Abstract
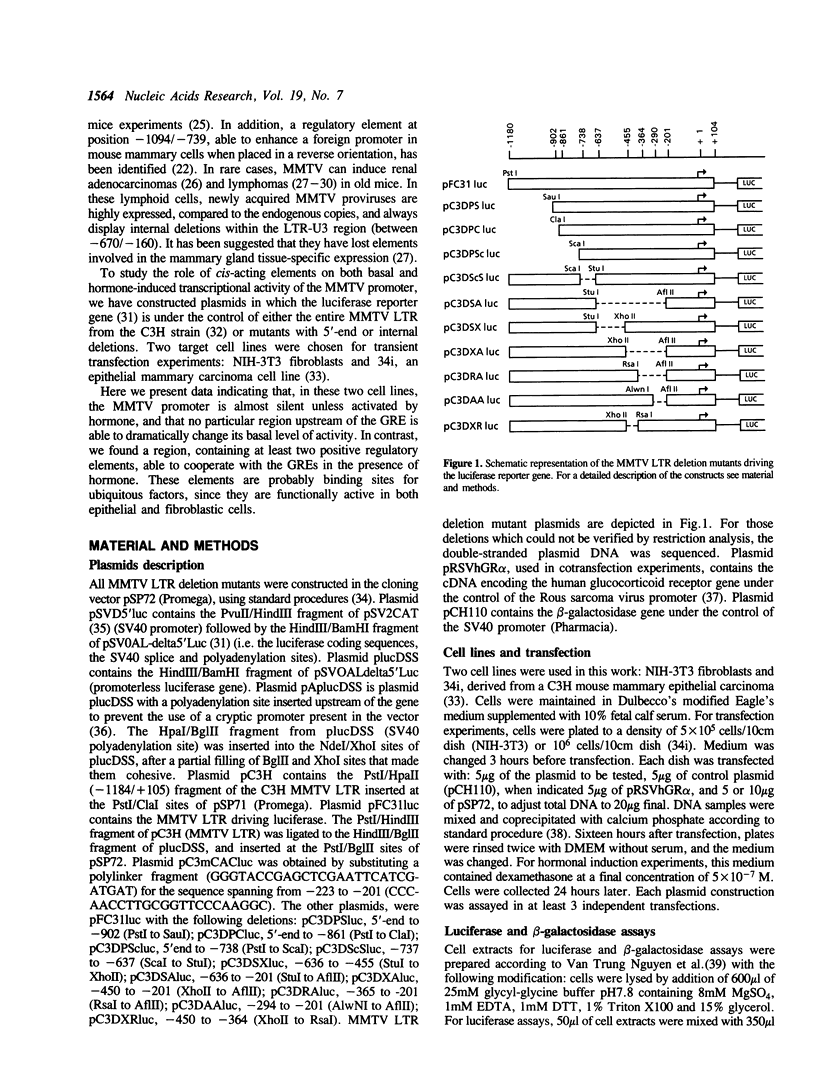
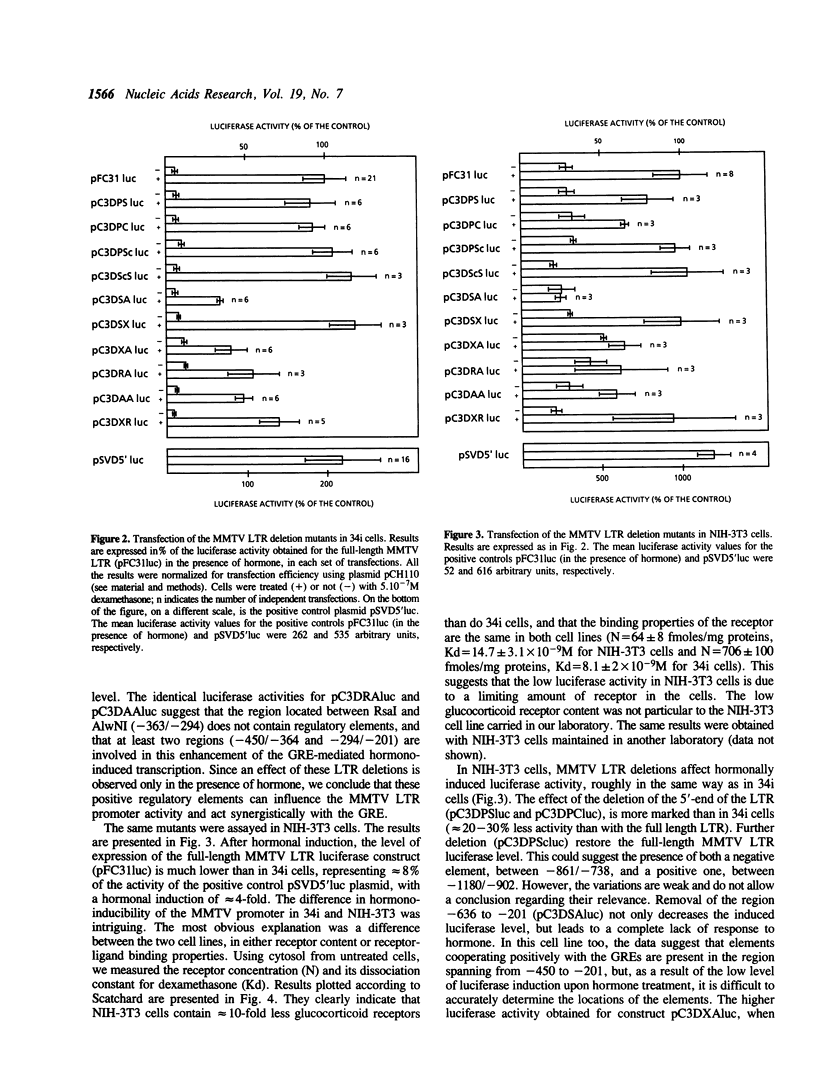
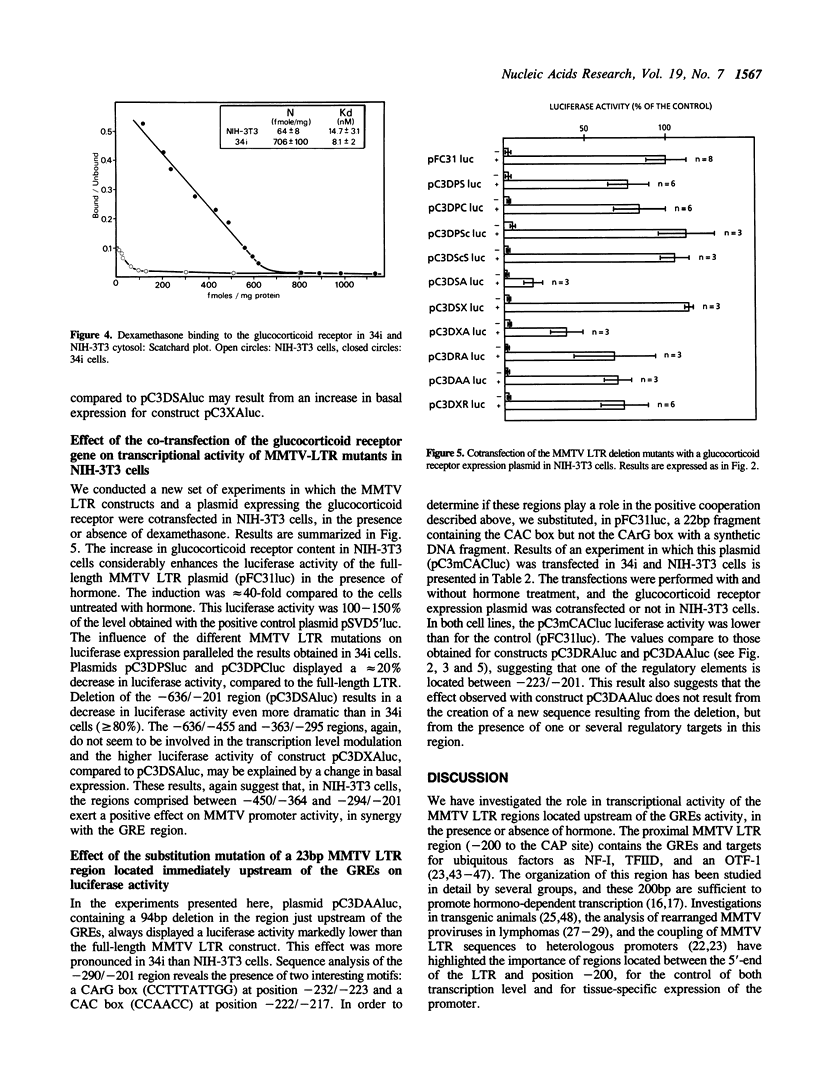
The mouse mammary tumor virus (MMTV) promoter is under the control of several types of regulatory agents. The proximal promoter within the long terminal repeat (LTR), from -200 to the CAP site and its regulation by steroid hormones have been extensively studied. However the precise role of sequences located upstream of this region remain unclear. We have constructed MMTV LTR deletion mutants coupled to the luciferase reporter gene and assayed their activities after transient transfection into transformed mammary epithelial cells (34i) and immortalized fibroblasts (NIH-3T3). In the absence of hormone, the MMTV promoter is almost silent, and deletions in the LTR have no significant effect on basal activity. In the presence of hormone, deletions spanning from the 5'-end to -455 have only slight effects on luciferase levels. In contrast, deletion of the region spanning from -450 to -201 leads to a dramatic decrease in transcription. A substantial decrease, more marked in 34i cells, is also clear when 90bp between -290 and -201 are deleted. At least one element cooperating positively with the glucocorticoid response element (GRE) is present between -223 and -201, as supported by the results of substitution mutation experiments. In 34i cell line, dexamethasone stimulates the MMTV LTR transcriptional activity to a level comparable to that of SV40. In contrast, in NIH-3T3 cells, MMTV promoter inducibility is weak. This results from a glucocorticoid receptor content 10-fold lower in NIH-3T3 cells than in 34i cells. Transfection of a glucocorticoid receptor expression plasmid allows recovery of a high inducibility of the MMTV promoter. This was true with all the MMTV LTR mutants studied here and suggests that NIH-3T3 cells possess all the factors necessary to cooperate with the steroid hormone in order to achieve a high transcriptional activity.
Full text
PDF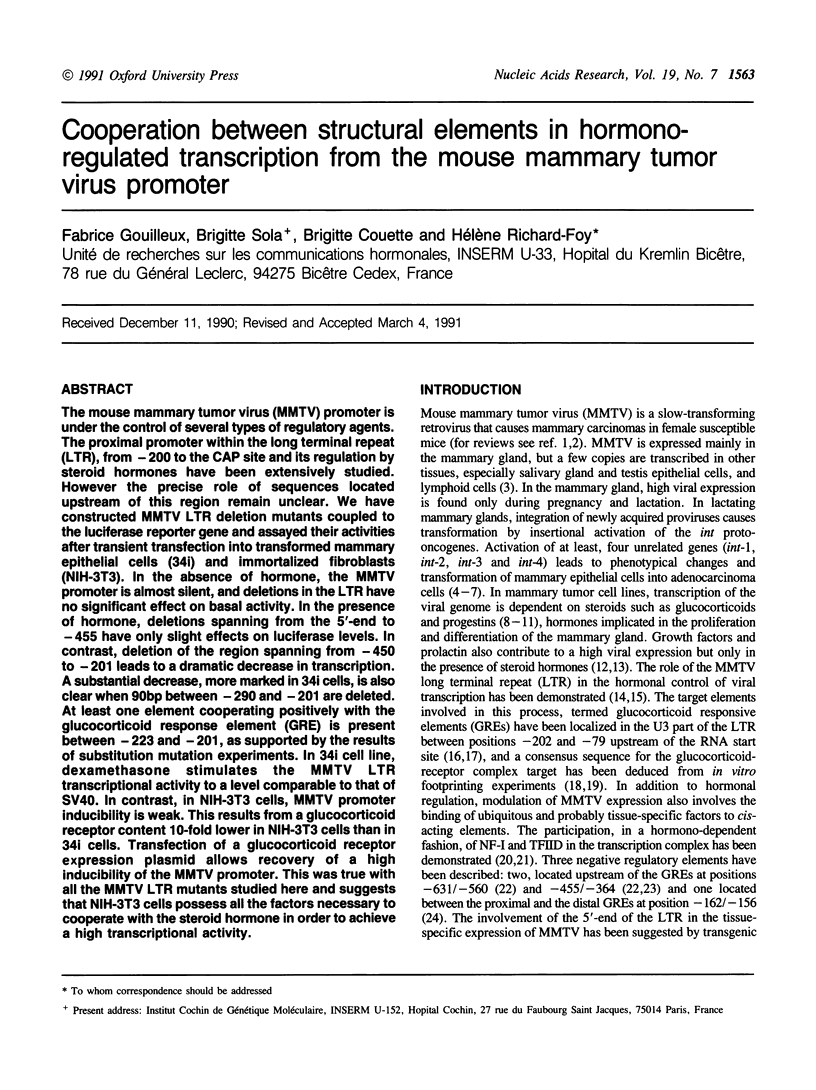



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Akerblom I. E., Slater E. P., Beato M., Baxter J. D., Mellon P. L. Negative regulation by glucocorticoids through interference with a cAMP responsive enhancer. Science. 1988 Jul 15;241(4863):350–353. doi: 10.1126/science.2838908. [DOI] [PubMed] [Google Scholar]
- Araki E., Shimada F., Shichiri M., Mori M., Ebina Y. pSV00CAT: low background CAT plasmid. Nucleic Acids Res. 1988 Feb 25;16(4):1627–1627. doi: 10.1093/nar/16.4.1627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boxer L. M., Prywes R., Roeder R. G., Kedes L. The sarcomeric actin CArG-binding factor is indistinguishable from the c-fos serum response factor. Mol Cell Biol. 1989 Feb;9(2):515–522. doi: 10.1128/mcb.9.2.515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brüggemeier U., Kalff M., Franke S., Scheidereit C., Beato M. Ubiquitous transcription factor OTF-1 mediates induction of the MMTV promoter through synergistic interaction with hormone receptors. Cell. 1991 Feb 8;64(3):565–572. doi: 10.1016/0092-8674(91)90240-y. [DOI] [PubMed] [Google Scholar]
- Buetti E., Kühnel B. Distinct sequence elements involved in the glucocorticoid regulation of the mouse mammary tumor virus promoter identified by linker scanning mutagenesis. J Mol Biol. 1986 Aug 5;190(3):379–389. doi: 10.1016/0022-2836(86)90009-4. [DOI] [PubMed] [Google Scholar]
- Cato A. C., Miksicek R., Schütz G., Arnemann J., Beato M. The hormone regulatory element of mouse mammary tumour virus mediates progesterone induction. EMBO J. 1986 Sep;5(9):2237–2240. doi: 10.1002/j.1460-2075.1986.tb04490.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cato A. C., Skroch P., Weinmann J., Butkeraitis P., Ponta H. DNA sequences outside the receptor-binding sites differently modulate the responsiveness of the mouse mammary tumour virus promoter to various steroid hormones. EMBO J. 1988 May;7(5):1403–1410. doi: 10.1002/j.1460-2075.1988.tb02957.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cato A. C., Weinmann J., Mink S., Ponta H., Henderson D., Sonnenberg A. The regulation of expression of mouse mammary tumor virus DNA by steroid hormones and growth factors. J Steroid Biochem. 1989;34(1-6):139–143. doi: 10.1016/0022-4731(89)90074-5. [DOI] [PubMed] [Google Scholar]
- Chapman A. B., Costello M. A., Lee F., Ringold G. M. Amplification and hormone-regulated expression of a mouse mammary tumor virus-Eco gpt fusion plasmid in mouse 3T6 cells. Mol Cell Biol. 1983 Aug;3(8):1421–1429. doi: 10.1128/mcb.3.8.1421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Claire M., Rafestin-Oblin M. E., Michaud A., Corvol P., Venot A., Roth-Meyer C., Boisvieux J. F., Mallet A. Statistical test of models and computerised parameter estimation for aldosterone binding in rat kidney. FEBS Lett. 1978 Apr 15;88(2):295–299. doi: 10.1016/0014-5793(78)80197-5. [DOI] [PubMed] [Google Scholar]
- Cordingley M. G., Hager G. L. Binding of multiple factors to the MMTV promoter in crude and fractionated nuclear extracts. Nucleic Acids Res. 1988 Jan 25;16(2):609–628. doi: 10.1093/nar/16.2.609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cordingley M. G., Riegel A. T., Hager G. L. Steroid-dependent interaction of transcription factors with the inducible promoter of mouse mammary tumor virus in vivo. Cell. 1987 Jan 30;48(2):261–270. doi: 10.1016/0092-8674(87)90429-6. [DOI] [PubMed] [Google Scholar]
- Diamond M. I., Miner J. N., Yoshinaga S. K., Yamamoto K. R. Transcription factor interactions: selectors of positive or negative regulation from a single DNA element. Science. 1990 Sep 14;249(4974):1266–1272. doi: 10.1126/science.2119054. [DOI] [PubMed] [Google Scholar]
- Dickson C., Haslam S., Nandi S. Conditions for optimal MTV synthesis in vitro and the effect of steroid hormones on virus production. Virology. 1974 Nov;62(1):242–252. doi: 10.1016/0042-6822(74)90319-5. [DOI] [PubMed] [Google Scholar]
- Dickson C., Smith R., Brookes S., Peters G. Tumorigenesis by mouse mammary tumor virus: proviral activation of a cellular gene in the common integration region int-2. Cell. 1984 Jun;37(2):529–536. doi: 10.1016/0092-8674(84)90383-0. [DOI] [PubMed] [Google Scholar]
- Donehower L. A., Huang A. L., Hager G. L. Regulatory and coding potential of the mouse mammary tumor virus long terminal redundancy. J Virol. 1981 Jan;37(1):226–238. doi: 10.1128/jvi.37.1.226-238.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fasel N., Pearson K., Buetti E., Diggelmann H. The region of mouse mammary tumor virus DNA containing the long terminal repeat includes a long coding sequence and signals for hormonally regulated transcription. EMBO J. 1982;1(1):3–7. doi: 10.1002/j.1460-2075.1982.tb01115.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallahan D., Kozak C., Callahan R. A new common integration region (int-3) for mouse mammary tumor virus on mouse chromosome 17. J Virol. 1987 Jan;61(1):218–220. doi: 10.1128/jvi.61.1.218-220.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giguère V., Hollenberg S. M., Rosenfeld M. G., Evans R. M. Functional domains of the human glucocorticoid receptor. Cell. 1986 Aug 29;46(5):645–652. doi: 10.1016/0092-8674(86)90339-9. [DOI] [PubMed] [Google Scholar]
- Gorman C. M., Moffat L. F., Howard B. H. Recombinant genomes which express chloramphenicol acetyltransferase in mammalian cells. Mol Cell Biol. 1982 Sep;2(9):1044–1051. doi: 10.1128/mcb.2.9.1044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graham F. L., van der Eb A. J. A new technique for the assay of infectivity of human adenovirus 5 DNA. Virology. 1973 Apr;52(2):456–467. doi: 10.1016/0042-6822(73)90341-3. [DOI] [PubMed] [Google Scholar]
- Henrard D., Ross S. R. Endogenous mouse mammary tumor virus is expressed in several organs in addition to the lactating mammary gland. J Virol. 1988 Aug;62(8):3046–3049. doi: 10.1128/jvi.62.8.3046-3049.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsu C. L., Fabritius C., Dudley J. Mouse mammary tumor virus proviruses in T-cell lymphomas lack a negative regulatory element in the long terminal repeat. J Virol. 1988 Dec;62(12):4644–4652. doi: 10.1128/jvi.62.12.4644-4652.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang A. L., Ostrowski M. C., Berard D., Hager G. L. Glucocorticoid regulation of the Ha-MuSV p21 gene conferred by sequences from mouse mammary tumor virus. Cell. 1981 Dec;27(2 Pt 1):245–255. doi: 10.1016/0092-8674(81)90408-6. [DOI] [PubMed] [Google Scholar]
- Hynes N., van Ooyen A. J., Kennedy N., Herrlich P., Ponta H., Groner B. Subfragments of the large terminal repeat cause glucocorticoid-responsive expression of mouse mammary tumor virus and of an adjacent gene. Proc Natl Acad Sci U S A. 1983 Jun;80(12):3637–3641. doi: 10.1073/pnas.80.12.3637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kühnel B., Buetti E., Diggelmann H. Functional analysis of the glucocorticoid regulatory elements present in the mouse mammary tumor virus long terminal repeat. A synthetic distal binding site can replace the proximal binding domain. J Mol Biol. 1986 Aug 5;190(3):367–378. doi: 10.1016/0022-2836(86)90008-2. [DOI] [PubMed] [Google Scholar]
- Langer S. J., Ostrowski M. C. Negative regulation of transcription in vitro by a glucocorticoid response element is mediated by a trans-acting factor. Mol Cell Biol. 1988 Sep;8(9):3872–3881. doi: 10.1128/mcb.8.9.3872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee F., Mulligan R., Berg P., Ringold G. Glucocorticoids regulate expression of dihydrofolate reductase cDNA in mouse mammary tumour virus chimaeric plasmids. Nature. 1981 Nov 19;294(5838):228–232. doi: 10.1038/294228a0. [DOI] [PubMed] [Google Scholar]
- Lee W. T., Prakash O., Klein D., Sarkar N. H. Structural alterations in the long terminal repeat of an acquired mouse mammary tumor virus provirus in a T-cell leukemia of DBA/2 mice. Virology. 1987 Jul;159(1):39–48. doi: 10.1016/0042-6822(87)90345-x. [DOI] [PubMed] [Google Scholar]
- Majors J., Varmus H. E. A small region of the mouse mammary tumor virus long terminal repeat confers glucocorticoid hormone regulation on a linked heterologous gene. Proc Natl Acad Sci U S A. 1983 Oct;80(19):5866–5870. doi: 10.1073/pnas.80.19.5866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michalides R., Wagenaar E. Site-specific rearrangements in the long terminal repeat of extra mouse mammary tumor proviruses in murine T-cell leukemias. Virology. 1986 Oct 15;154(1):76–84. doi: 10.1016/0042-6822(86)90431-9. [DOI] [PubMed] [Google Scholar]
- Mink S., Ponta H., Cato A. C. The long terminal repeat region of the mouse mammary tumour virus contains multiple regulatory elements. Nucleic Acids Res. 1990 Apr 25;18(8):2017–2024. doi: 10.1093/nar/18.8.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morley K. L., Toohey M. G., Peterson D. O. Transcriptional repression of a hormone-responsive promoter. Nucleic Acids Res. 1987 Sep 11;15(17):6973–6989. doi: 10.1093/nar/15.17.6973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muller W. J., Lee F. S., Dickson C., Peters G., Pattengale P., Leder P. The int-2 gene product acts as an epithelial growth factor in transgenic mice. EMBO J. 1990 Mar;9(3):907–913. doi: 10.1002/j.1460-2075.1990.tb08188.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muñoz B., Bolander F. F., Jr Prolactin regulation of mouse mammary tumor virus (MMTV) expression in normal mouse mammary epithelium. Mol Cell Endocrinol. 1989 Mar;62(1):23–29. doi: 10.1016/0303-7207(89)90109-3. [DOI] [PubMed] [Google Scholar]
- Nguyen V. T., Morange M., Bensaude O. Firefly luciferase luminescence assays using scintillation counters for quantitation in transfected mammalian cells. Anal Biochem. 1988 Jun;171(2):404–408. doi: 10.1016/0003-2697(88)90505-2. [DOI] [PubMed] [Google Scholar]
- Nowock J., Borgmeyer U., Püschel A. W., Rupp R. A., Sippel A. E. The TGGCA protein binds to the MMTV-LTR, the adenovirus origin of replication, and the BK virus enhancer. Nucleic Acids Res. 1985 Mar 25;13(6):2045–2061. doi: 10.1093/nar/13.6.2045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nusse R., Varmus H. E. Many tumors induced by the mouse mammary tumor virus contain a provirus integrated in the same region of the host genome. Cell. 1982 Nov;31(1):99–109. doi: 10.1016/0092-8674(82)90409-3. [DOI] [PubMed] [Google Scholar]
- Parks W. P., Hubbell E. S., Goldberg R. J., O'Neill F. J., Scolnick E. M. High frequency variation in mammary tumor virus expression in cell culture. Cell. 1976 May;8(1):87–93. doi: 10.1016/0092-8674(76)90189-6. [DOI] [PubMed] [Google Scholar]
- Parks W. P., Scolnick E. M., Kozikowski E. H. Dexamethasone stimulation of murine mammary tumor virus expression: a tissue culture source of virus. Science. 1974 Apr 12;184(4133):158–160. doi: 10.1126/science.184.4133.158. [DOI] [PubMed] [Google Scholar]
- Payvar F., DeFranco D., Firestone G. L., Edgar B., Wrange O., Okret S., Gustafsson J. A., Yamamoto K. R. Sequence-specific binding of glucocorticoid receptor to MTV DNA at sites within and upstream of the transcribed region. Cell. 1983 Dec;35(2 Pt 1):381–392. doi: 10.1016/0092-8674(83)90171-x. [DOI] [PubMed] [Google Scholar]
- Ponta H., Günzburg W. H., Salmons B., Groner B., Herrlich P. Mouse mammary tumour virus: a proviral gene contributes to the understanding of eukaryotic gene expression and mammary tumorigenesis. J Gen Virol. 1985 May;66(Pt 5):931–943. doi: 10.1099/0022-1317-66-5-931. [DOI] [PubMed] [Google Scholar]
- Rafestin-Oblin M. E., Couette B., Radanyi C., Lombes M., Baulieu E. E. Mineralocorticosteroid receptor of the chick intestine. Oligomeric structure and transformation. J Biol Chem. 1989 Jun 5;264(16):9304–9309. [PubMed] [Google Scholar]
- Ringold G. M., Yamamoto K. R., Bishop J. M., Varmus H. E. Glucocorticoid-stimulated accumulation of mouse mammary tumor virus RNA: increased rate of synthesis of viral RNA. Proc Natl Acad Sci U S A. 1977 Jul;74(7):2879–2883. doi: 10.1073/pnas.74.7.2879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salmons B., Günzburg W. H. Current perspectives in the biology of mouse mammary tumour virus. Virus Res. 1987 Aug;8(2):81–102. doi: 10.1016/0168-1702(87)90022-0. [DOI] [PubMed] [Google Scholar]
- Schüle R., Muller M., Otsuka-Murakami H., Renkawitz R. Cooperativity of the glucocorticoid receptor and the CACCC-box binding factor. Nature. 1988 Mar 3;332(6159):87–90. doi: 10.1038/332087a0. [DOI] [PubMed] [Google Scholar]
- Stewart T. A., Hollingshead P. G., Pitts S. L. Multiple regulatory domains in the mouse mammary tumor virus long terminal repeat revealed by analysis of fusion genes in transgenic mice. Mol Cell Biol. 1988 Jan;8(1):473–479. doi: 10.1128/mcb.8.1.473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strähle U., Schmid W., Schütz G. Synergistic action of the glucocorticoid receptor with transcription factors. EMBO J. 1988 Nov;7(11):3389–3395. doi: 10.1002/j.1460-2075.1988.tb03212.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toohey M. G., Lee J. W., Huang M., Peterson D. O. Functional elements of the steroid hormone-responsive promoter of mouse mammary tumor virus. J Virol. 1990 Sep;64(9):4477–4488. doi: 10.1128/jvi.64.9.4477-4488.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wellinger R. J., Garcia M., Vessaz A., Diggelmann H. Exogenous mouse mammary tumor virus proviral DNA isolated from a kidney adenocarcinoma cell line contains alterations in the U3 region of the long terminal repeat. J Virol. 1986 Oct;60(1):1–11. doi: 10.1128/jvi.60.1.1-11.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yanagawa S., Murakami A., Tanaka H. Extra mouse mammary tumor proviruses in DBA/2 mouse lymphomas acquire a selective advantage in lymphocytes by alteration in the U3 region of the long terminal repeat. J Virol. 1990 Jun;64(6):2474–2483. doi: 10.1128/jvi.64.6.2474-2483.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Wet J. R., Wood K. V., DeLuca M., Helinski D. R., Subramani S. Firefly luciferase gene: structure and expression in mammalian cells. Mol Cell Biol. 1987 Feb;7(2):725–737. doi: 10.1128/mcb.7.2.725. [DOI] [PMC free article] [PubMed] [Google Scholar]



