Abstract
The biological mechanisms responsible for aging remain poorly understood. We propose that increases in DNA damage and mutations that occur with age result from a reduced ability to repair DNA damage. To test this hypothesis, we have measured the ability to repair DNA damage in vitro by the base excision repair (BER) pathway in tissues of young (4-month-old) and old (24-month-old) C57BL/6 mice. We find in all tissues tested (brain, liver, spleen and testes), the ability to repair damage is significantly reduced (50–75%; P < 0.01) with age, and that the reduction in repair capacity seen with age correlates with decreased levels of DNA polymerase β (β-pol) enzymatic activity, protein and mRNA. To determine the biological relevance of this age-related decline in BER, we measured spontaneous and chemically induced lacI mutation frequency in young and old animals. In line with previous findings, we observed a three-fold increase in spontaneous mutation frequency in aged animals. Interestingly, lacI mutation frequency in response to dimethyl sulfate (DMS) does not significantly increase in young animals whereas identical exposure in aged animals results in a five-fold increase in mutation frequency. Because DMS induces DNA damage processed by the BER pathway, it is suggested that the increased mutagenicity of DMS with age is related to the decline in BER capacity that occurs with age. The inability of the BER pathway to repair damages that accumulate with age may provide a mechanistic explanation for the well-established phenotype of DNA damage accumulation with age.
Keywords: Aging, Base excision repair, DNA polymerase β, Dimethyl sulfate, Mutation frequency
1. Introduction
Aging affects all multicellular organisms and is associated with a gradual decline in the functional reserve capacity of most organ systems. Presently, the phenotype of aging is well characterized, yet the underlying mechanisms by which aging occurs are unknown. The somatic mutation theory of aging proposes DNA damage as a fundamental mechanism underlying the aging process, and is supported by frequently observed declines in genomic integrity seen with age [1–5]. It is interesting to consider that these increases in DNA damage and mutations may be the result of a reduced ability to repair DNA damage. A decreased ability to repair damage to DNA is also important in carcinogenesis, as mutations are known to be early events in cancer development [6]. Accordingly, many cancers are age-related diseases, and perhaps any decline in DNA repair seen with age may also explain the increase in cancer incidence that occurs with age.
A variety of repair mechanisms exist to maintain the stability of the genome. Of these, base excision repair (BER) repairs small, non-helix-distorting lesions that arise spontaneously or those that may be induced by oxidizing and alkylating agents. BER is estimated to be responsible for the repair of one million nucleotides per cell per day [7] and is suggested as having evolved in response to in vivo exposure to reactive oxygen species and endogenous alkylation [8]. Thus, BER may be the pathway with primary responsibility for repairing the types of DNA damage that arise spontaneously with age. Although over the past decade studies have addressed the question of increased DNA damage and sensitivity to carcinogens with age, these data are largely inconclusive with respect to any role that DNA repair mechanisms may play, in part because the types of DNA lesions induced by the agents studied are not well characterized and because the repair pathways for these agents may be diverse. We have chosen to investigate the effects of age on the BER pathway and further to determine whether an age-related sensitivity to dimethyl sulfate (DMS) occurs. We use DMS because it induces lesions that are repaired almost entirely by the BER pathway. Thus, we hypothesize that if BER capacity declines with age, then the mutagenicity of carcinogens, e.g. DMS, would also increase with age as a function of a reduced ability to repair the damage induced by DMS.
BER functions by glycosylase-initiated removal of a damaged base, which is followed by incision of the DNA backbone, synthesis of new DNA, excision of the deoxyribose phosphate (dRp) flap, and ligation [9]. Various glycosylases recognizing specific types of damage initiate BER. Incision of the phosphate backbone is accomplished by an AP endonuclease, Ape (Hap1, Apex, Ref 1). The DNA synthesis step in the predominant short-patch BER pathway is carried out by DNA polymerase β (β-pol) [10]. Importantly, β-pol also removes the dRp moiety, which has been determined to be the rate-limiting step in BER [11]. While variations within the BER pathway exist, it is estimated that 70–90% of all BER takes place via the short-patch pathway described above [12]. In a minor sub-pathway termed long-patch BER, modified abasic sites are resistant to dRp excision by β-pol and may not be as dependent on β-pol as the short-patch pathway. Long-patch repair has been shown to be β-pol independent and DNA polymerase δ/PCNA-dependent [13–15], as well as being PCNA-independent and β-pol and FEN1-dependent [14,16]. Recent evidence demonstrates that β-pol initiates repair synthesis in this long-patch pathway [17].
The purpose of this research is to determine the effect of age on BER capacity in a variety of different tissues, and to determine whether this decline in activity corresponds to decreased levels of β-pol activity, as well as decreased β-pol protein and mRNA levels. Additionally, we have tested whether there is an increased sensitivity to alkylation damage with age. If aged animals exhibit a reduced BER capacity and they are more sensitive to a direct-acting carcinogen that induces damage repaired by this pathway, then we have demonstrated that the decline in genomic stability seen with age is due in part to a decline in BER capacity.
2. Materials and methods
2.1. Animals
Experiments were performed in young (4–6 months) and old (22–26 months) male C57BL/6 spe-cific pathogen-free mice in accordance with the NIH guidelines for the use and care of laboratory animals and the animal protocol was approved by the Wayne State University Animal Investigation Committee. Mice were maintained on a 12 h light/dark cycle and were fed a standard mouse lab chow and water ad libitum. Mice were sacrificed at appropriate ages by cervical dislocation. Organs were flash frozen in liquid nitrogen and stored at −70 °C for later enzyme studies and western blot analyses. Tissue for RNA isolation and northern blot analysis were immediately homogenized in TRIzol® Reagent (GibcoBRL, Rockville, MD) and RNA was isolated according to the manufacturer’s protocol.
The dimethyl sulfate (DMS, CAS# 77-78-1, Sigma, St. Louis, MO) experiments were carried out in young (4–6 months) and old (18–20 months) male C57BL/6 mice harboring the lacI transgene (Stratagene, La Jolla, CA). Animals were injected with a total dose of 50 mg DMS/kg body weight administered as a single 30 mg/kg intraperitoneal injection followed by two weekly injections of 10 mg/kg each, or with vehicle only. An expression period of 2 weeks was allowed before animals were sacrificed and liver DNA was collected for mutational analysis as described in the subsequent sections.
2.2. Isolation of crude nuclear extract
All tissues were handled on ice or at 4 °C during isolation of nuclear proteins. Tissues were homogenized in a buffer (10 mM HEPES pH 8.0, 1.5 m MgCl2, 10 mM NaCl, 10 mM NaS2O5, 0.5 mM DTT, 0.5 mM PMSF, 1 μg/ml Pepstatin A), and then centrifuged for 10 min at 10,000×g at 4 °C. The pellet was mixed with 1.5 volumes of homogenization buffer plus 1 M NaCl and homogenized again, then centrifuged at 100,000× g at 4 °C. The nuclear proteins were precipitated by addition of 40% (NH)4SO4 with stirring for 30 min. Precipitated materials were collected by centrifugation at 15,000 × g for 20 min at 4 °C. The resultant pellet was dissolved in a minimal volume of dialysis buffer (20 mM Tris, pH 8.0, 100 mM KCl, 10 mM NaS2O5, 0.1 mM DTT, 0.1 mM PMSF, 1 μg/ml Pepstatin A) and dialyzed against the buffer for 1 h at 4 °C using Slide-A-Lyzer® dialysis cassettes (Pierce, Rockford, IL). Insoluble materials were removed by centrifugation at 12,000 × g for 10 min at 4 °C. The supernatant was stored at −20 °C for use in repair assay, gap-filling assay and western blot analysis. Protein concentration of nuclear extracts was determined according to Bradford [18].
2.3. DNA repair assay
Oligonucleotides (upper strand: 5′–ATATACCGC-GGUCGGCCGATCAAGCTTATTdd–3′; lower strand: 3′–ddTATATGGCGCCGGCCGGCTAGTTCG-AATAA–5′) flanked with dideoxy ends contained either a G:U mismatch or an 8-OHG:C pair. The oligonucleotides were incubated with nuclear extract from tissues of young and aged mice in a reaction mixture containing 100 mM Tris, pH 7.5; 5 mM MgCl2; 1 mM DTT; 0.1 mM ATP; 0.5 mM NAD;, 5 mM diTris–phosphocreatine; 10 U Creatine phosphokinase; 20 μM dATP, dTTP and dGTP with 2 μM dCTP plus 10 μCi radiolabeled dCTP were used in the G:U mismatch reaction, while 10 μCi radiolabeled dGTP and 2 μM unlabeled dGTP were added in the 8-OHG:C repair reaction. The mixtures were incubated for 10 min at 37 °C, and the DNA was extracted with phenol–chloroform and precipitated. The purified oligonucleotides were separated on a 12% polyacrylamide sequencing gel. Repair of the synthetic oligonucleotide results in the incorporation of the radiolabeled dNTP, which is visualized and quantified using a Molecular Imager® System (Bio-Rad, Hercules, CA). The data are expressed as machine counts per μg of protein.
2.4. Gap-filling assay/β-pol activity
A gapped oligonucleotide (upper strand: 5′–GCTT-GCATGCCTGCAGGTGTACGT-GATCCCCGGGT-ACCGAGC–3′, the 5-nucleotide gap indicated by the dashes; lower strand: 3′–CGAACGTACGGACGTCC-ACATGCAATTGCCTAGGGGCCCATGGCTCG–5′) is end-labeled and incubated at 37 °C for 30 min with a DNA synthesis reaction buffer (4× buffer:200 mM Tris, pH 8.0, 40 mM MgCl2, 80 mM NaCl, 10% glycerol; 1.25 mM dATP, dCTP, dGTP, dTTP; 100 mM DTT; crude nuclear extract, extraction procedure described above; 5 mg/ml Aphidicolin (DMSO)). DNA was resolved on a 12% polyacrylamide sequencing gel and visualized and quantified using a Molecular Imager® System (Bio-Rad, Hercules, CA). The data are expressed as machine counts per μg of protein. DNA resolving at 24 bases indicates an absence of gap-filling while DNA resolving at 47 bases indicates that gap-filling synthesis has occurred.
2.5. Western blot analysis
Nuclear extracts from tissues of young and aged animals were subjected to SDS–PAGE and transferred to nitrocellulose using a semi-dry transfer apparatus (Bio-Rad, Hercules, CA) according to the manufacturer’s protocol. SDS–PAGE was conducted in duplicate; one gel was stained with coomassie blue to ensure that the same quantity of protein was loaded onto the gels, and the other gel was used to quantify β-pol protein levels. Western analysis was accomplished using affinity purified polyclonal antisera developed against mouse β-pol. The bands were detected by ChemiImager™ (AlphaInnotech, San Leandro, CA) after incubation in a SuperSignal® Substrate System (Pierce, Rockford, IL). Intensity of the bands was quantified using a densitometer (Molecular Dynamics, Sunnyvale, CA) and the data are expressed as the integrated intensity of the band/μg protein loaded.
2.6. Northern analysis
The levels of β-pol mRNA were measured by cRNA/RNA hybridization using α-[32P] cRNA probe to β-pol [19]. The levels of β-pol mRNA were expressed relative to the 18S rRNA and glyceraldehyde-3-phosphate dehydrogenase (GAPDH) mRNA levels.
2.7. Mutation analysis
The mutation analysis was performed as described by Walter et al. [20]. In brief, genomic DNA was isolated using the RecoverEase™ DNA isolation kit (Stratagene, La Jolla, CA) according to the manufacturer’s protocol. The lacI transgene was recovered from genomic liver DNA by the Transpack® in vitro packaging kit (Stratagene, La Jolla, CA) according to manufacturer’s protocol. Packaged phage was mixed with E. coli SCS-8 cells (Stratagene, La Jolla, CA) and plated on NZY agar assay trays containing X-gal. The number of mutant lacI genes was divided by the total number of plaque-forming units to determine the mutation frequency.
2.8. Statistical analysis
Statistical significance between means was determined using ANOVA followed by the Fisher’s least significant difference test where appropriate [21].
3. Results
In this study, the effect of age on BER in young and old mice is analyzed. In young C57BL/6 mice, ages 4–6 months, and old C57BL/6 mice, ages 22–24 months, BER activity has been measured by an in vitro repair assay as described by Singhal et al. [10]. This assay measures the ability of crude nuclear extracts to repair a G:U mismatch contained within a synthetic oligonucleotide, which is quantified by measuring the incorporation of radio-labeled dCTP into the oligonucleotide. Uracil-initiated BER measures short-patch repair, which is estimated as being responsible for 70–90% of all BER [12]. When BER activity is measured by this in vitro repair assay, we observe a decrease in activity with advancing age in all tissues tested. That is, the ability of crude nuclear extracts from various tissues to repair the G:U mismatch significantly and consistently declines with age. The ability of nuclear extracts from brain, spleen and testes of old animals to repair the synthetic oligonucleotide is reduced by approximately 50% as compared to extracts from the same tissues of young animals (Fig. 1). The decrease in BER activity in liver extracts from old animals is even greater as we observe a 75% decrease in this tissue (Fig. 1). Interestingly, we observe similar decreases in BER with age in testes, liver and brain when repair is initiated by an oxidized base in the form of an 8-OHdG lesion across from a cytosine (Fig. 2).
Fig. 1.
Effect of age on base excision repair activity as determined by an in vitro G:U mismatch repair assay. The experiment was conducted using crude nuclear extracts as described in Section 2. (A) An autoradiograph of a sequence gel indicating repair activity as visualized by the appearance of a 30b fragment. Samples were pooled from extracts obtained from 3 to 4 animals in each group. (B) The relative level of base excision repair in various tissues of mice were quantified using a Molecular Imager® System (Bio-Rad, Hercules, CA), and the data were normalized based on the amount of protein used in each reaction. Values represent an average (±S.E.M.) for data obtained from at least three animals in each group. (Y, □): young mice; (O, ■): old mice; (+Ab): addition of antibody against β-pol to inhibit repair activity and to serve as a control; (*): value significantly different from control at P < 0.01.
Fig. 2.

Effect of age on BER activity as determined by removal of 8-OHdG. The experiment was conducted using crude nuclear extracts as described in Section 2. The relative level of BER in various tissues of mice were quantified using a Molecular Imager® System (Bio-Rad, Hercules, CA), and the data were normalized based on the amount of protein used in each reaction. Values represent an average (±S.E.M.) for data obtained from at least three animals in each group. (□): young mice; (■): old mice; (8-OHdG): 7,8-dihydro-8-oxoguanine; (*): value significantly different from control at P < 0.01; (◆): value significantly different from control at P < 0.05.
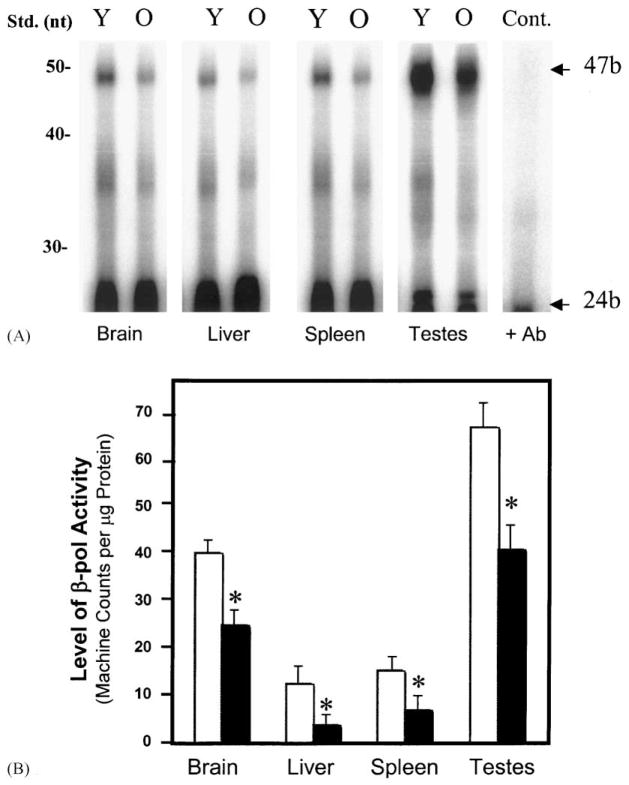
In addition to decreased BER activity, we also observed that the activity of the β-pol protein declines with age. A 47-mer gapped oligonucleotide is incubated with crude nuclear extract from young and old mice. Aphidicolin is present in the reaction mixture to block DNA polymerases α and δ, demonstrating that any gap-filling DNA synthesis occurring is not a function of these polymerases. Additionally, to ensure that we are looking specifically at β-pol activity, the reactions were also run in the presence of a neutralizing antibody to β-pol, which completely blocked gap-filling synthesis (Fig. 3A, lane 9). Resolution of DNA at 24 bases represents a lack of gap-filling, or DNA synthesis activity while resolution at 47 bases represents the presence of gap-filling. Additionally, we added DNA ligase I in excess and found no difference in activity, indicating that our results are not due to ligation effects.
Fig. 3.
Effect of age on β-pol activity as determined by an in vitro gap-filling reaction. The experiment was conducted using crude nuclear extracts as described in Section 2. (A) An autoradiograph of a sequence gel indicating repair activity as visualized by the appearance of a 47b fragment. Samples were pooled from extracts obtained from 3 to 4 animals in each group. (B) The relative level of BER in various tissues of mice were quantified using a Molecular Imager® System (Bio-Rad, Hercules, CA), and the data were normalized based on the amount of protein used in each reaction. Values represent an average (±S.E.M.) for data obtained from at least three animals in each group. (Y, □): young mice; (O, ■): old mice; (+Ab): addition of antibody against β-pol to inhibit repair activity and to serve as a control; (*): value significantly different from control at P < 0.01.
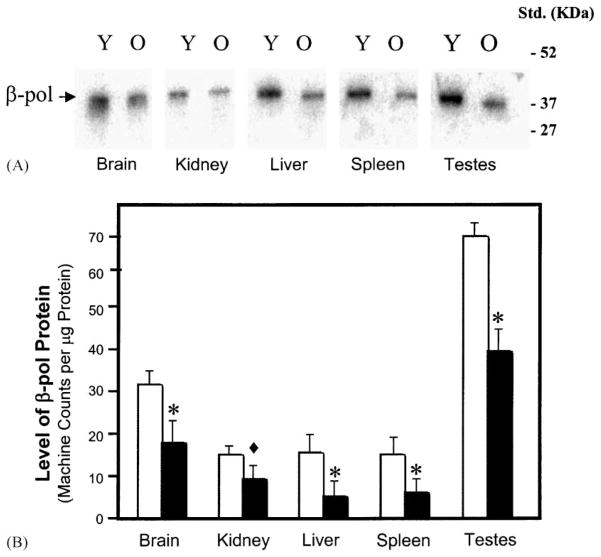
Having found age-related decreases in BER activity as well as β-pol activity, we chose to investigate whether these declines corresponded to decreased levels of β-pol protein and mRNA. Rao et al. [22] had shown that in mouse brain tissue β-pol activity and β-pol mRNA declined with age. We find that the decline in BER activity we observe with age corresponds with an age-related decline in levels of the 39 kDa β-pol protein (Fig. 4) and β-pol mRNA (Fig. 5) in all tissues tested (brain, liver, kidney, spleen and testes). In fact, graphical representation of β-pol activity (Fig. 4B) shows that the age-related decrease in β-pol activity corresponds exactly to the decreases seen in β-pol protein and mRNA levels. This suggests that the observed decrease in BER activity may be the result of age-related changes in the transcriptional regulation of β-pol. Importantly, addition of neutralizing antibody to β-pol in the reaction mixture completely blocked repair, demonstrating that no alternative polymerase was able to substitute for β-pol in these reactions.
Fig. 4.
Effect of age on the levels of β-pol protein. Nuclear extracts were isolated from various tissues of young and old mice as described in Section 2. (A) The level of the 39 kDa β-pol protein in 20 μg of nuclear extract was determined by western blot analysis using an antibody against β-pol protein and ECL detection kit (Pierce Chemical). Samples were pooled from extracts obtained from 3 to 4 animals in each group. (B) The relative level of β-pol protein in various tissues was quantified using an Alpha Innotech MultiImage™ system, and the data were normalized based on the amount of protein loaded on each gel. Values represent an average (±S.E.M.) for data obtained from at least three animals in each group. (Y, □): young mice; (O, ■): old mice; (β-pol): DNA β-pol; (*): value significantly different from control at P < 0.01; (◆): value significantly different from control at P < 0.05.
Fig. 5.
Effect of age on the levels of β-pol mRNA. Poly(A)+ RNA was isolated from various tissues of young and old mice as described in Section 2. (A) An autoradiograph depicting the level of β-pol mRNA in 5 μg of the poly(A)+ RNA as determined by northern blot hybridization using a cRNA probe to β-pol. Samples were pooled from extracts obtained from 3 to 4 animals in each group. (B) The relative level of β-pol mRNA in various tissues were normalized based on the 18S ribosomal RNA and GAPDH mRNA levels and quantified using a Molecular Imager® System (Bio-Rad, Hercules, CA). Values represent an average (±S.E.M.) for data obtained from at least three animals in each group. (Y, □): young mice; (O, ■): old mice; (β-pol): DNA β-pol; GAPDH: glyceraldehyde-3-phosphate dehydrogenase; (*): value significantly different from control at P < 0.01; (◆): value significantly different from control at P < 0.05.
We have also measured the effect of age on the spontaneous and chemically induced mutation frequencies in the lacI gene. Mutation frequency was measured in liver DNA from young mice (4–6 months) and old mice (18–20 months) harboring the lacI transgene to determine the effect of age on spontaneous mutation frequency. Mutation frequency was determined by dividing the number of mutant lacI genes by the total number of plaque-forming units (Table 1). We observe a three-fold increase in the spontaneous mutation frequency in liver from old mice as compared to young mice (Table 1). These data are in very good agreement with data from other labs measuring spontaneous mutation frequencies seen with increasing age [23–25].
Table 1.
Mutation frequencies in liver tissues obtained from mice of various ages exposed to DMS
| Age (months) | Number | Treatment (DMS)
|
Total pfua (×105) | Mutant plaques | MFb (×10−6 ± S.D.) | ||
|---|---|---|---|---|---|---|---|
| Value (mg/kg) | Route | Total | |||||
| 4–6 | 4◆ | – | – | – | 1.02 | 5 | 5.1± 3.7 a |
| 5 | 30 | 1 × ip | |||||
| 10 | 2 × ip | 50 | 1.64 | 22 | 13.1 ± 3.9 ab | ||
| 18–20 | 5◆ | – | – | – | 0.82 | 14 | 17.1 ± 7.4 b |
| 4 | 30 | 1 × ip | |||||
| 10 | 2 × ip | 50 | 0.97 | 86 | 88.7 ± 18.9 c | ||
Animals were treated with DMS or olive oil (◆: control) as described in Section 2. Animals were sacrificed 14 days after the last dose of the treatment, and the mutation frequency of the lacI transgene in liver tissues of control and DMS treated mice were quantified.
pfu: plaque-forming units.
MF: mutation frequency. MF values with different letters are significantly different from each other (P < 0.01).
We measure chemically induced mutation frequency in response to treatment with DMS because the spectrum of damage induced by DMS closely mimics the spectrum of endogenous alkylation [26]. Additionally, it is a direct-acting carcinogen. Because activation and deactivation of carcinogens change with age [27], the use of a direct-acting carcinogen allows us to test the effects of age without the confounding of age-related changes in xenobiotic metabolism. Young (4–6 months) and old (18–20 months) male lacI mice were treated with DMS as described in Section 2. In response to DMS the increase in mutation frequency in young mice is not statistically significant at P < 0.01 (Table 1), which is not surprising as the damage induced by DMS is generally well tolerated and of relatively low mutagenicity. What we find very interesting, however, is that in the old mice there is a five-fold increase in mutation frequency in response to the same dose of DMS that had no effect in the young mice. It is inviting to suggest that this dramatic change in the ability to tolerate DMS is related to the decline in BER activity that is occurring with age.
4. Discussion
In this study, we have demonstrated that BER activity declines with age, and that this decline corresponds to demonstrated declines in levels of β-pol activity, protein and mRNA. To our knowledge, our study is the first animal study showing a decline in BER activity with age and the first report of an age-related decline in the rate-limiting enzyme in this critical pathway. Additionally, we have demonstrated for the first time, an age-related decline in the ability to process DNA damage that is repaired by the BER pathway. Cell studies support our findings of decreased BER capacity with age. For example, aged rat neuronal extracts repair a synthetic oligonucleotide less efficiently than young neuronal extracts, and this age-related decline in repair is corrected by the addition of exogenous β-pol [28]. Additionally, IMR90 cells exhibit a decline in AP site repair and glycosylase activity as the cells senesce [29]. Contrarily, the ability of mito-chondrial extracts to excise 8-OHdG lesions (a BER substrate) actually increases with age, while nuclear extracts from these same aged animals seem to show no difference in the ability to remove this lesion [30].
We have also shown that as BER capacity declines, both spontaneous and induced mutation frequency increases. Interestingly, it appears that tissues with low BER activity exhibit the highest mutation frequency while tissues with high BER activity seem to be protected from mutation induction, or damage fixation. Hirose et al. [31] found that in mice β-pol mRNA levels were highest in the testis, then brain and thymus, while levels were lowest in heart, kidney and liver. Our findings support these data and additionally show low β-pol levels and low BER activity in these tissues and in spleen as well. Several labs have shown that in mice, mutation frequency increases with age more in tissues that we have shown to exhibit low BER activity. For example, Dolle et al. [23] found that in both liver and brain mutation frequency increased with age, but the increase was much greater in the liver. Interestingly, they found that the mutation frequency stopped increasing in brain after the age of 4–6 months, a tissue in which β-pol has been shown to be the predominant polymerase [32]. Ono et al. [24] found that from birth to 25 months of age mutation frequency significantly increased in all tissues but the rates of change in mutations differed between tissues. The highest rates of mutation frequency occurred in spleen, liver and heart, and the lowest rates were seen in the brain, skin and testes. Stuart et al. [25] found that from the age of 1.5–25 months mutation frequency increased in all tissues tested. Here, a three-fold increase in mutation frequency was found in liver, while the increase in mutation frequency in the brain was less than two-fold. Additional support for the idea that high BER activity may protect against mutation induction comes from data demonstrating that germ cells have very high levels of BER activity and very low mutation frequency [20]. Thus, data from several labs consistently show that mutation frequency is highest in tissues with lowest BER activity. The decrease in BER activity that we have observed with age may provide a mechanistic explanation for the phenomenon of increasing spontaneous mutation with age, perhaps more adequately than the proliferative status of a tissue can.
The BER pathway also repairs damage induced by oxidizing and alkylating agents, which often mimic endogenous damage. For this reason, we have chosen to test whether the decreased BER capacity seen with age would result in increased sensitivity to an environmental agent that induces a spectrum of damage almost entirely processed by the BER pathway. Other studies have addressed this issue of increased susceptibility to carcinogens with age. Anisimov [33] failed to find an age-related sensitivity to 1,2-diethylhydrazine in aged female L10 rats. Mullaart et al. [34] measured the effect of age on the induction and removal of lesions after exposure to 2-AAF, and failed to find a strong age-related effect. Dass et al. [35] failed to show increased sensitivity to Mitomycin C with age. However, none of these studies have used DNA damaging agents that induce damage repaired by the BER pathway. In contrast to the studies that failed to find an increased sensitivity to carcinogens with age, we have found that when the damage induced is the type repaired primarily by the BER pathway that there is an age-related increase in the mutagenicity of the carcinogen.
Generally, DMS is not a potent mutagen, likely because the amount of O6-methylguanine induced is less than 0.3% of total lesions [26]. Because of the high potency of this lesion, it is important to control for its effects when possible. Fortunately, levels of MGMT, the enzyme responsible for repair of O6-methylguanine, do not change with age [36,37]. Therefore, the age-related changes we see are not due to the processing of this lesion. The predominant lesion induced by DMS is N7-methylguanine, which is efficiently repaired by BER. In our young mice that have been shown to have high levels of BER activity, we see no statistically significant increase in mutation frequency in response to DMS. Yet we see a five-fold increase in mutation frequency in aged mice in response to DMS. This supports the concept that as animals age they become less able to tolerate levels and types of damage that were well tolerated in youth. This is important because Stuart et al. [25] have shown that while mutation frequency increases with age, no change in mutational specificity is seen. This suggests that animals are accumulating more of the same types of DNA damage throughout life, and perhaps they are less able to repair these types of damage. It is important to emphasize that in this study, we have measured the effect of DMS in liver tissues only, and considering that Dolle et al. [23] observed greater increases in mutation frequency in liver tissue with age, other tissues might not show same-fold differences in mutation frequency in response to DMS.
Our data demonstrate a strong correlation between a loss of BER activity with age and increased sensitivity to a DNA damaging agent. To directly test the role of BER in maintaining genomic integrity and to determine mechanistically whether the decline in BER activity directly increases the mutagenicity of DNA damaging agents the use of animals with targeted disruptions in BER genes will prove useful. In fact, the homozygous APNG knockout mouse which is defective in removing alkylated bases including N7-methylguanine, shows a higher mutagenic response to MMS than is seen in the wild type animals [38]. The homozygous OGG1 knockout mouse exhibits a mild phenotype including small but significant increases in spontaneous mutation frequency [39]. Because glycosylases recognize relatively specific types of damage and because the endogenous spectrum of damage is much broader than that recognized by any one glycosylase, we hypothesize that we will see even greater effects in animals that are deficient in BER enzymes responsible for repair of all BER intermediates. Support for this is already apparent in that homozygous deletions of Ape, β-pol and XRCC1 [40] are embryonic lethal. The use of these animal models as heterozygotes or rescued homozygotes in aging and carcinogenicity research will be instrumental in defining a role for BER in these processes.
Acknowledgments
We thank Drs. S.H. Wilson and R.W. Sobol (National Institute of Environmental Health Sciences) for their generous gifts of the DNA β-pol cDNA probe and antibody. This work was supported in part by grants from the American Institute for Cancer Research (AICR97A113), the National Institute on Aging (AG14242), and by a Pilot Project Program Grant from the Wayne State University NIEHS Center for Molecular and Cellular Toxicology (ES06639).
References
- 1.Warner HR, Price AR. Involvement of DNA repair in cancer and aging. J Gerontol. 1989;44:45–54. doi: 10.1093/geronj/44.6.45. [DOI] [PubMed] [Google Scholar]
- 2.Mullaart E, Lohman PH, Berends F, Vijg J. DNA damage metabolism and aging. Mutat Res. 1990;237:189–210. doi: 10.1016/0921-8734(90)90001-8. [DOI] [PubMed] [Google Scholar]
- 3.Bohr VA, Anson RM. DNA damage mutation and fine structure DNA repair in aging. Mutat Res. 1995;338:25–34. doi: 10.1016/0921-8734(95)00008-t. [DOI] [PubMed] [Google Scholar]
- 4.Walter CA, Grabowski DT, Street KA, Conrad CC, Richardson A. Analysis and modulation of DNA repair in aging. Mech Ageing Dev. 1997;98:203–222. doi: 10.1016/s0047-6374(97)00108-5. [DOI] [PubMed] [Google Scholar]
- 5.Vijg J, Dolle ME, Martus HJ, Boerrigter ME. Transgenic mouse models for studying mutations in vivo: applications in aging research. Mech Ageing Dev. 1997;98:189–202. doi: 10.1016/s0047-6374(97)00107-3. [DOI] [PubMed] [Google Scholar]
- 6.Loeb KR, Loeb LA. Significance of multiple mutations in cancer. Carcinogenesis. 2000;21:379–385. doi: 10.1093/carcin/21.3.379. [DOI] [PubMed] [Google Scholar]
- 7.Holmquist GP. Endogenous lesions, S-phase-independent spontaneous mutations, and evolutionary strategies for base excision repair. Mutat Res. 1998;400:59–68. doi: 10.1016/s0027-5107(98)00051-7. [DOI] [PubMed] [Google Scholar]
- 8.Lindahl T. Suppression of spontaneous mutagenesis in human cells by DNA base excision-repair. Mutat Res. 2000;462:129–135. doi: 10.1016/s1383-5742(00)00024-7. [DOI] [PubMed] [Google Scholar]
- 9.Hanawalt PC. Evolution of concepts in DNA repair. Environ Mol Mutagen. 1994;23:78–85. doi: 10.1002/em.2850230617. [DOI] [PubMed] [Google Scholar]
- 10.Singhal RK, Prasad R, Wilson SH. DNA polymerase β conducts the gap-filling step in uracilinitiated base excision repair in a bovine testis nuclear extract. J Biol Chem. 1995;270:949–957. doi: 10.1074/jbc.270.2.949. [DOI] [PubMed] [Google Scholar]
- 11.Srivastava DK, Berg BJ, Prasad R, Molina JT, Beard WA, Tomkinson AE, Wilson SH. Mammalian abasic site base excision repair: identification of the reaction sequence and rate-determining steps. J Biol Chem. 1998;273:21203–21209. doi: 10.1074/jbc.273.33.21203. [DOI] [PubMed] [Google Scholar]
- 12.Nilsen H, Krokan HE. Base excision repair in a network of defense and tolerance. Carcinogenesis. 2001;22:987–998. doi: 10.1093/carcin/22.7.987. [DOI] [PubMed] [Google Scholar]
- 13.Fortini P, Pascucci B, Parlanti E, Sobol RW, Wilson SH, Dogliotti E. Different DNA polymerases are involved in the short- and long-patch base excision repair in mammalian cells. Biochemistry. 1998;37:3575–3580. doi: 10.1021/bi972999h. [DOI] [PubMed] [Google Scholar]
- 14.Dianov GL, Prasad R, Wilson SH, Bohr VA. Role of DNA polymerase β in the excision step of long-patch mammalian base excision repair. J Biol Chem. 1999;274:13741–13743. doi: 10.1074/jbc.274.20.13741. [DOI] [PubMed] [Google Scholar]
- 15.Pascucci B, Stucki M, Jonsson ZO, Dogliotti E, Hubscher U. Long-patch base excision repair with purified human proteins. J Biol Chem. 1999;274:33696–33702. doi: 10.1074/jbc.274.47.33696. [DOI] [PubMed] [Google Scholar]
- 16.Klungland A, Lindahl T. Second pathway for completion of human DNA base excision-repair: reconstitution with purified proteins and requirement for DNase IV (FEN1) EMBO J. 1997;16:3341–3348. doi: 10.1093/emboj/16.11.3341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Podlutsky AJ, Dianova II, Podust VN, Bohr VA, Dianov GL. Human DNA polymerase beta initiates DNA synthesis during long-patch repair of reduced AP sites in DNA. EMBO J. 2001;20:1477–1482. doi: 10.1093/emboj/20.6.1477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein–dye binding. Anal Biochem. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- 19.Heydari AR, Wu B, Takahashi R, Strong R, Richardson A. Expression of heat shock protein 70 is altered by age and diet at the level of transcription. Mol Cell Biol. 1993;13:2909–2918. doi: 10.1128/mcb.13.5.2909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Walter CA, Intano GW, McCarrey JR, McMahan CA, Walter RB. Mutation frequency declines during spermatogenesis in young mice but increases in old mice. Proc Natl Acad Sci USA. 1998;95:1001510019. doi: 10.1073/pnas.95.17.10015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sokal RR, Rohlf FJ. Biometry. W.H. Freeman and Company; New York: 1981. Single classification analysis of variance; pp. 208–270. [Google Scholar]
- 22.Rao KS, Vinay Kumar D, Bhaskar MS, Sripad G. On the active molecules of DNA polymerase (in aging rat brain (erratum) Biochem Mol Biol Int. 1994;34:287–294. [PubMed] [Google Scholar]
- 23.Dolle ME, Giese H, Hopkins CL, Martus HJ, Hausdorff JM, Vijg J. Rapid accumulation of genome rearrangements in liver but not in brain of old mice. Nat Genet. 1997;17:431–434. doi: 10.1038/ng1297-431. [DOI] [PubMed] [Google Scholar]
- 24.Ono T, Ikehata H, Nakamura S, Saito Y, Hosoi Y, Takai Y, Yamada S, Onodera J, Yamamoto K. Age-associated increase of spontaneous mutant frequency and molecular nature of mutation in newborn and old lacI transgenic mouse. Mutat Res. 2000;447:165–177. doi: 10.1016/s0027-5107(99)00200-6. [DOI] [PubMed] [Google Scholar]
- 25.Stuart GR, Oda Y, deBoer JG, Glickman BW. Mutation frequency and specificity with age in liver, bladder and brain of lacI transgenic mice. Genetics. 2000;154:1291–1300. doi: 10.1093/genetics/154.3.1291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hoffmann GR. Genetic effects of dimethyl sulfate, diethyl sulfate, and related compounds. Mutat Res. 1980;75:63–129. doi: 10.1016/0165-1110(80)90028-7. [DOI] [PubMed] [Google Scholar]
- 27.Masuda M, Nukuzuma C, Kazusaka A, Fujita S. Alterations in activation and deactivation of mutagens in aging rat liver. J Gerontol A Biol Sci Med Sci. 1995;50:B303–B306. doi: 10.1093/gerona/50a.5.b303. [DOI] [PubMed] [Google Scholar]
- 28.Rao KS, Annapurna VV, Raji NS, Harikrishna T. Loss of base excision repair in aging rat neurons and its restoration by DNA polymerase beta. Brain Res Mol Brain Res. 2000;85:251–259. doi: 10.1016/s0169-328x(00)00266-7. [DOI] [PubMed] [Google Scholar]
- 29.Atamna H, Cheung I, Ames BN. A method for detecting abasic sites in living cells: age-dependent changes in base excision repair. Proc Natl Acad Sci USA. 2000;97:686–691. doi: 10.1073/pnas.97.2.686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.de Souza-Pinto NC, Hogue BA, Bohr VA. DNA repair and aging in mouse liver: 8-oxodG glycosylase activity increase in mitochondrial but not in nuclear extracts. Free Radical Biol Med. 2001;30:916–923. doi: 10.1016/s0891-5849(01)00483-x. [DOI] [PubMed] [Google Scholar]
- 31.Hirose F, Hotta Y, Yamaguchi M, Matsukage A. Difference in the expression level of DNA polymerase beta among mouse tissues: high expression in the pachytene spermatocyte. Exp Cell Res. 1989;181:169–180. doi: 10.1016/0014-4827(89)90191-2. [DOI] [PubMed] [Google Scholar]
- 32.Prapurna DR, Rao KS. DNA polymerases delta and epsilon in developing and aging rat brain. Int J Dev Neurosci. 1997;15:67–73. doi: 10.1016/s0736-5748(96)00047-0. [DOI] [PubMed] [Google Scholar]
- 33.Anisimov VN. Carcinogenesis induced by a single administration of 1,2-diethylhydrazine in female rats of various ages. Cancer Lett. 1992;67:21–25. doi: 10.1016/0304-3835(92)90004-f. [DOI] [PubMed] [Google Scholar]
- 34.Mullaart E, Boerrigter ME, Lohman PH, Vijg J. Age-related induction and disappearance of carcinogen-DNA-adducts in livers of rats exposed to low levels of 2-acetylaminofluorene. Chem Biol Interact. 1989;69:373–384. doi: 10.1016/0009-2797(89)90123-3. [DOI] [PubMed] [Google Scholar]
- 35.Dass SB, Ali SF, Heflich RH, Casciano DA. Frequency of spontaneous and induced micronuclei in the peripheral blood of aging mice. Mutat Res. 1997;381:105–110. doi: 10.1016/s0027-5107(97)00156-5. [DOI] [PubMed] [Google Scholar]
- 36.Washington WJ, Foote RS, Dunn WC, Generoso WM, Mitra S. Age-dependent modulation of tissue-specific repair activity for 3-methyladenine and O6-methylguanine in DNA in inbred mice. Mech Ageing Dev. 1989;48:43–52. doi: 10.1016/0047-6374(89)90024-9. [DOI] [PubMed] [Google Scholar]
- 37.Mizoguchi M, Naito H, Kurata Y, Shibata MA, Tsuda H, Wild CP, Montesano R, Fukushima S. Influence of aging on multi-organ carcinogenesis in rats induced by N-methyl-N-nitrosourea. Jpn J Cancer Res. 1993;84:139–146. doi: 10.1111/j.1349-7006.1993.tb02847.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Elder RH, Jansen JG, Weeks RJ, Wellington MA, Deans B, Watson AJ, Mynett KJ, Bailey JA, Cooper DP, Rafferty JA, Heeran MC, Wijnhoven SWP, vanZeeland AA, Margison GP. Alkylpurine–DNA–glycosylase knockout mice show increased susceptibility to induction of mutations by methyl methanesulfonate. Mol Cell Biol. 1998;18:5828–5837. doi: 10.1128/mcb.18.10.5828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Klungland A, Rosewell I, Hollenbach S, Larsen E, Daly G, Epe B, Seeberg E, Lindahl T, Barnes DE. Accumulation of premutagenic DNA lesions in mice defective in removal of oxidative base damage. Proc Natl Acad Sci USA. 1999;96:13300–13305. doi: 10.1073/pnas.96.23.13300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Friedberg EC, Meira LB. Database of mouse strains carrying targeted mutations in genes affecting cellular responses to DNA damage (Version 4) Mutat Res. 2001;459:243–274. doi: 10.1016/s0921-8777(00)00006-9. [DOI] [PubMed] [Google Scholar]