Abstract
Deoxyguanosine kinase (dGK) deficiency, a rare severe cause of mitochondrial DNA (mtDNA) depletion, has two forms of presentation: hepatocerebral syndrome and isolated hepatic disease. The authors report three cases with neonatal liver failure due to dGK deficiency. Consanguinity was present in all patients. One patient had a brother who died with a probable diagnosis of neonatal haemochromatosis. All patients had progressive cholestatic liver failure, hypoglycaemia, hyperlactacidaemia, elevated ferritin levels and nystagmus, since first day of life. Liver tissue study revealed: cholestasis, iron deposits, microvesicular steatosis and fibrosis/cirrhosis. Only one patient was submitted to liver transplantation. The other two died, at 2 and 5 months of age. mtDNA quantification and DGUOK gene study should be considered in infants/neonates with acute liver failure and systematically performed in patients with hepatocerebral presentation. Differential diagnosis with neonatal haemochromatosis is needed. Liver transplantation might be a therapeutic option. Early diagnosis is important for genetic counselling.
Background
Clinical manifestations of mitochondrial depletion syndrome in childhood include severe multi-systemic disease, affecting mainly liver, muscle, brain and kidneys.
Tissue mitochondrial DNA (mtDNA) depletion leads to inadequate supply of mtDNA gene products1 2 with subsequent functional deficiency of mitochondrial respiratory chain.
Nuclear DNA mutations affecting mtDNA maintenance may cause a severe decrease in mtDNA copy number (depletion).3 So far, mutations in eight nuclear genes causing mtDNA depletion (TP, TK2, SUCLA1, 2, POLG1, 2, DGUOK, MPV17, RRM2B) have been identified.4
Mutations in DGUOK gene, although rare (39 mutations have been identified so far), have been suggested to be the most common cause of mitochondrial hepatoencephalopathy.3 4 Liver involvement with cholestasis, hyperlactacidaemia, hypoglycaemia and increased ferritin levels, may lead to fatal hepatic failure, usually in neonatal period.5 Clinical features may also include nystagmus, hypotonia and encephalopathy.4 5
Diagnosis of deoxyguanosine kinase (dGK) deficiency is achieved by identification of tissue specific mtDNA depletion and DGUOK gene mutation.4
Currently, no satisfactory therapy is available for hepatoencephalopathy due to dGK deficiency. Liver transplantation may be indicated in patients with isolated liver involvement, since abnormal neurological features have been associated to poor survival.3
We report three cases, from unrelated families, with chronic severe liver failure due to depletion and DGUOK gene mutations, diagnosed in our hospital between 2008 and 2010.
Case presentation
Patient 1 (P1): male, first child of young Caucasian consanguineous parents (first degree cousins), born at term in Spain (May 2008) with low birth weight (2065 g). Family history was relevant: a first-degree cousin had been submitted to liver transplantation at 3 years old due to hepatitis A.
He was hospitalised in neonatal intensive care unit since first day of life, with persistent hypoglycaemia. Glucose infusion (≤13 mg/kg/min) and hydrocortisone (day 34 to 66) were required. During hospitalisation, failure to thrive (resistant to caloric supplementation), postnatal cytomegalovirus infection (confirmed by serology and protein chain reaction), hepatomegaly, progressive cholestasis, liver failure (4 weeks of age), neurological rotatory nystagmus (7 weeks of age), choreiform movements and arms and legs hypertonia (3 months of age) were noticed (table 1). Immunoglobulin, creatine kinase, amylase, α1-AT, ceruloplasmin and uric acid levels as well as hormonal study and karyotype were normal. Lactate level was high (4.5 mmol/l), with elevated lactate/pyruvate ratio (30). Plasma tyrosine and methionine levels were reported to be ‘increased’ (exact values are not available), with absence of succinylacetone in urine. Abdominal echography, liver scintigraphy, cerebral ultrasound scan, EEG (sleep and wakefulness), auditory evoked potentials and brain MRI were normal. No abnormalities were found in heart and eye examination. He was discharged at three and half months of age, by parents’ request. Two weeks later, he was admitted at our hospital due to hypoglycaemia, cholestasis and coagulopathy refractory to intravenous vitamin K. From investigation we highlight normal hormonal study during hypoglycaemia, normal plasma tyrosine and methionine levels, high alanine plasma level (485 µM), high blood lactate and high lactate/pyruvate ratio and magnetic resonance spectroscopy (MRS) with glutamine and lactate peaks (tables 2 and 3). End stage liver disease developed, with encephalopathy of level three. He died at 5 months of age, due to multi-organ failure. Liver biopsy disclosed intrahepatic cholestasis, with necrosis and septal steatosis (table 3). Enzymatic MRC function study showed multiple deficiencies in liver and complex V decreased activity in muscle. Genetic analysis revealed mtDNA depletion in liver and muscle and a sequence variation (c.677A>G, p.H226R, exon 5) in DGUOK gene (table 3).
Table 1.
Clinical presentation of patients with DGUOK mutation
| P1 | P2 | P3 | Total | |
| Consanguinity | + | + | + | 3/3 |
| Sex | M | F | M | 2M/1F |
| Age at onset (days) | 1 | 2 | 3 | (1–3) |
| Age death (months) | 5.5* | 2* | (2–5.5) | |
| Liver symptoms | ||||
| Hepatomegaly | + | + | − | 2/3 |
| Jaundice | + | + | + | 3/3 |
| Hypoglycaemia | + | + | + | 3/3 |
| Ascites | + | − | − | 1/3 |
| Neurologic symptoms | ||||
| Hypotonia | − | + | + | 2/3 |
| Nystagmus | + | + | + | 3/3 |
| Other symptoms | ||||
| Feeding difficulties | + | + | + | 3/3 |
| Failure to thrive | + | + | + | 3/3 |
| Renal tubulopathy | − | + | − | 1/3 |
F, female; M, male; P1, patient 1; P2, patient 2; P3, patient 3.
+, present; −, absent.
*Death.
Table 2.
Blood biochemical investigation of patients with DGUOK mutation
| Parameter | Normal values | P1 | P2 | P3 | Total of abnormal values |
| Lactate (mmol/l) | 0.7–2.1 | 7.6 | 10.9 | 4.0 | 3 |
| L/P ratio | 10–15 | 32 | 40 | 28 | 3 |
| Glycaemia (mmol/l) | 4.2-6.1 | 2.1 | 3.3 | 2.0 | 3 |
| AST (UI/l) | <50 | 151 | 95 | 359 | 3 |
| ALT (UI/l) | <45 | 655 | 198 | 237 | 3 |
| γGT(U/l) | 12–58 | 199 | 43 | 480 | 2 |
| Bilirubin (µmol/l) | |||||
| Total | <22 | 140 | 214 | 192 | 3 |
| Conjugated | <5 | 61 | 126 | 22 | 3 |
| Ammonaemia (µmol/l) | 21–50 | 27 | 65 | 149 | 2 |
| Albumin (g/l) | 34–42 | 27 | 25 | 18 | 3 |
| PTT (%) | 24–50 | >60 | 90 | 115 | 3 |
| INR | >6.0 | 3.3 | 3.0 | 3 | |
| Factor V/VII (%) | 48–132/39–143 | 56/12 | 32/7 | 13/10 | 3 |
| Ferritin (ng/ml) | 9–120 | 789 | 1931 | 1500 | 3 |
| AFP (U/ml) | <30 | 88970 | 35405 | 302300 | 3 |
| Tyrosine (µmol/l) | 28–96 | 68 | 538 | 543 | 2 |
| Creatine kinase (U/l) | 60–305 | 218 | 42 | 166 | 1 |
AFP, α-fetoprotein; ALT, alanine aminotransferase; AST, aspartate aminotransferase; γGT, γ glutaryl transferase; INR, international normalised ratio; L/P, lactate/pyruvate ratio; P1, patient 1; P2, patient 2; P3, patient 3; PTT, prothrombin time test.
Abnormal values are in bold
Table 3.
Results of brain imaging, liver morphology, mitochondrial respiratory chain functional study, mitochondrial DNA quantification and DGUOK gene studies
| P1 | P2 | P3 | Total | |
| Neuroimaging | ||||
| Age (months) | 4 | 1 | 0.75 | (0.75–4) |
| Brain MRI | N | N | N | 3/3 |
| Brain MRS | Lactate | Lactate | Lactate | 3/3 |
| Liver morphology | ||||
| Age (months) | 6 | 2 | 4.5 | (2–6) |
| Specimen | NE | NE | OLT | − |
| Morphology: | − | |||
| Microvesicular seatosis | + | ++ | − | 2/3 |
| Hepatocyte swelling | + | + | + | 3/3 |
| Syncytial cells | − | + | − | 1/3 |
| Cholestasis | +++ | +++ | +++ | 3/3 |
| Cirrhosis/Fibrosis | ++ | − | ++ | 2/3 |
| Iron deposits | + | + | − | 2/3 |
| Mitochondrial DNA quantification (% of control) | ||||
| Liver | 3.6 | 8.6 | − | 2/3 |
| Muscle | 42 | 60.9 | − | 2/3 |
| Lymphocytes | − | 25.9 | 7.1 | 2/3 |
| DGUOK gene studies | ||||
| c.677A>G (homozygous) | c.677A>G (homozygous) | c.749T>C (homozygous) | 3/3 | |
MRS, magnetic resonance spectroscopy; N, normal; NE, necropsy; OLT, orthotopic liver transplantation; P1, patient 1; P2, patient 2; P3, patient 3.
−, absent; +, mild; ++, moderate; +++, severe.
Abnormal values are in bold.
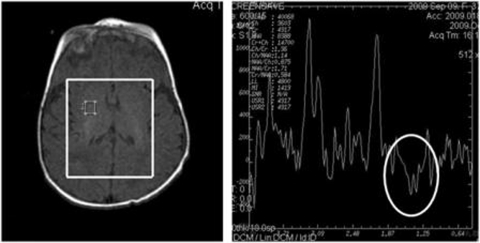
Patient 2 (P2): female, second child of consanguineous parents (first degree cousins), born in September 2009, after a normal pregnancy. Feeding difficulties, groaning, hypoglycaemia, hypotonia and jaundice were noticed since first week of life (table 1) and Escherichia coli sepsis was diagnosed. At 3 weeks of age, cholestatic liver dysfunction with hypoglycaemia and hyperlactacidaemia were disclosed. Ferritin level of 765 mg/dl (normal range 9–120 mg/dl), tyrosine plasma concentration of 538 µmol/l (normal range 28–96 µmol/l) and trace amounts of succinylacetone in urine were found. She was treated with desferroxamine and immunoglobulin, considering neonatal haemochromatosis. Her clinical condition deteriorated at 5 weeks of age, when she was referred to our centre for liver transplantation. She was then submitted to exchange transfusion (ferritin plasma levels ≤1931 mg/dl). Liver MRI showed iron overload (60 µmol/g; normal <36 µmol/g). Cholestatic liver failure progressed and neurologic dysfunction with rotary nystagmus emerged. Brain MRS revealed glutamine and lactate peaks (figure 1). No iron deposits were found in lip and salivary gland biopsies. She died at 2 months of age. Liver morphology showed microvesicular steatosis and cholestasis (table 3) and biochemical study revealed multiple MRC deficiencies. Genetic analysis revealed mtDNA depletion and a sequence variation (c.677A>G, p.H226R, exon 5) in DGUOK gene (table 3).
Figure 1.
Magnetic resonance spectroscopy of patient 2 showing a lactate peak (white circle).
Patient 3 (P3): male, third child of consanguineous parents (first degree cousins), born at term, in June 2010, with low birth weight (2272 g). A brother died during neonatal period with the diagnosis of haemochromatosis.
Since first day of life, he presented with generalised hypotonia, feeding difficulties with poor suction reflexes and hypoglycaemia (table 1). Wide anterior fontanel, hypotelorism and macroglossia were noticed. At third day of life, acute liver failure emerged. Neonatal haemochromatosis was considered and he was transferred to our hospital for liver transplantation. He had hyperlactacidaemia, high lactate/pyruvate ratio (28), high ferritin level (1500 ng/ml), elevated α-fetoprotein level (302300 U/ml) and elevated tyrosine plasma concentration (543 µmol/l) with absence of succinylacetone in urine (table 2).
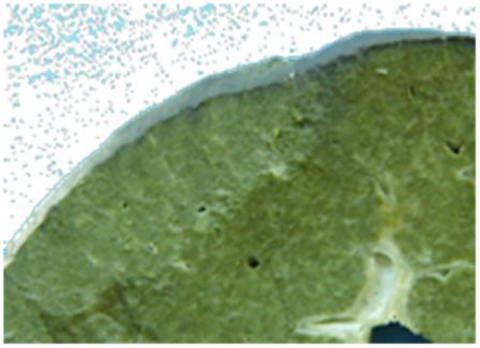
After a period of stabilisation, cholestatic liver dysfunction with frequent hypoglycaemia episodes and neurologic dysfunction with rotary nystagmus came out at 3 months of age. mtDNA depletion was found in blood and a sequence variation (c.749T>C, exon 6, p.L250S) in DGUOK gene was identified (table 3). He was submitted to liver transplantation at 5 months of age. Liver morphology showed cholestasis and cirrhosis (figure 2). One year later, graft function is normal (with minimal immunossuppression – tacrolimus and prednisolone). Nystagmus persisted and he has visual impairment, severe psychomotor retardation and failure to thrive (weight <5th percentile).
Figure 2.
Liver morphology of patient 3, showing cholestasis and cirrhosis.
Discussion
The association between liver failure and MRC deficiency in infants was first described in 1995.6 Later on, mtDNA depletion was considered the most relevant cause of energy impairment in liver. DGUOK gene mutations are the most frequent cause of liver mtDNA depletion in childhood,3 responsible for an important subset of acute liver failure/hepatoencephalopathy in neonatal period and infancy.
The three patients under study had disease onset in the first week of life, which is in accordance to literature.5
Patients with dGK deficiency typically present feeding difficulties, failure to thrive, and cholestatic neonatal liver failure, with or without neurological symptoms.7 This clinical presentation, with liver disease as the main clinical feature, occurred in the cases reported: all had early cholestasis, significant cytolysis, progressive acute to chronic liver failure and iron overload.
Differential diagnosis between dGK deficiency and neonatal haemochromatosis must be considered, since ferritin levels are frequently very high, with increased iron deposits in liver in both disorders.4 8 In our series, P2 had markedly elevated ferritin levels which leaded to liver MRI, mouth mucosal biopsies and iron chelation treatment (based on possible haemochromatosis diagnosis). In P3, haemochromatosis was also a strong hypothesis due to liver failure associated with high ferritin levels and history of probable neonatal haemochromatosis in a sibling.
Levels of AFP were markedly increased in all patients under study, as previously described in dGK deficiency.1 This is a non-specific finding, also observed in familiar intrahepatic cholestasis and other metabolic diseases, such as tyrosinemia type 1.
Increased tyrosine plasma levels were observed in all cases (P1–P3). High tyrosinemia level was detected in blood card in one of the two newborns (P2) submitted to extended neonatal screening. It has been suggested that dGK deficiency should be considered if elevated tyrosine levels are found in extended newborn screening.4 Tyrosinemia type 1 should also be ruled out by the analysis of succinilacetone in urine, as it was done in the reported cases. In P2 trace amounts were detected, probably as a secondary event.
Neurologic symptoms (hypotonia and/or nystagmus) were present in all patients. In dGK deficient patients these symptoms are usually associated to a poor prognosis.4 Neurologic symptoms in the context of multi-system disease may occur due to neuronal energy failure. The underlying mechanism is not always clear, since hypoglycaemia and encephalopathy are usually observed secondary to liver failure in patients with acute liver insufficiency.9
The majority of dGK deficient patients die in the first year of life,4 as occurred in our series. Patients 1 and 2 passed away before 6 months of age. Patient 3 is alive, 1 year after liver transplantation. This treatment option is known to be curative if the disease is restricted to the liver,9 which rarely happens. The presence of significant central nervous system involvement, including hypotonia, nystagmus or psychomotor retardation should preclude liver transplantation.3 4 10 It does not appear to prevent the progression of neurological disease or other extrahepatic manifestations, which may cause patients’ death.3 9 10 In fact, despite successful liver transplantation (until now), P3 shows severe neurological and ophthalmological deterioration.
In patients with dGK deficiency, clinical involvement is typically limited to liver and brain.7 However, other organs such as kidney may also be affected, as occurred in P2, who presented proximal tubulopathy. According to our knowledge, the occurrence of renal disease does not predict long-term survival and should not preclude liver transplantation as a therapeutical option.3
Morphological analysis of the liver, which may be difficult or impossible in the presence of clotting abnormalities secondary to hepatic failure, may be important for differential diagnosis. In the reported cases, liver histological analysis was done after death or liver transplantation. There are no specific morphological hepatic features of dGK deficiency, but irregular microvesicular and macrovesicular steatosis and cytoplasmic swelling appear to be characteristic.7 Hepatocyte swelling and cholestasis was disclosed in all patients described above, iron deposits, cirrhosis/fibrosis and microvesicular steatosis were noticed in two patients (P1 and P2) (table 3).
It is predictable that the sibling of P3, who died with a tentative diagnosis of neonatal haemochromatosis, had the same DGUOK gene mutation identified on his brother. The positive family history of severe liver disease in childhood (P2 and P3) and the high consanguinity rate in our series (P1, P2 and P3) point towards autosomal recessive inheritance, according to the more frequent inheritance mode of dGK deficiency described.2
Learning points.
-
▶
mtDNA depletion and subsequent DGUOK gene investigation should be considered in infants/neonates with acute liver failure (even in the absence of neuromuscular involvement) and systematically performed in patients with hepatocerebral presentation.
-
▶
Differential diagnosis with neonatal haemochromatosis is required, since ferritin levels are frequently extremely elevated.
-
▶
Liver transplantation might be a therapeutic option for these patients.
-
▶
Early diagnosis, which requires the study of the entire gene, is of utmost importance for genetic counselling.
Acknowledgments
The authors are grateful to Dr Filipe Silva, PhD (IBILI, Faculty of Medicine, University of Coimbra) for the critical review of the present paper. The authors also thank to Maria João Santos, Filipe Silva, Carla Pereira, Ana Isabel Padilha, João Pratas, Cândida Mendes and Marta Simões for the contribution to the laboratory assays.
Footnotes
Competing interests None.
Patient consent Obtained.
References
- 1.Freisinger P, Fütterer N, Lankes E, et al. Hepatocerebral mitochondrial DNA depletion syndrome caused by deoxyguanosine kinase (DGUOK) mutations. Arch Neurol 2006;63:1129–34 [DOI] [PubMed] [Google Scholar]
- 2.Slama A, Giurgea I, Debrey D, et al. Deoxyguanosine kinase mutations and combined deficiencies of the mitochondrial respiratory chain in patients with hepatic involvement. Mol Genet Metab 2005;86:462–5 [DOI] [PubMed] [Google Scholar]
- 3.Dimmock DP, Dunn JK, Feigenbaum A, et al. Abnormal neurological features predict poor survival and should preclude liver transplantation in patients with deoxyguanosine kinase deficiency. Liver Transpl 2008;14:1480–5 [DOI] [PubMed] [Google Scholar]
- 4.Dimmock DP, Zhang Q, Dionisi-Vici C, et al. Clinical and molecular features of mitochondrial DNA depletion due to mutations in deoxyguanosine kinase. Hum Mutat 2008;29:330–1 [DOI] [PubMed] [Google Scholar]
- 5.Spinazzola A, Invernizzi F, Carrara F, et al. Clinical and molecular features of mitochondrial DNA depletion syndromes. J Inherit Metab Dis 2009;32:143–58 [DOI] [PubMed] [Google Scholar]
- 6.Goncalves I, Hermans D, Chretien D, et al. Mitochondrial respiratory chain defect: a new etiology for neonatal cholestasis and early liver insufficiency. J Hepatol 1995;23:290–4 [PubMed] [Google Scholar]
- 7.Labarthe F, Dobbelaere D, Devisme L, et al. Clinical, biochemical and morphological features of hepatocerebral syndrome with mitochondrial DNA depletion due to deoxyguanosine kinase deficiency. J Hepatol 2005;43:333–41 [DOI] [PubMed] [Google Scholar]
- 8.Hanchard NA, Shchelochkov OA, Roy A, et al. Deoxyguanosine kinase deficiency presenting as neonatal hemochromatosis. Mol Genet Metab 2011;103:262–7 [DOI] [PubMed] [Google Scholar]
- 9.Sokal EM, Sokol R, Cormier V, et al. Liver transplantation in mitochondrial respiratory chain disorders. Eur J Pediatr 1999;158 Suppl 2:S81–4 [DOI] [PubMed] [Google Scholar]
- 10.Dubern B, Broue P, Dubuisson C, et al. Orthotopic liver transplantation for mitochondrial respiratory chain disorders: a study of 5 children. Transplantation 2001;71:633–7 [DOI] [PubMed] [Google Scholar]