Abstract
Phosgene is a highly toxic gas to which accidental exposure may occur in occupational workers. This case report describes the clinical presentation and management of accidental phosgene poisoning happened after the leakage of phosgene gas from nearby pipeline. The need to suspect phosgene gas exposure and observe such patients is crucial for life saving, especially in view of the delay in clinical deterioration observed in some patients who subsequently develop adult respiratory distress syndrome.
Background
Phosgene gas, also known as carbonyl chloride, is a synthetic and colourless irritant gas with a somewhat musty odour that is heavier than air. It first developed a worldwide reputation during World War I, when it was used in chemical warfare. Phosgene was the principal agent used, accounting for approximately 80% of the 100 000 gas-induced casualties.1–3 Today, phosgene gas most commonly arises from welding and from the combustion of volatile substances including organochloride compounds (ie, polyvinyl chloride and isocyanates).4 These substances are found commonly in many household products such as solvents, paint removers, dry cleaning fluids, home and office furnishings, floor coverings and electrical insulation. We report a case of accidental phosgene inhalation leading to pulmonary oedema in a 20-year-old male. The author attempts to briefly discuss the clinical presentation and the management of phosgene poisoning.
Case presentation
A 20-year-old male was brought to the emergency department with severe dyspnoea, wheeze and cough following inhalation of phosgene gas. The patient immediately after exposure noted a peculiar, pungent smelling gas then he experienced lacrimation and a burning sensation in his mouth and throat also. On arrival to the emergency department the patient was dyspnoeic and had chest tightness and palpitations. On clinical examination, his conjunctiva was congested, pulse rate 130/min, blood pressure 90/60 and he was tachypnoeic (respiratory rate 40/min). Central venous pressure was 14 mm Hg, and oxygen saturation was 58% on a non-re-breather facemask. On chest auscultation, bilateral coarse crepitations with rales were audible over whole of the chest. Other systemic examinations were within normal limit. Chemistries and complete blood count were within normal limits. An arterial blood gas was done which showed type 1 respiratory failure with severe metabolic acidosis; ECG showed sinus tachycardia. With this clinical scenario, the patient was diagnosed as a case of acute non-cardiogenic pulmonary oedema and due to poor respiratory efforts, he was put on mechanical ventilation (volume control ventilatory mode) and vasopressor support. Following Centres for Disease Control and Prevention and Occupational Safety and Health Administration recommendations, appropriate measures were taken to minimise risk of poisoning hospital personnel including monitoring of the patients exhaled gas phosgene levels.
Investigations
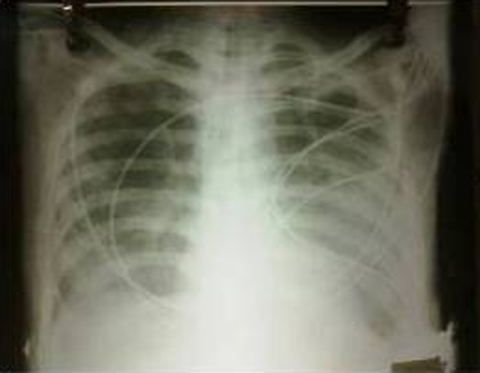
Arterial blood gas analysis showed pH 7.16, pO2 47 mm Hg, pCO2 30 mm Hg, HCO3 10.4 mmol/l, lactate 5.2 mmol/l suggestive of type 1 respiratory failure. Chest x-ray (figure 1) showed bilateral fluffy infiltration with normal cardiac size suggesting of non-cardiogenic pulmonary oedema. By the mean time, other investigations were also available which showed haemoglobin 14.2 g%, total leucocyte count 11500/mm3, differential leucocyte count P76 L20, platelet count 1.59 Lac, random blood sugar-109 mg/dl, serum bilirubin 0.5 mg%, serum glutamic oxaloacetic transaminase-39, serum glutamic pyruvic transaminase-42, serum alkaline phosphatase-56. Kidney function tests were normal. The patient was regularly followed by intensive blood pressure monitoring along with serial arterial blood gas to monitor the response to therapy. The ventilatory support was withdrawn after third of admission and during the hospital stay, the patient made uneventful recovery and was discharged on 7th day.
Figure 1.
Chest x-ray posteroanterior view shows bilateral fluffy infiltration with normal cardiac size.
Treatment
We used N-acetyl cystein via nebulisation, 1 to 10 ml of the 20% solution or 2 to 20 ml of the 10% solution every 2 to 6 h.
Outcome and follow-up
The patient was vigorously managed in the medical intensive care unit with improvement of cardiopulmonary parameters within 3 days so as ventilatory support was withdrawn within 2 days and vasopressor support within 3 days and the patient was discharged on 7th day.
Discussion
Phosgene is a chemical used to make plastics and pesticides. At room temperature (70°F), phosgene is a poisonous gas. With cooling and pressure, phosgene gas can be changed into a liquid so that it can be shipped and stored. When liquid phosgene is released, it quickly turns into a gas that stays close to the ground and spreads rapidly. Poisoning caused by phosgene depends on the amount of phosgene to which a person is exposed, the route of exposure and the length of time that a person is exposed.
The mechanism of phosgene poisoning in a study demonstrated by the significantly increase in tracheal pressure, the rate of lung weight gain, leukotrienes C4, D4 and E4, lipid peroxidation (thiobarbituric acid-reactive substances) and oxidised glutathione over a time following exposure to phosgene.5
Inhalation of phosgene at high concentrations results in a sequence of events, including an initial bio protective phase, a symptom-free latent period, and a terminal phase characterised by pulmonary oedema.
In the initial phase, high concentrations (>3 ppm) may result in a vagal reflex action that causes frequent, shallow respiration and decreased respiratory vital capacity and volume. This, in turn, leads to a decreased arterial CO2 pressure increase and decreased blood pH. After cessation of exposure, the reflex syndrome shows a tendency to regress.6
In the second phase, which may last for several hours postexposure, clinical signs and symptoms are generally lacking. However, histologic examination reveals the beginnings of an oedematous swelling, with blood plasma increasingly entering the pulmonary interstitium and alveoli. This may result in damage to the alveolar type I cells and a rise in haematocrit. In exposed humans, the individual is unaware of these processes; thus, this phase is termed the ‘clinical latent phase.’ The length of this phase varies inversely with the inhaled dose.6
In the third clinical phase of phosgene toxicity, the accumulating fluid in the lung results in the oedema becoming apparent both directly and indirectly. The severity of the oedema increases, potentially resulting in decreased gas exchange as the fluid gradually rises from the alveoli to the proximal segments of the respiratory tract. Agitated respiration may cause the protein-rich fluid to take on a frothy consistency. A severe oedema may result in an increased concentration of haemoglobin in the blood and congestion of the alveolar capillaries. At sufficiently high exposure levels, the heart also may be affected, resulting in cardiac failure due to pulmonary congestion. In general, this phase peaks approximately 24 h after an acute exposure and, assuming lethality does not occur, recedes over the next 3 to 5 days.6
Signs and symptoms of acute phosgene gas exposure include irritation to the eyes; skin contact can result in lesions similar to those from frostbite or burns, respiratory and gastrointestinal systems. In addition to the acute irritant symptoms, non-cardiogenic pulmonary oedema can develop within min and up to 72 h, leading to progressive respiratory insufficiency and acute respiratory distress syndrome (ARDS). The respiratory symptoms may be accompanied by hypovolemia, hypotension and haemoconcentration. The development of these delayed symptoms is usually preceded by a relatively asymptomatic period. The length of the latent period is thought to be inversely proportional to the severity of the initial symptoms. In several cases, infectious pneumonitis develops 3 to 5 weeks after exposure. Mortality is also rare, however, in such cases it does occur within 24 to 48 h after exposure. Most people who recover after an exposure to phosgene make a complete recovery. However, chronic bronchitis and emphysema have been reported as a result of phosgene exposure.
There is no specific laboratory test for confirmatory diagnosis of phosgene gas exposure; however, various means of monitoring pulmonary status should be undertaken. These include a chest x-ray, oxygen saturation, arterial blood gas (if deemed necessary) and volume status assessment (initially via vital signs and examination of mucous membranes). Chest x-ray typically shows signs of pulmonary oedema with enlargement of the hila as the earliest finding (4 to 8 h after exposure) and/or ill-defined patchy infiltrates.
In the management as in any poisoning the decontamination is also paramount here; initially, the patient should be removed from the exposed environment and stripped of his/her clothes. If any area of the skin or eyes has been exposed, thorough irrigation with tepid water should be performed. In areas where direct skin contact has occurred, one should perform a thorough rinse and wash with soap and water.
Pulmonary support should initially be maintained by oxygen therapy as needed. If the patient continues to be hypoxic, intubation may be necessary with or without positive end-expiratory pressure. Pulmonary oedema should be managed with special attention to maintain a net negative fluid balance. Diuretics should be avoided since the pulmonary oedema is not secondary to fluid overload. If necessary, haemodynamic should be monitored through a central line or Swan-Ganz catheter. Antibiotics should not be started empirically, but instead should be reserved for the cases in which there is clinical evidence of pneumonia or bronchitis. The effective treatment of phosgene-induced lung injury involves early postexposure intervention that could reduce free radical species responsible for lipid peroxidation, correct the imbalance in the glutathione redox state and prevent the release of biological mediators such as leukotrienes, which are accountable for increased permeability. Bronchodilators may improve existing bronchospasm. In animal studies, beneficial effect has been shown with the administration of numerous drugs, including leukotriene antagonists, ibuprofen,7 colchicine, cyclophosphamide,8 terbutaline, aminophylline and isoproterenol. Steroids are effective in reducing inflammation secondary to irritant effects of phosgene gas. Specific treatments that have been studied in the past include the use of hexamethylenetetramine (HMT) and N-acetylcysteine (NAC).9 10 HMT, once considered a specific antidote, has been proven only to be effective if administered in a prophylactic manner. There has been no evidence of benefit from HMT in acute phosgene exposure. NAC is thought to ‘trap’ phosgene and convert it to a less harmful metabolite. It has also been postulated that NAC’s antioxidant properties play a role in the decrease in direct toxicity to pulmonary parenchyma.9 However, invivo, it has not been proven effective in reducing morbidity and mortality with the administration of NAC.9 10 Nebulised sodium bicarbonate treatment theoretically may be beneficial; however, consider it as second line after the drugs noted above.
Learning points.
-
▶
Phosgene exposure is associated with significant morbidity and mortality.
-
▶
Physicians should be aware of the risk of phosgene exposure when heat is applied to freon, commonly used in a variety of industries.
-
▶
Patients with a history of exposure should be admitted to the hospital for minimum of 24 h of observation because of the potential for delayed onset respiratory failure and ARDS.
-
▶
The patient must remain asymptomatic and have no chest x-ray changes or hypoxemia after observation to be released from the inpatient ward.
Footnotes
Competing interests None.
Patient consent Obtained.
References
- 1.Eckert WG. Mass deaths by gas or chemical poisoning. A historical perspective. Am J Forensic Med Pathol 1991;12:119–25 [DOI] [PubMed] [Google Scholar]
- 2.Bradley BL, Unger KM. Phosgene inhalation: a case report. Tex Med 1982;78:51–3 [PubMed] [Google Scholar]
- 3.Goldfrank LR, Flomenbaum NE, Lewin NA, et al. Goldfrank’s Toxicologic Emergencies. Fifth Edition East Norwalk, Connecticut: Appleton and Lange; 1994 [Google Scholar]
- 4.Doig AT, Challen PJ. Respiratory hazards in welding. Ann Occup Hyg 1964;7:223–31 [DOI] [PubMed] [Google Scholar]
- 5.Sciuto AM, Strickland PT, Kennedy TP, et al. Protective effects of N-acetylcysteine treatment after phosgene exposure in rabbits. Am J Respir Crit Care Med 1995;151(3 Pt 1):768–72 [DOI] [PubMed] [Google Scholar]
- 6.Diller WF. The methenamine misunderstanding in the therapy of phosgene poisoning. Arch Toxicol 1980;46:199–206 [DOI] [PubMed] [Google Scholar]
- 7.Sciuto AM, Stotts RR, Hurt HH. Efficacy of ibuprofen and pentoxifylline in the treatment of phosgene-induced acute lung injury. J Appl Toxicol 1996;16:381–4 [DOI] [PubMed] [Google Scholar]
- 8.Sciuto AM. Assessment of early acute lung injury in rodents exposed to phosgene. Arch Toxicol 1998;72:283–8 [DOI] [PubMed] [Google Scholar]
- 9.Schelble DT. Phosgene and phosphine. In: Haddad LM, Shannon MW, Winchester J, eds. Clinical Management of Poisoning and Drug Overdose. Third Edition Philadelphia, PA: WB Saunders; 1998:960–3 [Google Scholar]
- 10.Borak J, Diller WF. Phosgene exposure: mechanisms of injury and treatment strategies. J Occup Environ Med 2001;43:110–9 [DOI] [PubMed] [Google Scholar]