Abstract
Purpose
Appropriate treatment of the lower neck when using IMRT is controversial. Our study tried to determine differences in clinical outcomes using IMRT or a standard LNF to treat low neck.
Methods and Materials
This is a retrospective, single institution study. Ninety-one patients with squamous cell carcinoma of head and neck cancer were treated with curative intent. Based on physician preference, some patients were treated with LNF (PTV3) field using a single anterior photon field matched to the IMRT field. Field junctions were not feathered. The endpoints were time to failure and use of PEG tube (as a surrogate of laryngeal edema causing aspiration) and analysis done with chi-square and the log-rank tests.
Results
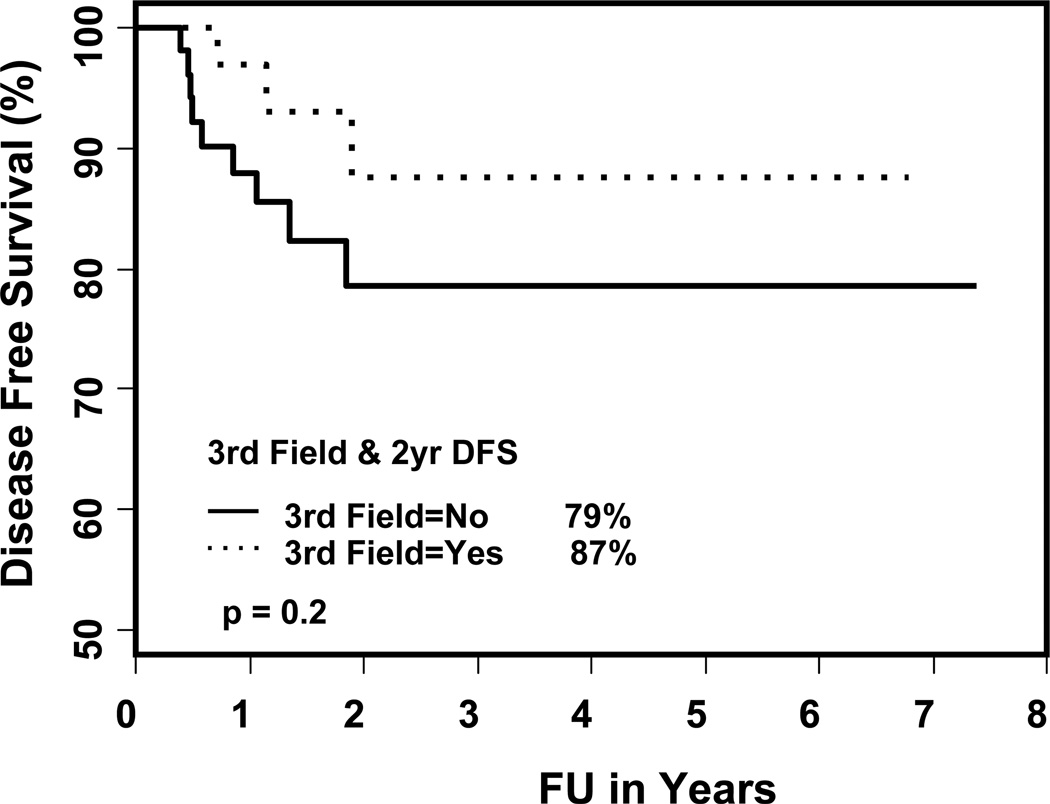
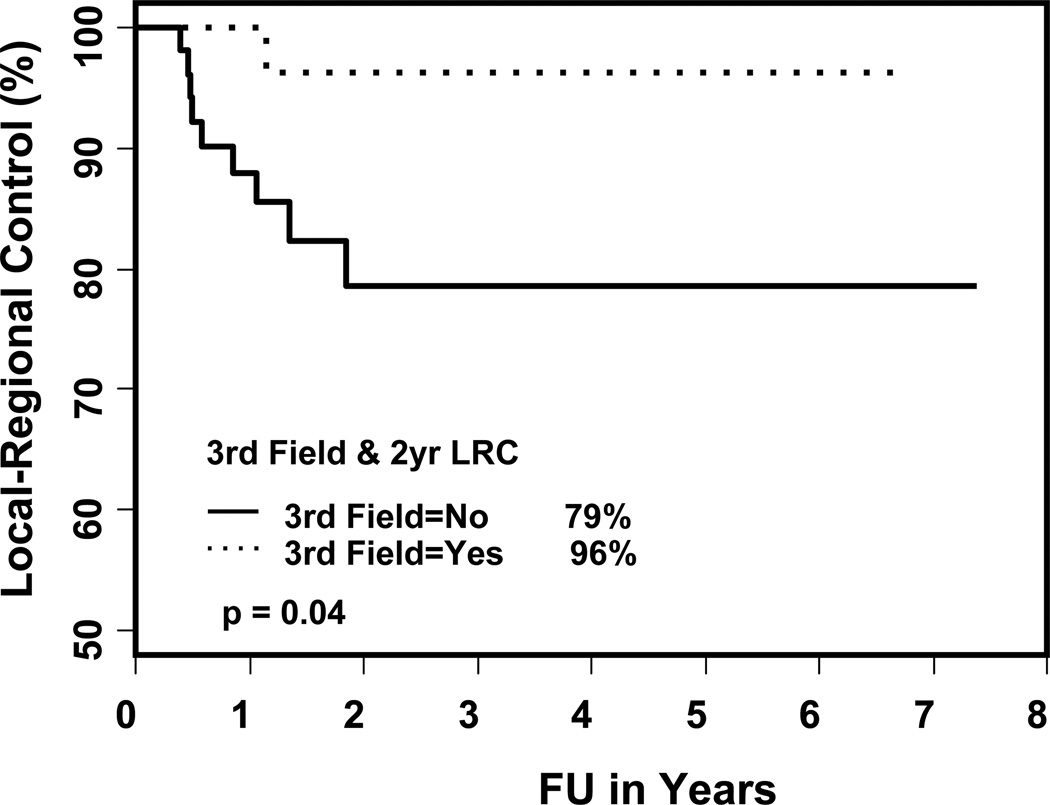
Median follow up 21 months (range 2 – 89). The median age 60 years. Thirty seven (41%) were treated with LNF, 84% were stage III or IV. PEG tube was required in 30% as opposed to 33% without the use of LNF. N2 or 3 neck disease was treated more commonly without a LNF (38% vs. 24%, p = 0.009). Failures occurred in 12 patients (13%). Only one patient treated with LNF failed regionally, 4.5 cm above the match line. The 3-year disease-free survival rate was 87%, 79% with LNF and without LNF respectively (p = 0.2) and the 3-year LR failure rate was 4%, 21% respectively, (p = 0.04).
Conclusions
Using LNF to treat the low neck did not increase the risk of regional failure “in early T& early N diseases” or decrease PEG tube requirements.
Keywords: IMRT, Head and Neck cancer, Low neck field, RT toxicities, PEG tube
Introduction
Treatment of head and neck cancers with standard radiation therapy has traditionally consisted of two parallel-opposed lateral portals matched to an anterior supraclavicular field. With the introduction of 3-dimensional conformal techniques (3D-CRT) using intensity modulated radiation therapy (IMRT), there has been an improvement in tumor coverage in certain tumors (i.e. nasopharyngeal carcinoma) and a decrease in dose to certain normal structures (i.e. reducing the dose to the parotid glands1 has decreased the risk of late Xerostomia) compared with conventional radiation portals. This improved tumor coverage appears to have improved treatment outcomes by lowering loco-regional recurrence rates2–7. When using IMRT, it is controversial how to treat the low neck. There are two basic approaches: Using IMRT as a single field/or whole field versus using a split field technique using IMRT to treat the primary tumor matched to a conventional low neck field to treat low neck. Several authors described different techniques for matching the IMRT fields8–14. There are advantages and disadvantages for each approach which were described in several recent papers8, 14–17. Using a matching (i.e. split field IMRT), the dose inhomogeneities contribute to setup errors leading to either cold or hot spots over the entire treatment course which could lead to increased failures or complications, respectively. The concern with the whole field approach is the increased dose to the larynx, causing laryngeal edema which could cause speech or swallowing difficulties. When there is gross adenopathy at the level of the larynx prior to treat, split field technique is not recommended unless a neck dissection is planned due to a potential risk of regional failures. This retrospective study was performed to determine differences in clinical outcomes using whole-field or split-field IMRT.
Methods and Materials
Between May 2001 and June 2008, 153 patients with head and neck cancer were treated with IMRT at Fox Chase Cancer Center. Among these, 91 (59.5%) had squamous cell carcinoma and were treated with curative intent. The patient, tumor and treatment related demographic data were obtained retrospectively. The distribution of these variables is shown in Tables 1 to 3. The IMRT technique, dose prescription and treatment planning methods at FCCC were described in detail previously7.
Table 1.
Patient- related characteristics with and without LNF (n = 91)
| Variable | Without LNF No. of. patients (%) |
With LNF No. of. patients (%) |
p* value |
|---|---|---|---|
| Age, median in years | 63 (28–85) | 57 (30–88) | 0.3 |
| Gender | 0.3 | ||
| Male | 39 (72) | 30 (81) | |
| Smoking | |||
| Yes | 42 (78) | 29 (78) | 1.00 |
| Alcohol intake | |||
| Yes | 42 (81) | 28 (76) | 0.61 |
| Race | |||
| White | 51 (94) | 37 (100) | 0.71 |
| African-American | 2 (4) | 0 (0) | |
| Asian | 1 (2) | 0 (0) |
Abbreviations: LNF= low neck field.
p < 0.05 considered statistically significant.
Table 3.
RTOG Acute toxicity with and without LNF
| Grade 2, N (%) | Grade 3, N (%) | Grade 4, N (%) | p* value | ||||
|---|---|---|---|---|---|---|---|
| Without LNF (N=54) |
With LNF (N=37) |
Without LNF (N=54) |
With LNF (N=37) |
Without LNF (N=54) |
With LNF (N=37) |
||
| Skin | 23 (43) | 20 (54) | 2 (4) | 0 (0) | 0 | 0 | 0.56 |
| Mucosa | 28 (52) | 17 (46) | 17 (32) | 15 (41) | 0 | 0 | 0.54 |
| Pharynx/Esophagitis | 29 (54) | 20 (54) | 9 (17) | 5 (14) | 0 | 1 (3) | 0.21 |
| Larynx | 4 (7) | 1 (3) | 1 (2) | 0 | 0 | 0 | 0.43 |
| Taste changes | 37 (69) | 29 (78) | 4 (7) | 1 (3) | 0 | 1 (3) | 0.43 |
| Xerostomia | 34 (63) | 27 (73) | 1 (2) | 2 (5) | 0 | 0 | 0.38 |
| Hematological | 5 (9) | 6 (16) | 1 (2) | 0 | 0 | 0 | 0.58 |
Abbreviations: N= number of patients.
p < 0.05 considered statistically significant.
Radiotherapy details
All patients were treated with IMRT using a smart boost18, which uses the inherent hot spots generated by the IMRT plan to treat different volumes at different doses at the same time. Based on physician preference, some patients receive the radiation to the supraclavicular fossa using a single anterior photon field matched to the IMRT fields. In the definitive treatment setting, IMRT was used to decrease the dose to the parotid gland only if there were no clinically involved lymph nodes on that side of the neck. The high risk volume was prescribed to 70 Gy in 35 fractions, 2 Gy per fraction over 7 weeks duration (clinically evident disease at the primary site and neck with a 1 to 2 cm margin, PTV1) while electively treated neck nodes were treated to 56 Gy (PTV2). An intermediate risk volume was generally not used. When a low neck field (LNF) was treated, the dose was prescribed to 46–50 Gy to a depth of 3 cm. Field junctions were not feathered. The inferior 100 cGy isodose line of the IMRT radiation fields was matched to superior border of supraclavicular fossa facilitated by asymmetric collimation providing half beam blocking, ensuring no cold spots. In the postoperative setting, the PTV1 (the post-operative bed and regions of pathologic lymph node involvement) was prescribed to 60 to 66 Gy while the PTV2 was prescribed to 54 Gy (elective regional lymph nodes). Normal tissue dose constraints were: spinal cord less than 48 Gy, brain stem less than 54 Gy, mean parotid dose less than 26 Gy, limit larynx dose to less than 40 Gy and optic chiasm to less than 54 Gy. The Corvus inverse treatment planning system was used for the majority of patients (version 3.0, NOMOS Corp., Cranberry Township, PA). The median total dose to the PTV1 was 70 Gy in 35 fractions (range: 42–75 Gy in 21–50 fractions); PTV2 was 56 Gy (range: 39–68 Gy) and PTV3 was 48.6 Gy (range: 44–56 Gy). All patients were treated with once daily fractionation, 2 Gy per fraction over a period of 6 to 7 weeks except 3 patients who were treated using a University of Chicago approach (ClinicalTrials.gov Identifier: NCT00117572) with 75 Gy, 1.5 Gy per fraction, twice daily, one week on and one week off concomitant with chemotherapy. The median duration of treatment was 46 days (range: 29–67 days). The median numbers of beams used were seven (range: 4–17) with IMRT.
Chemotherapy
Fifty-two patients (57%) were treated with various chemotherapy regimens along with radiation therapy; 24/37 (65%) and 28/54 (52%) patients each with or without LNF (p = 0.28). Concomitant Cisplatin was most commonly used (n = 29, 32%) at 100 mg/m2 every three weeks, along with RT. Weekly Cetuximab was used in 11 patients (12%). Cisplatin and cetuximab combination was given to 6 patients (7%). Six patients were treated with combination regimens (3 with docetaxel, fluorouracil, hydroxyurea, one with carboplatin, taxol and cetuximab, one patient with cisplatin and docetaxel and one with carboplatin and taxol). Concurrent chemotherapy was given to 57% in the definitive and 5% in the postoperative setting for patients treated with LNF. The distributions of patients treated with chemotherapy along with different RT methods with and without LNF (definitive versus postoperative setting) are shown in Table 3.
Neck dissection
All patients were assessed by a head and neck surgeon 4 weeks post-radiation for neck dissection. During the interval studied, planned neck dissections were performed when lymph nodes > 3 cm at presentation. In addition, patients with persistent nodes following radiation went on to receive a neck dissection. Thirty nine patients (43%) had neck dissection performed: 12 (32%) and 27 (50%) patients treated with and without LNF.
Complications
Acute and late toxicity were recorded as per the Radiation Therapy Oncology Group (RTOG) toxicity criteria. The use of a PEG (percutaneous endoscopic gastrostomy) tube was used as a surrogate marker of laryngeal edema causing aspiration and the toxicities were analyzed for the patients treated with or without the LNF.
Statistical analysis
All the analyses were performed using the statistical analysis systems (SAS Institute, Cary, NC). Patient, tumor- and treatment-related factors, including T stage (T1, 2, versus T3, 4), N stage (N0,1 vs. N2, 3), tobacco history (yes versus no), alcohol intake (yes versus no), location of primary (oral cavity versus other sites), treatment type (definitive RT versus PORT), concurrent chemotherapy (yes versus no), use of amifostine (yes versus no), use of a third field (yes versus no) and RT duration (< 46 days versus > 46 days) were analyzed for an impact on disease-free survival, patterns of failure and toxicity via the chi-square test and log-rank test. Values of ‘p’ less than 0.05 were considered statistically significant. Local failures (LF) were defined by persistence/recurrence within the primary site. Regional failures occurred in the draining lymph nodes in the neck. Marginal failure was defined as a failure that occurred at a region of dose falloff or outside the RT field.
Results
Thirty seven (41%) patients were treated with a LNF. Patient characteristics are shown in Table 1. The median ages were 57 years and 63 years for patients treated with and without LNF. There were no differences between groups based on tobacco use (p = 1.0), alcohol intake (p = 0.61) and ethnicity (p = 0.71). Tumor-related characteristics are shown in Table 2. The plurality of patients (48%) had tumor in the oropharynx (24 and 20 patients in each group) and 24% had oral cavity tumors (p = 0.16). There were 6 (7%) patients with laryngeal and two with hypopharyngeal primary cancers. Twenty patients had T3 tumors and 15 T4; 28 patients had N2 nodal stage and 31 and 41 belong to stage III and IV, respectively. N2 or 3 neck disease was treated more commonly without a LNF (38% versus 24%, p = 0.009). In patients treated with LNF, 31/37 (84%) belonged to stage III or IV. PORT was given to 29% of patients (24% RT alone and 5% concurrent chemoradiation), while 71% were treated with definitive radiation (19% RT alone and 52% concurrent chemoradiation), p = 0.24.
Table 2.
Tumor and treatment- related characteristics (n = 91)
| Variable | Without LNF No. of. patients (%) |
With LNF No. of. patients (%) |
p* value |
|---|---|---|---|
| Primary site | 0.16 | ||
| Nasopharynx | 1 (2) | 5 (13) | |
| Oropharynx | 24 (44) | 20 (54) | |
| Oral cavity | 14 (26) | 8 (22) | |
| Hypopharynx | 1 (2) | 1 (3) | |
| Larynx | 5 (9) | 1 (3) | |
| PNS & Nasal cavity | 7 (13) | 2 (5) | |
| Others | 2 (4) | 0 (0) | |
| T stage | 0.16 | ||
| T1 | 12 (24) | 12 (33) | |
| T2 | 15 (29) | 13 (36) | |
| T3 | 16 (31) | 4 (11) | |
| T4 | 8 (16) | 7 (20) | |
| Missing | 3 | 1 | |
| N stage | 0.009 | ||
| N0 | 24 (45) | 10 (27) | |
| N1 | 9 (17) | 18 (49) | |
| N2 | 19 (36) | 9 (24) | |
| N3 | 1 (2) | 0 (0) | |
| Missing | 1 | 0 | |
| Overall stage | 0.21 | ||
| I | 3 (6) | 3 (8) | |
| II | 9 (17) | 3 (8) | |
| III | 14 (26) | 17 (46) | |
| IVA/B | 27 (51) | 14 (38) | |
| Missing | 1 | 0 | |
| Type of treatment | 0.24 | ||
| Definitive RT | 35 (65) | 30 (81) | |
| RT alone | 9 (17) | 9 (24) | |
| Concurrent CRT | 26 (48) | 21 (57) | |
| Postoperative RT | 19 (35) | 7 (19) | |
| RT alone | 17 (31) | 5 (14) | |
| Concurrent CRT | 2 (4) | 2 (5) | |
| Chemotherapy | 0.28 | ||
| Yes | 28 (52) | 24 (65) | |
| Neck dissection | 0.13 | ||
| Yes | 27 (50) | 12 (32) | |
| PEG tube use | 0.82 | ||
| Yes | 18 (33) | 11 (30) |
Abbreviations: PNS= Para nasal sinuses, T= tumor, N= nodal stage, PEG= percutaneous endoscopic gastrostomy, RT= radiation therapy, CRT= definitive chemoradiotherapy.
p < 0.05 considered statistically significant.
The acute and late toxicities are shown in Tables 3 and 4. Most patients developed grade 2 and 3 skin and mucosal reactions whether treated with or without LNF. Grade 4 toxicity occurred in 3% of patients with LNF. There were no other major grade 3 or 4 toxicities in either group. 33% needed PEG tube as opposed to 29% without the use of a LNF during the course of treatment (p = 0.82). The median time at which the PEG tube was taken out was 7 months (range: 1–72 months). Among the 37 patients in the total group with PEG tube use, 7 were dependant for more than one year (1 and 6 patients with and without LNF, respectively.
Table 4.
RTOG Late toxicity with and without LNF
| Grade 2, N (%) | Grade 3, N (%) | Grade 4, N (%) | p* value |
||||
|---|---|---|---|---|---|---|---|
| Without LNF (N=54) |
With LNF (N=37) |
Without LNF (N=54) |
With LNF (N=37) |
Without LNF (N=54) |
With LNF (N=37) |
||
| Skin | 4 (8) | 2 (6) | 0 | 0 | 0 | 0 | 0.52 |
| Mucosa | 0 | 0 | 0 | 0 | 0 | 0 | 0.56 |
| Pharynx/Esophagitis | 4 (8) | 1 (3) | 0 | 0 | 0 | 0 | 0.84 |
| Larynx | 2 (4) | 0 | 0 | 0 | 0 | 0 | 0.41 |
| Xerostomia | 42 (81) | 29 (88) | 0 | 0 | 0 | 0 | 0.55 |
| Hematological | 1 (2) | 2 (6) | 0 | 0 | 0 | 0 | 0.56 |
p < 0.05 considered statistically significant.
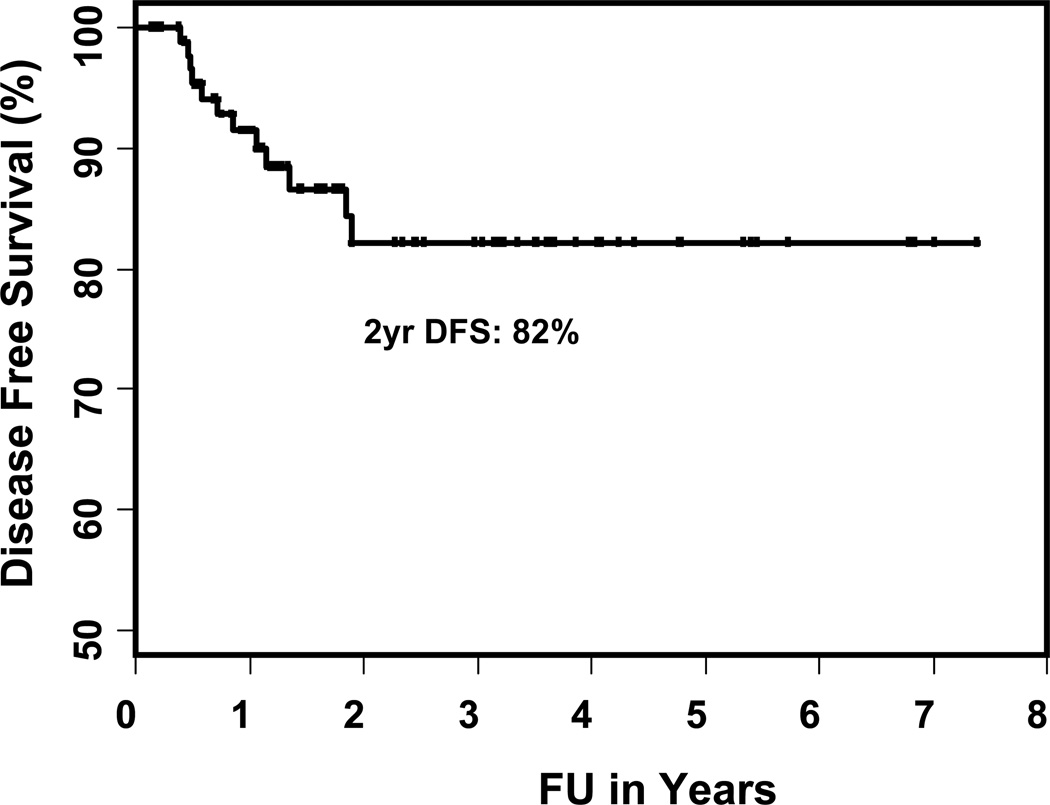
The Kaplan-Meier estimates of two- and three-year local control rates, locoregional control rates and overall survival are shown in Table 5. The two-year local control rates, loco-regional (LR) control and overall survival were 100% and 89% (p = 0.06), 96% and 79% (p = 0.04), and 78% and 81%, (p = 0.98) for patients treated with and without LNF respectively. The two-year actuarial rate of failure was 18% for the total cohort, 13% and 21% in patients treated with and without LNF (p = 0.2, shown in Figures 1 and 2). Failures occurred in 12 patients (13%): 5 local, 2 regional, 1 locoregional, 1 local-regional-distant, 2 distant and 1 regional-distant (shown in Table 6). Only one patient treated with a LNF failed regionally, 4.5 cm above the match line (p = 0.04). On univariate analysis, the type of treatment (p = 0.004; definitive RT versus PORT), overall stage (p = 0.05; I/II versus III/IV) and location of primary (p = 0.008; oral cavity versus other sites) were significant predictors of disease free survival (shown in Table 7). The use of LNF did not predict for regional failure (p = 0.5) or the need of PEG acutely (p = 0.82). The predictors for loco-regional failure were overall stage (p = 0.02; I, II, III versus IV), location of primary (p = 0.01; oral cavity versus other sites), chemotherapy (p = 0.04; yes versus no) and the use of LNF (p = 0.04). There was no difference in the overall survival rates.
Table 5.
Local control and survival rates with and without LNF
| Type of treatment |
Local control rate | Loco-regional control rate |
Overall Survival | ||||||
|---|---|---|---|---|---|---|---|---|---|
| 2 year |
3 year |
p value |
2 year |
3 year |
p value |
2 year |
3 year |
p* value |
|
| Total group | 94% | 94% | 86% | 86% | 80% | 71% | |||
| Without LNF | 89% | 89% | 0.06 | 79% | 79% | 0.04 | 81% | 73% | 0.98 |
| With LNF | 100% | 100% | 96% | 96% | 78% | 68% | |||
Abbreviations: CRT= concurrent chemoradiation; RT= definitive radiation therapy; PORT+CT= postoperative concurrent chemoradiation.
p < 0.05 considered statistically significant.
Figure 1.
The DFS rates for total cohort treated with IMRT
Figure 2.
The DFS rates in patients with and without LNF
Table 6.
Failure patterns with and without LNF
| Variable | Without LNF No. of. Patients (%) |
With LNF No. of. Patients (%) |
p* value |
|---|---|---|---|
| Total (n = 12) | 9 (17) | 3 (8) | 0.35 |
| Local only | 5 (9) | 0 (0) | 0.08 |
| Loco-regional | 9 (17) | 1 (3) | 0.04 |
| Regional | 2 (4) | 0 (0) | 0.5 |
| Distant | 1 (2) | 3 (8) | 0.3 |
p < 0.05 considered statistically significant.
Table 7.
Factors associated with locoregional failures (LRF) and disease free survival (DFS)
| Variable | LRF p* value | DFS p* value |
|---|---|---|
| Type of treatment (definitive CRT versus Postop CRT) | 0.2 | 0.004 |
| Type of treatment (definitive RT versus PORT) | 0.1 | 0.07 |
| Smoking (yes versus no) | 0.67 | 0.99 |
| Alcohol intake (yes versus no) | 0.69 | 0.5 |
| T stage (T1, 2 versus T3, 4) | 0.8 | 0.8 |
| N stage (N0,1 versus N2, 3) | 0.6 | 0.8 |
| Overall stage (I, II, III versus IV) | 0.02 | 0.05 |
| Location of primary (oral cavity versus other sites) | 0.01 | 0.008 |
| Chemotherapy (yes versus no) | 0.04 | 0.07 |
| Neck dissection (yes versus no) | 0.1 | 0.04 |
| PEG tube use (yes versus no) | 0.5 | 0.3 |
| Amifostine (yes versus no) | 0.1 | 0.2 |
| Third field use (yes versus no) | 0.04 | 0.2 |
| RT duration (< 46 days versus > 46 days) | 0.7 | 0.5 |
Abbreviations: LRF= loco-regional failure, DFS= disease-free survival, others as in the above tables.
p value < 0.05 considered statistically significant.
Discussion
Proper selection of IMRT technique (wide field versus split field) is controversial. The major arguments are the risk of marginal failures with the use of SF-IMRT as opposed to the unnecessary larynx irradiation17, 19, 20 with the WF-IMRT. There are no clinical studies on outcomes with and without use of LNF with IMRT. This is the first dataset looking at the clinical outcomes. In our study, we did not see any difference in the patterns of failure or the side effects profile (Tables 2–6) including PEG tube dependency in patients treated with and without LNF. Matching an anterior low neck field (LNF) with a larynx block decreases the dose to the larynx while increasing the uncertainty of dose delivery at the match line. Rosenthal et al19 described that independent jaw collimation at the junction of the abutting fields corrects the errors with either over-dose or under-dose at the match line. Duan et al9 compared the conventional single-isocenter and half-beam (SIHB) technique with dynamic field matching IMRT in 10 head and neck cancer patients. To avoid field overlap beyond the match line, the inferior border of IMRT fields were adjusted automatically by the treatment planning system to fit the dynamic MLC fields, 1.5 cm inferior to the match line by the use of collimator jaw before dose calculation. The average inhomogeneity ranged between −1.6% to + 1.6% using dynamic IMRT versus −3.7% to + 3.8% with SIHB. Dabaja et al17 studied the target volume coverage using different IMRT techniques in 13 patients with early-stage oropharynx cancers. Treatment plans were created with half-beam (HB-IMRT) and wide field (WFIMRT) IMRT techniques and comparisons included coverage to the planning target volume of the primary (PTV 66) and subclinical disease (PTV 54). The mean volume (PTV 66) receiving >110% for all patients planned with WF-IMRT was 9.3% compared with 13.7% with HB-IMRT (p = 0.09). The mean doses to all critical structures were comparable except those to larynx. The mean dose to the larynx was significantly less with HB-IMRT (18.7 Gy versus 47 Gy) compared with WF-IMRT (p = 0.001). Amdur et al16 performed a dosimetry evaluation of two IMRT techniques in a model patient with a stage T2N2b carcinoma of the tonsil with adenopathy extending inferiorly to cricoid cartilage. The mean dose to the larynx was higher with the WF-IMRT (35 Gy) compared with the split-field including the LNF (17 Gy). Amdur et al15 from University of Florida (UF) reviewed all the techniques being used to date in matching the IMRT fields to the LNF, including the method followed at UF from a head and neck cancer symposium. At UF, to minimize the concern about hot or cold spots from collimator misalignment, the position of the collimator was moved 3 mm superior to the central axis after completing one third of the treatment and then 3 mm inferior to the central axis after delivering two thirds of treatment. Lee et al14 proposed guidelines to select the IMRT technique for treating head and neck cancers in 6 patients. Dosimetric parameters were compared for each patient with split-field (SF) and extended whole-field (WF) IMRT. Target-dose coverage and doses delivered to the critical normal structures were similar between the two treatment techniques. SF-IMRT was preferred to nasopharyngeal and oropharyngeal primary sites to minimize the dose to the glottic larynx. WF-IMRT was preferred for carcinoma of the larynx, hypopharynx and unknown head-and-neck primary sites where the glottic larynx was considered part of the target. Regardless of the primary site of head and neck cancer, WF-IMRT was preferred if there was evidence of enlarged lymph nodes measuring > 3 cm in the lower neck.
Conclusions
Both the techniques are good for treating subclinical disease and do not increase the risk of regional failure “in early T & early N diseases” or decrease the risk of needing a PEG tube. Careful attention to matching the IMRT field with the conventional low neck field will minimize the problems with matching and also to avoid matching when the disease is present in the low neck > 3 cm. Longer follow up and larger patient numbers are needed to confirm these findings.
Figure 3.
The loco-regional control rates with and without LNF
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Part of the work accepted as Poster to the scientific session at the 51st Annual Meeting American Society for Therapeutic Radiology and Oncology (ASTRO), November 1–5, 2009, Chicago, IL.
“Conflicts of Interest Notification”: None of the authors have any financial interests in the paper.
References
- 1.Eisbruch A, Ship JA, Dawson LA, et al. Salivary gland sparing and improved target irradiation by conformal and intensity modulated irradiation of head and neck cancer. World J Surg. 2003;27:832–837. doi: 10.1007/s00268-003-7105-6. [DOI] [PubMed] [Google Scholar]
- 2.Chao KS, Ozyigit G, Tran BN, et al. Patterns of failure in patients receiving definitive and postoperative IMRT for head-and-neck cancer. Int J Radiat Oncol Biol Phys. 2003;55:312–321. doi: 10.1016/s0360-3016(02)03940-8. [DOI] [PubMed] [Google Scholar]
- 3.Lee N, Xia P, Fischbein NJ, et al. Intensity-modulated radiation therapy for headand- neck cancer: the UCSF experience focusing on target volume delineation. Int J Radiat Oncol Biol Phys. 2003;57:49–60. doi: 10.1016/s0360-3016(03)00405-x. [DOI] [PubMed] [Google Scholar]
- 4.Eisbruch A, Marsh LH, Dawson LA, et al. Recurrences near base of skull after IMRT for head-and-neck cancer: implications for target delineation in high neck and for parotid gland sparing. Int J Radiat Oncol Biol Phys. 2004;59:28–42. doi: 10.1016/j.ijrobp.2003.10.032. [DOI] [PubMed] [Google Scholar]
- 5.Schoenfeld GO, Amdur RJ, Morris CG, et al. Patterns of failure and toxicity after intensity-modulated radiotherapy for head and neck cancer. Int J Radiat Oncol Biol Phys. 2008;71:377–385. doi: 10.1016/j.ijrobp.2007.10.010. [DOI] [PubMed] [Google Scholar]
- 6.Cannon DM, Lee NY. Recurrence in region of spared parotid gland after definitive intensity-modulated radiotherapy for head and neck cancer. Int J Radiat Oncol Biol Phys. 2008;70:660–665. doi: 10.1016/j.ijrobp.2007.09.018. [DOI] [PubMed] [Google Scholar]
- 7.Turaka A, Li T, Sharma N, et al. Increased Recurrences Using Intensity Modulated Radiation Therapy in the Postoperative setting. Am J Clin Oncol. 2009 doi: 10.1097/COC.0b013e3181c4c3cc. Accpeted for publication: 13. [DOI] [PubMed] [Google Scholar]
- 8.Li JG, Liu C, Kim S, et al. Matching IMRT fields with static photon field in the treatment of head-and-neck cancer. Med Dosim. 2005;30:135–138. doi: 10.1016/j.meddos.2005.03.007. [DOI] [PubMed] [Google Scholar]
- 9.Duan J, Shen S, Spencer SA, et al. A dynamic supraclavicular field-matching technique for head-and-neck cancer patients treated with IMRT. Int J Radiat Oncol Biol Phys. 2004;60:959–972. doi: 10.1016/j.ijrobp.2004.06.213. [DOI] [PubMed] [Google Scholar]
- 10.Wu Q, Arnfield M, Tong S, et al. Dynamic splitting of large intensity-modulated fields. Phys Med Biol. 2000;45:1731–1740. doi: 10.1088/0031-9155/45/7/302. [DOI] [PubMed] [Google Scholar]
- 11.Hong L, Alektiar K, Chui C, et al. IMRT of large fields: whole-abdomen irradiation. Int J Radiat Oncol Biol Phys. 2002;54:278–289. doi: 10.1016/s0360-3016(02)02921-8. [DOI] [PubMed] [Google Scholar]
- 12.Li JG, Xing L, Boyer AL, et al. Matching photon and electron fields with dynamic intensity modulation. Med Phys. 1999;26:2379–2384. doi: 10.1118/1.598753. [DOI] [PubMed] [Google Scholar]
- 13.Dogan N, Leybovich LB, Sethi A, et al. Improvement of dose distributions in abutment regions of intensity modulated radiation therapy and electron fields. Med Phys. 2002;29:38–44. doi: 10.1118/1.1428757. [DOI] [PubMed] [Google Scholar]
- 14.Lee N, Mechalakos J, Puri DR, et al. Choosing an intensity-modulated radiation therapy technique in the treatment of head-and-neck cancer. Int J Radiat Oncol Biol Phys. 2007;68:1299–1309. doi: 10.1016/j.ijrobp.2006.11.019. [DOI] [PubMed] [Google Scholar]
- 15.Amdur RJ, Liu C, Li J, et al. Matching intensity-modulated radiation therapy to an anterior low neck field. Int J Radiat Oncol Biol Phys. 2007;69:S46–S48. doi: 10.1016/j.ijrobp.2007.04.091. [DOI] [PubMed] [Google Scholar]
- 16.Amdur RJ, Li JG, Liu C, et al. Unnecessary laryngeal irradiation in the IMRT era. Head Neck. 2004;26:257–263. doi: 10.1002/hed.10379. discussion 263–264. [DOI] [PubMed] [Google Scholar]
- 17.Dabaja B, Salehpour MR, Rosen I, et al. Intensity-modulated radiation therapy (IMRT) of cancers of the head and neck: comparison of split-field and whole-field techniques. Int J Radiat Oncol Biol Phys. 2005;63:1000–1005. doi: 10.1016/j.ijrobp.2005.03.069. [DOI] [PubMed] [Google Scholar]
- 18.Butler EB, Teh BS, Grant WH, 3rd, et al. Smart (simultaneous modulated accelerated radiation therapy) boost: a new accelerated fractionation schedule for the treatment of head and neck cancer with intensity modulated radiotherapy. Int J Radiat Oncol Biol Phys. 1999;45:21–32. doi: 10.1016/s0360-3016(99)00101-7. [DOI] [PubMed] [Google Scholar]
- 19.Rosenthal DI, McDonough J, Kassaee A. The effect of independent collimator misalignment on the dosimetry of abutted half-beam blocked fields for the treatment of head and neck cancer. Radiother Oncol. 1998;49:273–278. doi: 10.1016/s0167-8140(98)00128-5. [DOI] [PubMed] [Google Scholar]
- 20.Chuang C, Xia P, Akazawa C, et al. Comparison of Three Treatment Techniques Involving IMRT Fields for Head and Neck Cancers. Int J Radiat Oncol Biol Phys. 2002;54:293. [Google Scholar]