller segments and hence to a greater difficulty to measure. The pitch variation and FW selection affect the time that chambers are exposed. Exposure time could also affect the performance of the ion chamber array and therefore must be evaluated. These unique aspects of tomotherapy can be altered by modifying the pitch, FW, and modulation factor. Dependencies on these three aspects are necessary to determine whether or not an ion chamber with a coarse resolution, 1 cm for the Seven29, can give accurate results in order to quantify the acceptability of a treatment plan.
Previously, Spezi et al.[10] tested the reproducibility and linearity for the Seven29 array for a linear accelerator; Chandraraj et al. examined the use of the Seven29 array for use with volume-modulated arc therapy, RapidArc, and sliding window quality assurance techniques;[12,13] and Van Esch et al.[14] evaluated the directional dependence of the Seven29 using the Octavius phantom for tomotherapy. This work intends to build upon these projects by evaluating tomotherapy-specific parameter dependencies and then characterizing the Seven29 with Octavius for use on actual patient plans. The Seven29/Octavius has never been examined this specifically for tomotherapy and for actual tomotherapy patient plan verifications. Previously, the Seven29/Octavius has been cross-checked with point ion chamber measurements, but this work will allow a more thorough comparison based on cross-comparing planar doses with planar doses measured with a trusted diode array device.[14] The main objective of this work was to evaluate the Seven29/Octavius for tomotherapy based on treatment planning parameters and patient quality assurance evaluation.
Materials and Methods
For this work, the first objective was to evaluate the Seven29/Octavius setup for use with the TomoTherapy Hi-ART system (TomoTherapy Inc., Madison, WI, USA) by testing for dependencies on pitch, FW, and modulation factor. The second objective was to compare the PTW Seven29/Octavius setup against the Delta4 phantom (ScandiDos AB, Uppsala, Sweden) for 15 clinical patient DQA plans. The current method for performing patient DQA tests for tomotherapy at our institution is with the Delta4 and therefore served as a standard for the PTW Seven29/Octavius device as well as film and ion chamber measurements using the cheese phantom that is the historical gold standard for tomotherapy.[9,15,16] Plans were evaluated using the gamma index planar dose distribution and dose profile comparison.
Measurements were performed on a TomoTherapy Hi-ART unit. The PTW Seven29 detector array was inserted in the Octavius phantom and scanned using a GE LightSpeed CT scanner (GE Medical, Waukesha, WI, USA) with a slice thickness of 2.5 mm as shown in Figure 1a.
Figure 1.
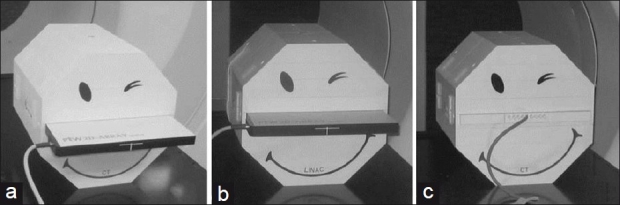
(a) Seven29 inserted into the cavity of the octagonal Octavius phantom with CT base. (b) Seven29 inserted into the cavity of the octagonal Octavius phantom. (c) Ion chamber and solid water slabs inserted into the cavity of the Octavius phantom
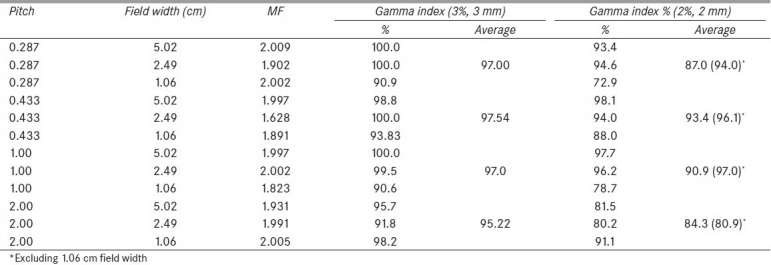
Twelve plans (n = 12) were created using the CT image set obtained after delineating a cylindrical, virtual target with a radius of approximately 5.0 cm and a length of approximately 10.0 cm centrally located in the phantom. For each plan, the pitch and the FW were varied [Table 1] and the prescribed dose was set to 2.0 Gy to 95% of the target volume. For those plans with a pitch = 2.0 and the plan for pitch = 1.0 and FW = 1.06 cm, the prescription was set to 0.5 Gy to 95%of the target volume due to the limitations of the tomotherapy treatment planning system TPS.
Table 1.
Gamma passing percentages for each plan based on the clinical, 3% DD and 3 mm DTA criteria, 2% DD and 2 mm DTA criteria, and modulation factors for each evaluation plan
Detectors
The 2d ionization chamber array
The PTW Seven29 2D-array consists of 729 vented, cubic ion chambers creating a field size of 27 × 27 cm2.[17] Each vented, parallel plate ion chamber is 0.5 × 0.5 × 0.5 cm3 with a resolution of 1 cm from center to center of neighboring chambers. The array weighs 3.2 kg with a thickness of 2.2 cm and the effective depth of the chambers is 0.5 cm. The linear dimensions of the 2D array are 2.2 × 30.0 × 42.0 cm3.
The Octavius phantom
The Octavius phantom is made of polystyrene with a physical density of 1.04 g/cm3 and relative electron density of 1.00. The phantom is 32 cm wide with a length of 32 cm. A 30.0 × 30.0 × 2.2 cm3 central cavity allows the user to insert the 2D ion chamber array into Octavius as shown in Figure 1b.
The position of the cavity is such that when the 2D array is inserted, the plane through the middle of the ion chambers is aligned with the physical center of the phantom. For single ion chamber measurements, three separate slabs with dimensions of 10.0 × 31.0 × 2.2 cm3 were constructed – two entirely solid and the third containing nine ion chamber inserts with center-to-center spacing of 1.05 cm (diameter of 0.69 cm). The slab with ion chamber inserts is tailored to accommodate the 0.125 cc T31010 Semiflex thimble chambers (PTW).[18] The three slabs and ion chamber inserts are shown in Figure 1c.
The bottom half of the Octavius phantom is removable. Two bases are provided with the Octavius phantom: a CT and linear accelerator base. The CT base does not contain a pocket of air which is present in the linear accelerator base. The role of the air pocket is to compensate for angular dependencies of the PTW Seven29 array. The CT base is pictured in Figure 1a and c .
ScandiDos Delta4 detector
The Delta4 phantom consists of 1069 p-type silicon diodes arranged in a matrix along two orthogonal planes. Each p-type diode has a cylindrical sensitive volume with a 0.78 mm2 area and a thickness of 0.05 mm. The detectors are spaced at 0.5 cm intervals in the central 6 cm × 6 cm area and at 1 cm intervals outside of this area, covering an area of 20 cm × 20 cm. The detector planes are placed in a polymethyl methacrylate PMMA cylindrical phantom, 22 cm in diameter and 40 cm in length. The normal planes are achieved by means of a main detector board, which passes through the entire diameter of the phantom, and two wing detector boards. Multichannel electrometers are located at the ends of the detector planes in an integrated module. Data are transferred to the control computer via a CAT-5 cable. The device was set up and allowed to warm up for 30 minutes prior to use, as shown in Figure 2.
Figure 2.
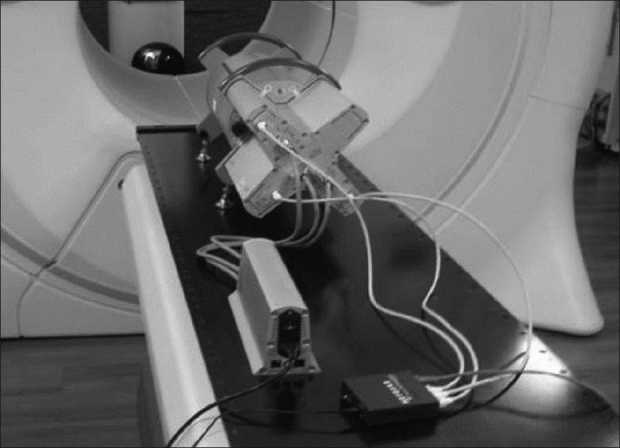
TomoTherapy setup for the ScandiDos Delta4 quality assurance phantom
Evaluation metrics
Initial calibration factor
In order to obtain an initial calibration factor for the VeriSoft software (PTW), first, a rotational QA plan was created on the tomotherapy TPS with a cylindrical target volume using the CT images of the Seven29/Octavius phantom. Figure 3 shows a screen capture of the rotational QA plan.
Figure 3.
Rotational QA plan created in the TomoTherapy TPS for initial calibration factor determination
The target prescription dose was 2.0 Gy per fraction and was prescribed to 98% of the target volume solely to obtain a highly conformal and homogenous dose distribution for the purposes of the initial calibration factor. The plan was approved and delivered using the Octavius phantom with the ion chamber slabs in place of the PTW Seven29 array. The CT base for the Octavius phantom was used for the point dose measurement. An accredited dosimetry calibration laboratory ADCL calibrated ion chamber, PTW 31013 Semiflex ionization chamber with a nominal sensitive volume of 0.3 cc (PTW), was inserted into the middle ion chamber insert such that the collection volume corresponded to the center of the planned target volume. The ionization chamber was connected to a calibrated CNMC Model 206 dosimetry electrometer (CNMC, Nashville, TN, USA). Figure 1c shows an image of the ion chamber calibration setup.
The electrometer reading was obtained and correction factors were then applied to the raw reading to obtain the dose to the chamber. These correction factors include an acrylic to water scatter correction, a temperature–pressure correction factor, an electrometer correction factor, an ion-recombination correction factor, a polarity correction factor, a quality conversion factor, and the absorbed-dose to water calibration factor.[19] The corrected reading was inserted into the VeriSoft software as the expected dose in order to complete the cross calibration of the PTW Seven29 array. The cross calibration is completed by exchanging the CT base of Octavius with the Linac base, inserting the Seven29, and repeating the same measurement with this setup. The measurement taken with the Seven29 is then compared to the expected value from the electrometer reading and a kuser factor is calculated by the VeriSoft software. This kuser factor can be used with “temperature and pressure” corrections thereafter for subsequent measurements on the following days.
Pitch and FW dependence
The rotational plan that was used to obtain the initial calibration factor was modified into 12 unique plans by altering the pitch and FW. The pitches used were 0.287, 0.433, 1.0, and 2.0. The pitch in tomotherapy is defined as the couch travel per rotation divided by the FW used. This gives an indication of the extent of the helical delivery and indicates overlapping when the pitch is less than 1. When the pitch is greater than 1, there is no overlap and the treatment field is spiral. A pitch of 0.287 is used most during planning at our clinic. For this study, we strived to evaluate pitches that would be clinically relevant, although extreme pitches of 1 and 2 were used in order to attempt to quantify the limitations of the array. Each of these four different pitches were planned in conjunction with each of the three different FWs (1.06 cm, 2.49 cm, and 5.02 cm), making 12 plans, in order to determine any dependencies. Once again, these FWs were selected in order to examine the array for use in clinically relevant situations at FWs that are most commonly used in clinical planning. The FW of 1.06 cm is a more extreme case as the other two FWs are most commonly used in our clinic. Each plan created with the TPS was then delivered using the Octavius/Seven29 setup. The gamma passing percentage was determined for each measurement compared to its corresponding planned dose.[20] The gamma passing criteria used to analyze the measurements were 3% maximum dose deviation (DD) and 3 mm distance to agreement (DTA). For a pixel to be considered passing, the gamma value must have been equal to or less than 1.0. Each of the 12 plans was given an objective to suppress data from regions that received doses less than 20% of the maximum measured dose value in order to exclude points that would provide a false impression of the array accuracy by inflating the gamma index value.
Modulation factor dependence
The modulation factor represents the complexity of the plan. This gives an indication of the sharpness of the dose gradients for a given plan and the dependence of the array based on the differing dose gradients is important to test. After completing each of the 12 cylindrical QA plans in the TPS, the actual modulation factors were taken for each plan and recorded. The actual modulation factors that the TPS used were compared between each of the 12 plans in order to assess whether or not a dependency existed based on this factor. This actual modulation factor was determined from the plan report for each completed plan.
To further evaluate the presence of modulation factor dependence, more complex plans were generated in the TomoTherapy TPS. Figure 4 shows the modulation factor specific plan that was created. The target – hemicylindrical shell – was prescribed a total dose of 2 Gy per fraction to 95% of the target volume. The cylindrical volume was designated as an organ at risk (OAR). All of the remaining volume of the phantom, excluding the target and cylindrical OAR, was contoured and denoted as RVR, remaining volume at risk. Both of these OARs were used in the treatment plan optimization. Three plans were created: a low modulation factor (LMF) plan (1.203), an intermediate modulation factor (IMF) plan (1.911), and a high modulation factor (HMF) plan (2.795). The higher modulation factors were a result of using a directional block, which prevents entrance of the primary beam through the specified OAR, on the cylindrical OAR for the IMF plan, and a complete block, which prevents entrance and exit beam, for the HMF plan. Most commonly, modulation factors are in the range of 1.8–2.0 for our clinic. Using this extended range of modulation factors, we were able to examine the response of the array at two extremes, both high complexity and low complexity, as well as one that is more clinically applicable. These three plans were then delivered with the tomotherapy unit and the Seven29/Octavius. The gamma passing percentages were compared to determine if a modulation factor dependency was indicated.
Figure 4.
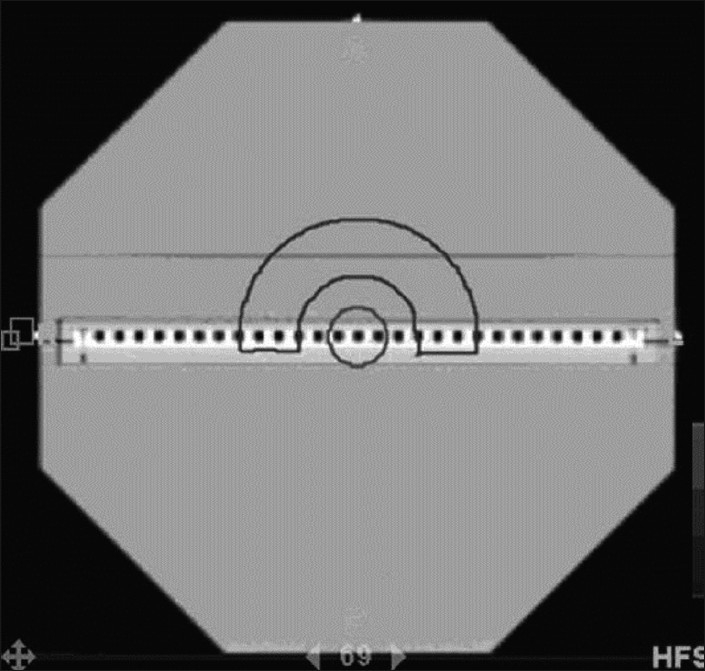
Modulation factor specific plan created with the TomoTherapy TPS
Patient QA comparison
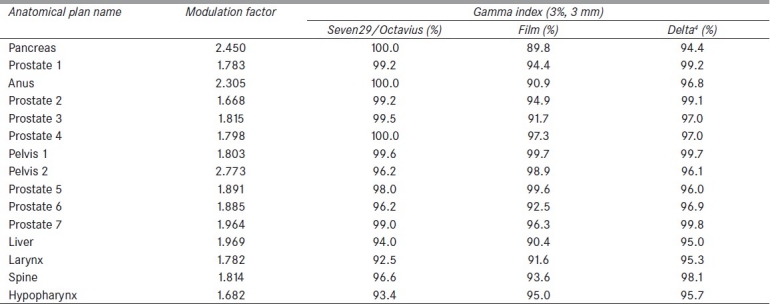
Fifteen (n = 15) consecutive patient QA plans that had been previously delivered using the Delta4 phantom were compared with the same plans delivered with the Octavius/Seven29 setup. Plans were chosen to represent many different anatomical disease sites [Table 2] in order to more universally evaluate the accuracy of the Seven29/Octavius phantom. The FW used for all plans was found to be 2.5 cm highlighting the clinical relevance of this particular field size. Each plan was delivered on the tomotherapy unit with both the Delta4 and Octavius/Seven29. The plans were then compared based on gamma passing percentages of 3% DD and 3 mm DTA, and 2% DD and 2 mm DTA. Although ICRU Report 83[21] recommends a less stringent gamma passing criteria of 5% and 5 mm, at our institution we require 3% and 3 mm and 90% of points to agree for a QA to be considered passing. Therefore, the plans were evaluated with this clinical gamma criterion. The modulation factor for each plan was also recorded from the TPS after completing each QA in order to determine whether the modulation factor influenced the results.
Table 2.
Gamma passing percentages for each quality assurance plan based on the clinical, 3% DD and 3 mm DTA criteria for the Seven29/Octavius, film measurements, and the Delta4, as well as the modulation factors for each plan
Gamma analysis was performed similarly for both devices in order to facilitate a comparison between the two. A median dose value from the planar dose map of each measurement was chosen for the gamma reference value in order to obtain a more universal comparison method. Each device uses the same gamma equation as that obtained from Low et al.[20] and the plans were normalized to a global dose value according to Equation (1) for the difference matrix:

where Dc is the dose value at a point in the calculated or planned dose matrix and Dr is the dose value at the corresponding point in the reference or measured dose matrix.
Film and ion chamber measurements were also taken in order to compare against a gold standard for tomotherapy plan evaluation. Film measurements, using KODAK EDR2 (Kodak, Rochester, NY, USA) film, were made of the same patient set using the tomotherapy cheese phantom and a Standard Imaging (Standard Imaging, Inc., Middleton, WI, USA) A1SL (0.056 cm3 active volume) ion chamber for a point dose measurement. These films were then analyzed using the RIT v5.3 (RIT Colorado Springs, CO, USA) software and a gamma analysis was performed comparing the film measurement to the exported planar dose from the tomotherapy TPS. Gamma criteria of 3% DD and 3 mm DTA were used and the passing percentages were then compared to those of the Seven29/Octavius phantom setup.
Results
Evaluation metrics
Initial calibration factor
Using the setup shown in Figure 1b, the delivered dose to the ionization chamber was measured to be 2.4% greater than the planned dose (2.099 Gy vs. 2.049 Gy). After retaking the same measurement with the Seven29, the kuser factor calculated by the VeriSoft software was found to be 1.083. This factor was used in the temperature and pressure correction window in the VeriSoft software thereafter for the consecutive measurements associated with this work.
Pitch and FW dependence
Table 1 shows the gamma passing percentages for each of the 12 plans arranged according to pitch. Clinically, pitches of 0.287 and 0.433 are most commonly used.[22] Pitches of 1.0 and 2.0 were added in order to test the limits of the Seven29/Octavius. With the exception of a pitch of 2.0, the pitch had little effect on the plan outcome. Plans optimized with a pitch of 2.0, in general, displayed lower gamma passing percentages for all but one given field size (1.06 cm).
Using the 3% DD and 3 mm DTA criteria, all plans optimized with various pitches performed well, with the percent of points less than 1.0 being greater than 90%. When reviewing pitches that are commonly used in the clinic (pitch = 0.287 and 0.433), all plans demonstrated higher gamma index passing percentages, except for those with FWs of 1.06 cm.
Our results show that the Seven29/Octavius phantom can accurately measure the predicted planar dose calculated by the TomoTherapy TPS for pitches of 0.287 to 2.0. Larger pitches did result in slightly lower gamma values overall, but these larger pitches are considered clinically unrealistic.
The clinical criteria of 3% DD and 3 mm DTA was satisfied for all FWs; however, the Seven29/Octavius demonstrated some FW dependence. The best average gamma passing percentage results were obtained with 5.02 cm FW, which was the largest FW used. The 2.49 cm FW resulted in a similar gamma percentage, although it was slightly less than that for the largest FW. Results are shown in Table 1. The average percent of points for each FW that satisfied the gamma index criteria of 3% DD and 3 mm DTA calculated over all pitch values used were 98.6, 97.8, and 93.4% for 5.02, 2.49, and 1.06 cm FW, respectively.
In the clinic, FWs of 5.02 and 2.49 cm are more commonly used due to their reduction in treatment time when compared to the 1.06 cm FW. On average, plans that were optimized with an FW of 5.02 cm had the greatest gamma passing percentage. The 2.49 cm FW gave a gamma percentage, on average, slightly less than that for the 5.02 cm FW, and the 1.06 cm FW produced lower gamma passing percentage.
Modulation factor dependence
Table 1 shows the actual modulation factors (MF) for each plan that were recorded from the plan report in the TPS. The actual MFs varied from 1.628 to 2.009. After analyzing the MF data from each plan with the Pearson product-moment correlation coefficient, we found an r value of –0.02, which indicates that there is very little correlation between the MF and the gamma passing percentage for the plans from Table 1. Because the MF did not influence the gamma passing percentage of the plans, it was determined that based on our sample the Seven29/Octavius does not show an intrinsic MF dependence.
The results of the three different plans generated with the structures visible in Figure 4 were also used to confirm the conclusion of the previous paragraph. After further evaluating the possibility of MF dependence of the Seven29 array, it was noted that the gamma passing percentage, based on a 3% DD and 3 mm DTA, varied from 88.5% to 90.3% between the three different MF plans. Because the gamma percentage differed by less than 2% at a maximum between MFs varying from 1.203 to 2.795, a lack of MF dependence was validated. Given these results and those from the previous paragraph, the Seven29/Octavius was shown to be independent of the MF.
Patient QA comparison
Table 2 shows the comparison of the percent of points that have a gamma index ≤1.0 using 3% DD and 3 mm DTA between the Seven29/Octavius, film, and the Delta4. The plans were normalized such that 100% corresponded to the median measured target dose. The anatomical locations represented in these plans include the pancreas, prostate, anus, pelvis, liver, larynx, head and neck, and spine. By using various types of anatomical sites for our patient-specific QA comparison, the overall accuracy and clinical relevance of the Seven29/Octavius compared to the film and Delta4 could be determined.
The average gamma percentage over all plans for the Seven29/Octavius was 97.6%, 94.4% for the film, and 97.0% for the Delta4. It should be noted that for all plans, the clinical standard of 90% or greater for the gamma index percentage was achieved for the Seven29/Octavius, film (with the exception of one plan), and Delta4, validating the accuracy of the Seven29/Octavius phantom for a wide range of treatment plans. The ionization chamber absolute dose values were an average percent difference of 2.1% (±1.2%) as compared with the planned data. A Bland–Altman analysis was performed in order to compare the Seven29 method with the film and Delta4 methods. Bland–Altman plots indicated that the Seven29/Octavius had higher gamma values overall for the 15 plans versus the Delta4 and film. The Bland–Altman bias values for the comparison with Delta4 (2.40%) and film (4.03%) also support this finding. Based on these results, the Seven29/Octavius was found to be an effective tool for quality assurance purposes of helical tomotherapy plans.
Figure 5 shows an example of the profile analysis that was performed for each plan. Figure 5a and c displays the horizontal and vertical profiles of an example plan using the Seven29/Octavius. The line depicts the planned profile and the diamonds overlaying it indicate ion chamber measurements along that profile. Figure 5b and d displays the horizontal and vertical profiles of the same plan using the Delta4. Similar to the profiles from the Seven29/Octavius, the solid line indicates the planned profile and the overlying circles indicate the measured diode points. A qualitative analysis of the profiles indicates that the measured values from both detectors are closely matching the planned dose profiles. Quantitatively, the measured points along all of the profiles are within the 3%/3 mm passing criteria. Profile analysis was performed for each plan.
Figure 5.
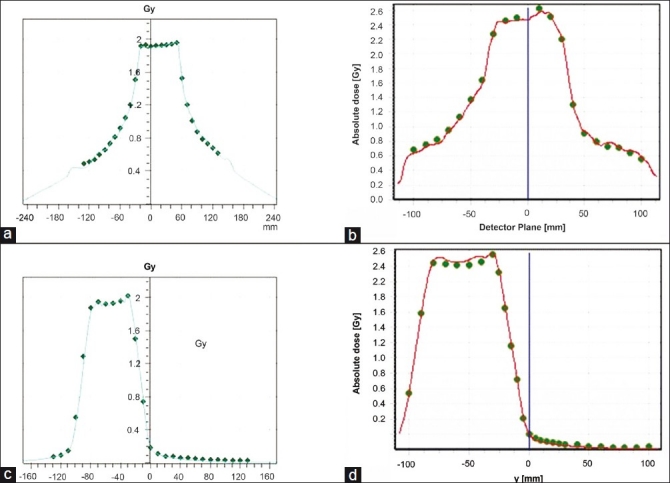
(a) Horizontal and (c) vertical profile measurements using the Seven29/Octavius (line, plan; points, measurements). (b) and (d) display the horizontal and vertical profiles of the same plan using the Delta4
The actual modulation factors for each of these plans are listed in Table 2. Analyzing the data from our measurements using the Pearson product-moment correlation, we found an r-value of 0.17 indicating very little correlation between the modulation factor and the gamma passing percentages from Table 2 for the Seven29/Octavius phantom. It was determined that for these plans, the modulation factor did not play a role in the accuracy of the plan delivery and outcome of the gamma index passing percentage.
Discussion
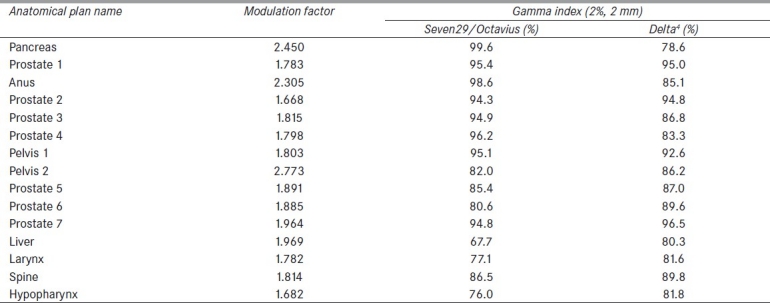
Our results showed that the Seven29 array had no dependency on the pitch used during optimization when the analysis was performed using 3% DD and 3 mm DTA. In order to further investigate the performance of the detector system, we analyzed our results using a stricter, 2% DD and 2 mm DTA. In this case, all the measurements based on the optimized plans, with the various pitch values, showed that the number of points with gamma index equal to or less than 1.0 was reduced compared to those when using the 3% DD and 3 mm DTA. For a field size of 1.06 cm, all plans with various pitch values had a gamma index value below our clinically acceptable tolerance of 90%, except in the case of pitch = 2.0. Moreover, the percentages of points with gamma index less than or equal to 1.0 using 2% DD and 2 mm DTA were further analyzed and the average gamma index per pitch value was calculated with and without the inclusion of the gamma index values of the 1.06 cm FW. The results are summarized in Table 1.
The Seven29/Octavius was shown to accurately measure planar dose as calculated by the TomoTherapy TPS without any dependency on the pitch used during optimization when the clinical, 3% and 3 mm, criteria are used for evaluation. However, plans with a pitch of 2.0 did display lower gamma passing percentages. Pitch dependency was only observed when stricter criteria were used.
Similar analysis as with the pitch dependence was performed for FW dependence using 2% DD and 2 mm DTA. Results of our analysis showed that the smallest FW, 1.06 cm, gave the poorest gamma passing percentage on average. With the clinical criteria of 3% and 3 mm, however, the 1.06 cm FW was able to pass the 90% or greater criterion. Our overall results demonstrate FW dependence. For both the strict and clinical criteria, the largest FW, 5.02 cm, showed better agreement between calculated and measured planar doses when analyzed using the gamma index method. As the FW value decreased, the gamma index passing percentage decreased. The largest discrepancy of the FW dependency analysis was seen with the smallest FW, 1.06 cm. While the medium FW, 2.49 cm, had gamma percentages slightly less than the 5.02 cm field, the 1.06 cm FW demonstrated the largest reduction in gamma index passing rate. Caution should be used by users who wish to use the smallest FW available. The reduction in gamma percentage for the smallest field size used could be contributed to leaf-timing inaccuracies for tomotherapy involving plans with small mean leaf open times which could be a factor when using a very small field size such as 1.06 cm. These inaccuracies have been described by Westerly et al.[23]
A stricter gamma index analysis was also carried out for the patient-specific QA comparison. Our results once again indicated that the Seven29 and Octavius performed equally well. These results are shown in Table 3. The average gamma percentage for all plans based on the stricter criteria was 88.3% for the Seven29/Octavius versus 87.3% for the Delta4. Gamma analysis compared the 2D planar dose data from each device. Although the Delta4 has the ability to measure 3D dose values, gamma evaluations of 2D planar dose maps were used so that comparisons could be drawn to the 2D Seven29 array.
Table 3.
Gamma passing percentages for each quality assurance plan based on the stringent 2% DD and 2 mm DTA criteria for the Seven29/Octavius and the Delta4
Conclusions
Accurate dose validation is a key component in the successful delivery of patient treatments. The PTW Seven29 with Octavius is accurate with the clinical criteria of 3% and 3 mm and is also accurate with more stringent criteria, 2% and 2 mm, for clinically used FWs and pitches. With more obscure settings of 1.06 cm FW and pitch of 2, higher discrepancies were observed using more stringent criteria. The Seven29/Octavius and Delta4 phantoms are comparable in performance based on average gamma index passing percents. Based on these measurements, the Seven29/Octavius performs accurately using the clinical passing criteria of 3%/3 mm. Based on these results, the Seven29/Octavius has proven to be an accurate tool for dose validations when used for patient-specific TomoTherapy treatment plans.
Conflict of Interest Statement
This work was partially funded by PTW, Freiburg, Germany.
Footnotes
Source of Support: PTW, Freiburg, Germany.
Conflict of Interest: None declared.
References
- 1.Bhide SA, Nutting CM. Recent advances in radiotherapy. BMC Med. 2010;8:25. doi: 10.1186/1741-7015-8-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jason Lee M. Intensity Modulated Radiation Therapy (IMRT): A Patient-Centered Guide, University of Pennsylvania. 2002 [Google Scholar]
- 3.Ezzell GA, Burmeister JW, Dogan N, LoSasso TJ, Mechalakos JG, Mihailidis D, et al. IMRT commissioning: Multiple institution planning and dosimetry comparisons, a report from AAPM Task Group 119. Med Phys. 2009;36:5359–73. doi: 10.1118/1.3238104. [DOI] [PubMed] [Google Scholar]
- 4.Bucciolini M, Buonamici FB, Casati M. Verification of IMRT fields by film dosimetry. Med Phys. 2004;31:161–8. doi: 10.1118/1.1631093. [DOI] [PubMed] [Google Scholar]
- 5.Ju SG, Ahn YC, Huh SJ, Yeo IJ. Film dosimetry for intensity modulated radiation therapy: Dosimetric evaluation. Med Phys. 2002;29:351–5. doi: 10.1118/1.1449493. [DOI] [PubMed] [Google Scholar]
- 6.Ting JY, Davis LW. Dose verification for patients undergoing IMRT. Med Dosim. 2001;26:205–13. doi: 10.1016/s0958-3947(01)00059-0. [DOI] [PubMed] [Google Scholar]
- 7.Xing L, Curran B, Hill R, Holmes T, Ma L, Forster KM, et al. Dosimetric verification of a commercial inverse treatment planning system. Phys Med Biol. 1999;44:463–78. doi: 10.1088/0031-9155/44/2/013. [DOI] [PubMed] [Google Scholar]
- 8.Buonamici FB, Compagnucci A, Marrazzo L, Russo S, Bucciolini M. An intercomparison between film dosimetry and diode matrix for IMRT quality assurance. Med Phys. 2007;34:1372–9. doi: 10.1118/1.2713426. [DOI] [PubMed] [Google Scholar]
- 9.Geurts M, Gonzalez J, Serrano-Ojeda P. Longitudinal study using a diode phantom for helical tomotherapy IMRT QA. Med Phys. 2009;36:4977–83. doi: 10.1118/1.3238153. [DOI] [PubMed] [Google Scholar]
- 10.Spezi E, Angelini AL, Romani F, Ferri A. Characterization of a 2D ion chamber array for the verification of radiotherapy treatments. Phys Med Biol. 2005;50:3361–73. doi: 10.1088/0031-9155/50/14/012. [DOI] [PubMed] [Google Scholar]
- 11.Stasi M, Giordanengo S, Cirio R, Boriano A, Bourhaleb F, Cornelius I, et al. D-IMRT verification with a 2D pixel ionization chamber: Dosimetric and clinical results in head and neck cancer. Phys Med Biol. 2005;50:4681–94. doi: 10.1088/0031-9155/50/19/017. [DOI] [PubMed] [Google Scholar]
- 12.Chandraraj V, Stathakis S, Manickam R, Esquivel C, Supe SS, Papanikolaou N. Comparison of four commercial devices for RapidArc and sliding window IMRT QA. J Appl Clin Med Phys. 2011;12:3367. doi: 10.1120/jacmp.v12i2.3367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chandraraj V, Stathakis S, Manickam R, Esquivel C, Supe SS, Papanikolaou N. Consistency and reproducibility of the VMAT plan delivery using three independent validation methods. J Appl Clin Med Phys. 2011;12:3373. doi: 10.1120/jacmp.v12i1.3373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Van Esch A, Clermont C, Devillers M, Iori M, Huyskens DP. On-line quality assurance of rotational radiotherapy treatment delivery by means of a 2D ion chamber array and the Octavius phantom. Med Phys. 2007;34:3825–37. doi: 10.1118/1.2777006. [DOI] [PubMed] [Google Scholar]
- 15.Bedford JL, Lee YK, Wai P, South CP, Warrington AP. Evaluation of the Delta4 phantom for IMRT and VMAT verification. Phys Med Biol. 2009;54:N167–76. doi: 10.1088/0031-9155/54/9/N04. [DOI] [PubMed] [Google Scholar]
- 16.Sadagopan R, Bencomo JA, Martin RL, Nilsson G, Matzen T, Balter PA. Characterization and clinical evaluation of a novel IMRT quality assurance system. J Appl Clin Med Phys. 2009;10:2928. doi: 10.1120/jacmp.v10i2.2928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.PTW: 2D-ARRAY seven29 with 729 Ion Chambers. Freiburg, PTW. 2006-2009 [Google Scholar]
- 18.PTW: User Manual OCTAVIUS Phantom. Freiburg, PTW-Freiburg. 2007 [Google Scholar]
- 19.Almond PR, Biggs PJ, Coursey BM, Hanson WF, Huq MS, Nath R, et al. AAPM's TG-51 protocol for clinical reference dosimetry of high-energy photon and electron beams. Med Phys. 1999;26:1847–70. doi: 10.1118/1.598691. [DOI] [PubMed] [Google Scholar]
- 20.Low DA, Harms WB, Mutic S, Purdy JA. A technique for the quantitative evaluation of dose distributions. Med Phys. 1998;25:656–61. doi: 10.1118/1.598248. [DOI] [PubMed] [Google Scholar]
- 21.Bethesda, MD, ICRU: 2010. ICRU: Prescribing, Recording, and Reporting Photon-Beam Intensity-Modulated Radiation Therapy (IMRT), in ICRU Jot (ed) pp. 1–96. [Google Scholar]
- 22.Kissick MW, Fenwick J, James JA, Jeraj R, Kapatoes JM, Keller H, et al. The helical tomotherapy thread effect. Med Phys. 2005;32:1414–23. doi: 10.1118/1.1896453. [DOI] [PubMed] [Google Scholar]
- 23.Westerly DC, Soisson E, Chen Q, Woch K, Schubert L, Olivera G, et al. Treatment planning to improve delivery accuracy and patient throughput in helical tomotherapy. Int J Radiat Oncol Biol Phys. 2009;74:1290–7. doi: 10.1016/j.ijrobp.2009.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]


