Abstract
PURPOSE:
Mediastinal lymphadenopathy (ML) is a cause for concern, especially in patients with previous malignancy. We report our experience with the use of endoscopic ultrasound-guided fine needle aspiration (EUS-FNA) with immunocytochemical stains in patients being evaluated for ML.
METHODS:
Retrospective analysis of patients with ML of unknown origin who underwent EUS-FNA. On-site evaluation was performed by experienced cytologist, and special immunocytochemical stains were requested as indicated.
RESULTS:
A total of 116 patients were included, and a total of 136 mediastinal LN were sampled. Prior malignancy was present in 45%. The most common site of examined lymph node (LN) were subcarinal (76%, 103 LN). The median long and short axis diameters were 28 mm and 13 mm, respectively. FNA was read on-site as malignant, 21 (16%); benign, 100 (76.9%); suspicious, six (4%); atypical, 3 (2%); and inadequate sample, six (4%). Sixty-four LN were deferred for additional studies; 22 for immunocytochemical and 26 for Gimesa (GMS) stain and 21 for flow cytometry. Final FNA read was malignant in 28 (21%), benign in 103 (76%), suspicious in three (2%), and atypical in two (1%). Metastatic malignancies disclosed included Hodgkin's and Non-Hodgkin's lymphoma, melanoma, hepatoma, breast, lung, colon, renal, endometrial, Fallopian tube, and unknown carcinoma. The sensitivity, specificity, and accuracy of the final FNA read to predict malignancy were 100%.
CONCLUSION:
EUS-guided FNA with additional ancillary studies is useful in disclosing metastatic ML from a variety of neoplasms. Due to its safety and accuracy profile, it should be considered the test of choice in evaluating abnormal ML in appropriately selected patients.
Keywords: Endoscopic ultrasound, fine needle aspiration, immunostains, lung cancer, metastatic disease
The mediastinum is a host for a number of un usual primary neoplasms, as well as the site of metastasis.[1] Several benign and inflammatory conditions can also present as mediastinal lymphadenopathy (ML). The presence of ML in patients without other primary lesions can present a diagnostic dilemma. The exact location and the context of the lymphadenopathy is a major determinant of what modality can be employed for imaging or diagnosis. Computed tomography (CT) scans and Positron emission tomography (PET) scans can determine the size and metabolic status, respectively, but tissue diagnosis is required for definitive diagnosis and patient management. This is especially true if the patient has had no previous malignancy or if a new primary is suspected. In addition, while PET scan and CT are accurate, we have previously shown that false-positive and false-negative diagnosis can frequently result, should one rely on the size of the lesion or the standard uptake value obtained from PET.[2] Mediastinoscopy, though considered a standard method for mediastinal lymph node staging, is limited by its invasiveness, cost, possible complications, and the need for general anesthesia. Since Wiersema et al.[3] described the first successful endoscopic ultrasound-guided fine needle aspiration (EUS-FNA) of a mediastinal lymph node in 1993, this method has proven to be a versatile, dynamic tool in our diagnostic arsenal.
In addition, the rapid advancement in immunohistochemical, molecular, and molecular diagnostic techniques available in cytopathology has further expanded the diagnostic horizons of EUS-FNA. We can increasingly do “much more with much less.” This holds particularly true for conditions and malignancies with unique and specific immunohistochemical properties or patterns.
On-site cytopathology interpretation has been previously shown to improve the diagnostic yield of EUS-guided FNA.[4,5] On-site assessment helps to ensure that cytopathological samples aspirated by endoscopist are both representative of the target organ and adequate for diagnosis.[6,7] This is particularly important when trying to differentiate between a suspected malignancy and a benign/inflammatory process. Preliminary assessment of specimen also allows the cytopathologist to prospectively identify cases that would benefit from additional aspirates for performing confirmatory immunocytochemical stains or flow cytometry.[8] In addition, ultrasonographic features, such as echogenicity, borders, contours, and size may add further information to help synthesize a diagnosis.[9]
We describe our institution's unique experience in the use of immunohistochemical staining with samples obtained with EUS-FNA in patients with ML.
Methods
Study population
After obtaining Institutional Review Board approval of the University of Alabama at Birmingham, data collected prospectively from all patients with CT documented ML who underwent EUS-guided FNA between September 2000 and May 2007 by the same endosonographer (M.A.E) were combined into the study database. Data were collected in 116 patients, including 136 LNs. Included patients were those with mediastinal adenopathy of unknown origin. Patients with known lung cancer or suspected lung cancer were excluded from this analysis, since these data were concerned mostly with idiopathic mediastinal lymph nodes. Patients with esophageal obstruction, coagulopathy, and those unable to consent or undergo endoscopy were also excluded from the study population.
Data collection
Data abstraction was done by retrospective review of a databank of patients who underwent EUS during the study period. Follow-up data were obtained with electronic medical records review.
EUS-guided FNA
EUS was performed under conscious sedation by a single endosonographer (M.A.E.) as previously described, who was aware of the presence of enlarged LN. A curvilinear echoendoscope (Olympus UC-30P or UCT 140) was inserted (Olympus America, Melville, NY) in the stomach where the examination started with full evaluation of the left adrenal gland by imaging it from the fundus of the stomach. The echoendoscope was then gradually withdrawn to evaluate the inferior pulmonary ligament nodal station (#9), the periesophageal areas (#8), the subcarinal space (#7), the aortopulmonary window (#5), and the upper and lower paratracheal LNs (#2 and 4). Once an LN was identified, EUS-FNA of the target lesion(s) was performed as previously described in the presence of a cytopathologist.[7] Additional passes were performed at the discretion of the pathologists for ancillary studies such as immunostains, flow cytometry, or culture (See Table 3 or supplemental table for specific immunostains performed).
Table 3.
Individual cases with selective use of immunohistochemical stains
All EUS-FNAs were performed with 22-gauge Echotip needles (Wilson-Cook Inc, Winston-Salem, NC). Cytologic diagnosis of the aspirated lesion was classified into the following four categories: (1) benign or reactive, (2) positive for malignancy, (3) atypical or suspicious for malignancy, or (4) non-diagnostic. The endosonographic criteria for malignant involvement of the LN were documented before cytologic evaluation as previously described.[9]
Diagnosis of malignancy was based on FNA findings and clinical follow-up data such as surgical specimens or unequivocal radiological findings of cancer progression. Patients were considered to have benign lymphadenopathy only if (1) the FNA was non-malignant, (2) surgical and pathologic findings of the target LN from thoracotomy or other surgical procedure were benign, and (3) results of clinical and radiological 6-month follow-up were negative for malignancy at that LN. If any of the above was positive for malignancy, the target LN was considered malignant.
Statistical analysis
Results are described in frequencies and percentages for categorical variables and medians and interquartile range (IQR) for continuous data. Given patients with atypical FNA reads are usually treated in a similar fashion as those with benign reads, atypical FNA reads were considered benign when calculating sensitivity, specificity, and accuracy of FNA. Similarly, suspicious FNA reads were considered malignant. All statistical analyses were performed using Stata 10.1 (StataCorp, College Station, TX).
Results
A total of 116 patients were included, and a total of 136 mediastinal LNs were sampled. Mean age of patients was 54.5 years (SD 16), 52.5% were men (61 patients) and 77.6% were white (90 patients). Of the 116 subjects, prior malignancy was present in 45% (52 patients). Prior chest CT was performed in 86% (99 patients); PET scan in 40% (46 patients). Prior LN biopsy was performed in 26 patients (22%).
The location of lymph nodes and their respective EUS characteristics are shown in Table 1. Sites of examined LNs were subcarinal (103 LN, 76%), lower paraesophageal (12 LN, 9%), aortopulmonary window (8 LN, 6%), other locations (9 LN, 7%), and 2 LN with missing location. The median long axis diameter was 28 mm (IQR, 18-37 mm) and short axis diameter was 13 mm (IQR, 9-20 mm). Sharp borders were seen in 33 LN (25%), round shape was noted in 21 LN (16%), and hypoechoic appearance in 54 LN (40%). Mean number of EUS-FNA passes was 3.8 (SD 1.3).
Table 1.
Lymph node location and EUS characteristics

FNA was read on-site as malignant in 21 (16%), benign in 100 (74%), suspicious in six (4%), atypical in three (2%), and inadequate sample in six (4%). Sixty-four LN were deferred for additional studies; 22 for immunocytochemical analysis and 26 for GMS stain and 21 for flow cytometry. Final FNA read was malignant in 28 (21%), benign in 103 (76%), suspicious in three (2%), and atypical in 2 (1%).
Final diagnosis of the LN was confirmed by pathology, imaging, or 6-month clinical follow-up in 114 LNs (84%). Among these cases, the sensitivity, specificity, and accuracy of the final FNA read to predict final LN diagnosis of malignancy was 100%.
In multivariable analysis, the significant independent predictors of malignancy in EUS-guided FNA were prior history of a cancer (Odds ratio [OR], 13.10; 95% Confidence interval (CI), 2.71-63.32; P=0.001), short axis (OR, 1.10; 95% CI, 1.00-1.22; P=0.041), and presence of sharp LN borders (OR, 5.47; 95% CI, 1.01-29.51; P=0.048). Patient age, race and gender, long axis, and round shape were not independently associated with FNA positive for malignancy in our cohort.
Final diagnosis of the 136 LN is shown in Table 2. Of those, disclosed metastatic malignancies included Hodgkin's lymphoma (3 patients, 5 LN), non-Hodgkin's lymphoma (5 patients), lung cancer (4 patients, 5 LN), breast cancer (6 patients, 6 LN) [Figure 1], renal cell carcinoma (2 patients) [Figures 2 and 3], colon carcinoma (3 patients), [Figure 4] endometrial carcinoma (1 patient), Fallopian tube carcinoma (1 patient), hepatoma (1 patient), melanoma (1 patient), Figure 5 and unknown primary site carcinoma (1 patinent). Summary of the subjects with metastatic malignancies and associated ancillary studies is shown in Table 3.
Table 2.
Final diagnosis of LNs
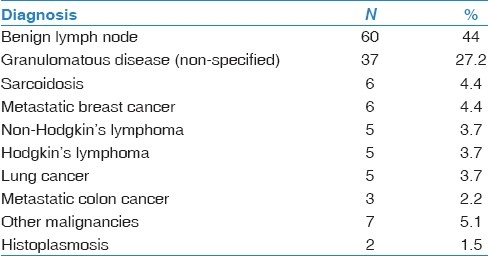
Figure 1.
(a) Aspirate smear, Diff-Quik stain, power, ×20 showing pleomorphic malignant-appearing cells, with large nuclei and nucleoli. (b) Cell block, H and E stain, power ×10, showing clumps of atypical cells. (c) Cell block, Estrogen receptor stain, showing the strong nuclear stain, some cells with intracytoplasmic mucin consistent with breast cancer, ×40
Figure 2.
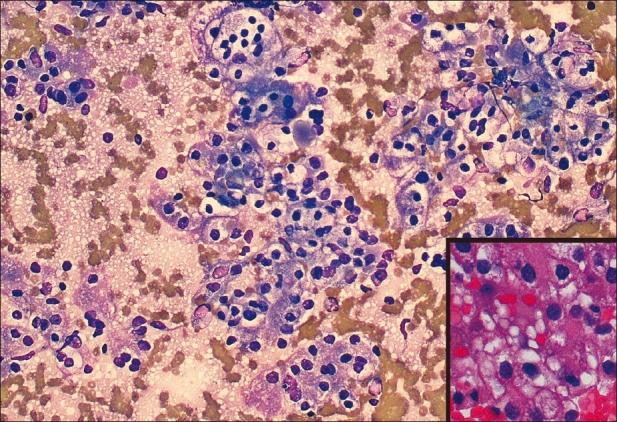
Diff-Quik smear: Metastatic carcinoma, renal cell type showing clumps of malignant-appearing cells with abundant clear cytoplasm, ×20. Atypical cells are immunoreactive for CD10, vimentin, and broad-spectrum cytokeratin, RCC. Inset: Higher magnification showing clear, vacuolated cytoplasm with large nuclei, prominent nucleoli typical of RCC (cell block, ×40)
Figure 3.
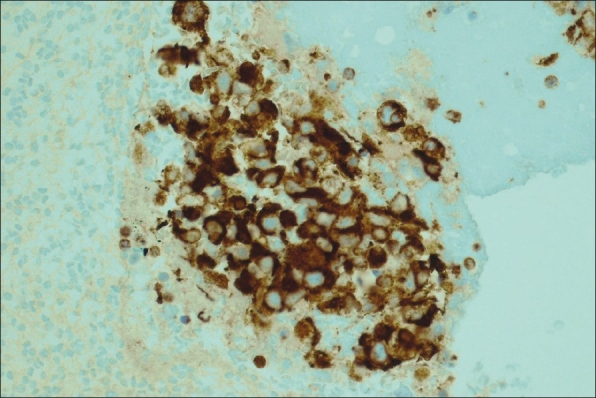
Left hilar node, cell block, immunohistochemical stain for vimentin ×20. The atypical cells described in Figure 2 stain positively with vimentin supporting the diagnosis of metastatic Renal Cell Carcinoma
Figure 4.
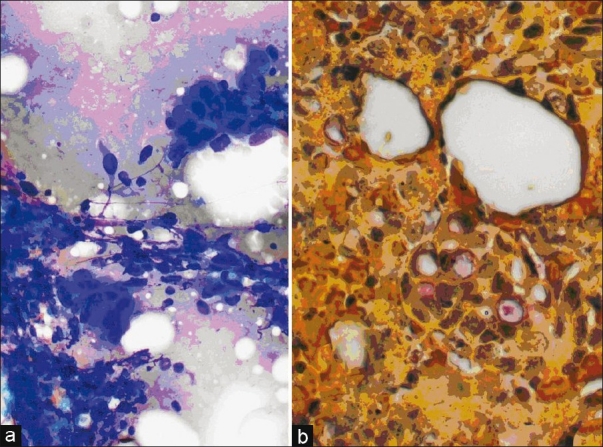
Colon Adenocarcinoma. (a) Aspirate smear, Diff-Quik stain, power, ×20 with large adherent groups of pleomorphic cells with increased nucleus to cytoplasm ratio. (b) Cell block, Mucicarmine stain indicating presence of mucin in the atypical cells which appear arranged in glandular structures, power, ×40
Figure 5.
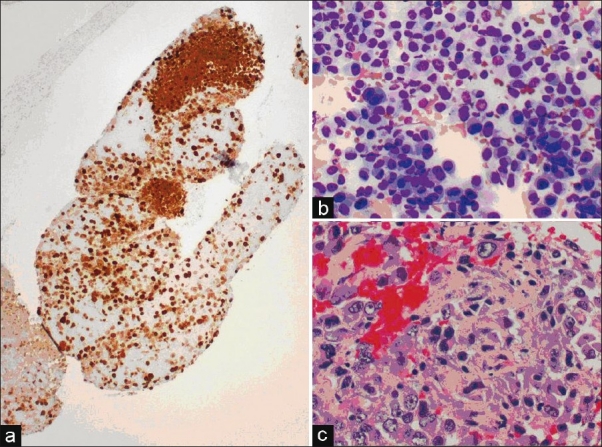
Melanoma (a) Cell block, S-100 stain showing a positive (brown) nuclear and cytoplasmic staining, ×10. (b) Aspirate smear, Diff-Quik stain, ×20 with highly pleomorphic cells, some binucleated with prominent nucleoli. (c) Cell block, higher magnification showing characteristic prominent cherry red nucleoli
Discussion
Just as the technology, imaging and sampling modalities available to clinicians have expanded, the Pathologist's diagnostic armament including new molecular techniques, novel antibodies in immunocytochemical staining, and specimen processing has expanded dramatically over the past decade as well.
EUS-FNA is a minimally invasive technique well suited for cytologic and immunohistochemical diagnosis of mediastinal lesions. Among the 116 patients in our study, the sensitivity, specificity, and accuracy of the final FNA read to predict final LN diagnosis of malignancy were 100%. Other smaller studies[10] have shown sensitivity and accuracy of EUS-FNA to be about 69% and 75%, respectively. In that study involving 20 patients, surgical diagnostic procedures were precluded in 60% of the patients. Similar results are mirrored in larger studies[11] in the context of patients with previous extrathoracic malignancy and suspected mediastinal metastases, where EUS-FNA consistently demonstrates high sensitivity (86%) and accuracy (91%).[11] Negative predictive value of EUS-FNA for mediastinal staging was found to be 72% by the some authors.
The role of EUS-FNA even in low-volume EUS centers (<50 mediastinal EUS-FNA/endoscopist/year) has been examined by a recent European study[12] involving 213 patients where the clinical impact of EUS-FNA was evaluated by using the classification put forward by Chen and Eloubeidi.[13] The study concluded that EUS-FNA had a positive impact on clinical management in 178 of 213 (84%) procedures, whereas only in 16 of 213 (8%) procedures, clinical management was considered to have been negatively affected by the cytological results by providing false-negative or inconclusive cytology. This overall positive effect of cost-reduction was also quantified by calculating the total cost of the actual diagnostic workup vs the total cost of the alternative diagnostic workup, including the costs of all the components of the diagnostic procedures (In the alternative diagnostic workup, for example, mediastinoscopy instead of EUS-FNA would have been performed in 143 patients with an estimated complication risk of 2%).
It is not surprising then that by performing 213 EUS-FNAs, €100 593 (or ~133 000) were saved[12] (not including the cost of surgery for non-diagnostic alternative interventions). The impact on cost reduction has been corroborated by other authors as well. Eloubeidi et al.[14] showed an even higher average cost reduction of $11 033 per patient by using EUS-FNA instead of mediastinoscopy as the primary staging tool in patients with non-small cell lung carcinoma. Combining EUS-FNA with PET has also been shown to reduce staging costs by 40% by obviating surgical staging.[15]
Traditionally, the use of EUS-FNA for ML has been in the context of lung cancer staging where it has clearly been shown to reduce the incidence of futile thoracotomies (especially where the stage of the disease is more advanced than expected preoperatively) from 25% to 9%.[16] It can also facilitate a more thorough examination of not only the mediastinal nodal stations but also the adrenals[17] and other infradiaphragmatic sites of potential spread. Although conventionally mediastinoscopy was regarded as the “gold-standard” for mediastinal staging, it is limited in sampling less accessible areas such as aortopulmonary, retrotracheal, posterior carinal, or inferior mediastinal lymph nodes. Also, with the lower complication rates following EUS-FNA in various cohorts,[11,18,19] EUS is now indicated as a minimally invasive alternative for surgical staging in recent lung cancer staging guidelines.[20]
Characteristic immunostaining and cytomorphological features help in diagnosing less common tumors in locations other the mediastinum sampled with EUS-FNA (such as the solid-pseudopapillary tumors of the pancreas with immunostaining for Neuron-specific enolase,α1-antitrypsin, and α1-antichymotrypsin[21] or either CD117 immunostaining or high-resolution amplicon melting analysis for CD 117/PDGFR mutations for Gastrointestinal stromal tumors[22,23]).
EUS with FNA provides a viable approach to the diagnosis and staging of tumors in the head and neck region as well, when there is a suggestion of esophageal invasion on CT or MRI, or enlarged mediastinal lymph nodes.[24] Additionally, for example, parathyroid hormone concentration measurement in the aspirated material in cases of suspected mediastinal parathyroid adenomas may make EUS-FNA a useful adjunct in diagnosing mediastinal parathyroid adenomas.[25]
Even for hepatic lesions, early experience[26,27] suggests that EUS-FNA is comparable with CT-FNA in terms of diagnostic utility. EUS FNA should be considered especially if the lesion is in the hilum and left lobe of the liver or the proximal biliary tract. The gallbladder, extrahepatic biliary system, and perihilar lymph nodes are readily accessible as well.
Our study outlines the role of EUS-FNA in diagnosing the non-malignant, inflammatory, or infectious causes of ML such as sarcoidosis, fungal infections, especially when special features help direct onsite evaluation toward the correct diagnosis. In one study,[28] sensitivity of EUS in detecting granulomas in patients with sarcoidosis causing ML was 87% (with cell-block analysis added to conventional cytological evaluation), with the optimal yield for granuloma detection reached with four needle passes. These non-malignant conditions are particularly suited for diagnosis through EUS-FNA when characteristic pathologic findings (such as asteroid bodies or epitheloid granulomas) are seen.
Working in association with an experienced cytopathologist aware of artifacts inherent in EUS-FNA sampling can minimize diagnostic errors.[26] This is especially relevant with lesions that have characteristic histopathologic and immunophenotypic features.[29–31] With some of these lesions where ancillary immunophenotyping may be key to diagnosis, EUS-FNA has been shown to be superior to CT-guided FNA.[32]
In our study, the history of prior malignancy was associated with detection of malignant lymph nodes in the mediastinum. From our observation and interactions with our pathology group, we note that it is of paramount importance to discuss the clinical history with our colleagues in cytopathology at the time of tissue collection, so that adequate material can be obtained at the time of onsite interpretation for further ancillary studies such as immunostains, flow cytometry, and culture.
Limitations of our study included the retrospective evaluation of data which were prospectively collected. In addition, the majority of the LNs of our cohort were located at level 7, which is most likely explained by referral bias regarding the utility of EUS-FNA in this subset of patients. The other limitation is the absence of surgery and 6-month follow-up in a small proportion of our patients. We used a compound reference standard similar to other investigations in the field.
With our study, we describe the unique experience in evaluation of ML at our institution and reiterate the expanding and central role of EUS-FNA in diagnosing mediastinal pathology.
Footnotes
Source of Support: Nil,
Conflict of Interest: None declared.
References
- 1.Geisinger KR. Differential diagnostic considerations and potential pitfalls in fine-needle aspiration biopsies of the mediastinum. Diagn Cytopathol. 1995;13:436–42. doi: 10.1002/dc.2840130512. [DOI] [PubMed] [Google Scholar]
- 2.Cerfolio RJ, Bryant AS, Ojha B, Eloubeidi M. Improving the inaccuracies of clinical staging of patients with NSCLC: A prospective trial. Ann Thorac Surg. 2005;80:1207–14. doi: 10.1016/j.athoracsur.2005.04.019. [DOI] [PubMed] [Google Scholar]
- 3.Wiersema MJ, Kochman ML, Chak A, Cramer HM, Kesler KA. Real time endoscopic ultrasound guided fine needle aspiration of mediastinal lymph node. Gastrointest Endosc. 1993;39:429–31. doi: 10.1016/s0016-5107(93)70122-4. [DOI] [PubMed] [Google Scholar]
- 4.Klapman JB, Logrono R, Dye CE, Waxman I. Clinical impact of on-site cytopathology interpretation on endoscopic ultrasound-guided fine needle aspiration. Am J Gastroenterol. 2003;98:1289–94. doi: 10.1111/j.1572-0241.2003.07472.x. [DOI] [PubMed] [Google Scholar]
- 5.Singh HK, Silverman JF, Powers CN, Geisinger KR, Frable WJ. Diagnostic pitfalls in fine-needle aspiration biopsy of the mediastinum. Diagn Cytopathol. 1997;17:121–6. doi: 10.1002/(sici)1097-0339(199708)17:2<121::aid-dc7>3.0.co;2-j. [DOI] [PubMed] [Google Scholar]
- 6.Logrono R, Waxman I. Interactive role of the cytopathologist in EUS-guided fine needle aspiration: An efficient approach. Gastrointest Endosc. 2001;54:485–90. doi: 10.1067/mge.2001.118445. [DOI] [PubMed] [Google Scholar]
- 7.Eloubeidi MA, Tamhane A, Jhala N, Chhieng D, Jhala D, Crowe DR, et al. Agreement between rapid onsite and final cytologic interpretations of EUS-guided FNA specimens: Implications for the endosonographer and patient management. Am J Gastroenterol. 2006;101:2841–7. doi: 10.1111/j.1572-0241.2006.00852.x. [DOI] [PubMed] [Google Scholar]
- 8.Bhutani MS, Logrono R. Endoscopic ultrasound-guided fine-needle aspiration cytology for diagnosis above and below the diaphragm. J Clin Ultrasound. 2005;33:401–11. doi: 10.1002/jcu.20149. [DOI] [PubMed] [Google Scholar]
- 9.Catalano MF, Sivak MV, Jr, Rice T, Gragg LA, Van Dam J. Endosonographic features predictive of lymph node metastasis. Gastrointest Endosc. 1994;40:442–6. doi: 10.1016/s0016-5107(94)70206-3. [DOI] [PubMed] [Google Scholar]
- 10.Kramer H, Koëter GH, Sleijfer DT, van Putten JW, Groen HJ. Endoscopic ultrasound-guided fine-needle aspiration in patients with mediastinal abnormalities and previous extrathoracic malignancy. Eur J Cancer. 2004;40:559–62. doi: 10.1016/j.ejca.2003.11.010. [DOI] [PubMed] [Google Scholar]
- 11.Peric R, Schuurbiers OC, Veseliç M, Rabe KF. Transesophageal endoscopic ultrasound-guided fine-needle aspiration for the mediastinal staging of extrathoracic tumors: A new perspective. Ann Oncol. 2010;21:1468–71. doi: 10.1093/annonc/mdp578. [DOI] [PubMed] [Google Scholar]
- 12.Hirdes MM, Schwartz MP, Tytgat KM, Schlösser NJ, Sie-Go DM, Brink MA, et al. Performance of EUS-FNA for mediastinal lymphadenopathy: Impact on patient management and costs in low-volume EUS centers. Surg Endosc. 2010;24:2260–7. doi: 10.1007/s00464-010-0946-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chen VK, Eloubeidi MA. Endoscopic ultrasound-guided fine-needle aspiration of intramural and extraintestinal mass lesions: Diagnostic accuracy, complication assessment, and impact on management. Endoscopy. 2005;37:984–9. doi: 10.1055/s-2005-870272. [DOI] [PubMed] [Google Scholar]
- 14.Eloubeidi MA, Tamhane A, Chen VK, Cerfolio RJ. Endoscopic ultrasound-guided fine-needle aspiration in patients with non-small cell lung cancer and prior negative mediastinoscopy. Ann Thorac Surg. 2005;80:1231–9. doi: 10.1016/j.athoracsur.2005.04.001. [DOI] [PubMed] [Google Scholar]
- 15.Kramer H, van Putten JW, Post WJ, van Dullemen HM, Bongaerts AH, Pruim J, et al. Oesophageal endoscopic ultrasound with fine needle aspiration improves and simplifies the staging of lung cancer. Thorax. 2004;59:596–601. doi: 10.1136/thx.2003.018028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Larsen SS, Vilmann P, Krasnik M, Dirksen A, Clementsen P, Maltbaek N, et al. Endoscopic ultrasound guided biopsy performed routinely in lung cancer staging spares futile thoracotomies: Preliminary results from a randomised clinical trial. Lung Cancer. 2005;49:377–85. doi: 10.1016/j.lungcan.2005.04.005. [DOI] [PubMed] [Google Scholar]
- 17.Jhala NC, Jhala D, Eloubeidi MA, Chhieng DC, Crowe DR, Roberson J, et al. Endoscopic ultrasound-guided fine-needle aspiration biopsy of the adrenal glands: Analysis of 24 patients. Cancer. 2004;102:308–14. doi: 10.1002/cncr.20498. [DOI] [PubMed] [Google Scholar]
- 18.Lee YT, Lai LH, Sung JJ, Ko FW, Hui DS. Endoscopic ultrasonography-guided fine-needle aspiration in the management of mediastinal diseases: Local experience of a novel investigation. Hong Kong Med J. 2010;16:121–5. [PubMed] [Google Scholar]
- 19.Aerts JG, Kloover J, Los J, van der Heijden O, Janssens A, Tournoy KG. EUS-FNA of enlarged necrotic lymph nodes may cause infectious mediastinitis. J Thorac Oncol. 2008;3:1191–3. doi: 10.1097/JTO.0b013e3181872752. [DOI] [PubMed] [Google Scholar]
- 20.De Leyn P, Lardinois D, Van Schil PE, Rami-Porta R, Passlick B, Zielinski M, et al. ESTS guidelines for preoperative lymph node staging for non-small cell lung cancer. Eur J Cardiothorac Surg. 2007;32:1–8. doi: 10.1016/j.ejcts.2007.01.075. [DOI] [PubMed] [Google Scholar]
- 21.Bardales RH, Centeno B, Mallery JS, Lai R, Pochapin M, Guiter G, et al. Endoscopic ultrasound-guided fine-needle aspiration cytology diagnosis of solid-pseudopapillary tumor of the pancreas: A rare neoplasm of elusive origin but characteristic cytomorphologic features. Am J Clin Pathol. 2004;121:654–62. doi: 10.1309/DKK2-B9V4-N0W2-6A8Q. [DOI] [PubMed] [Google Scholar]
- 22.Willmore-Payne C, Layfield LJ, Holden JA. c-KIT mutation analysis for diagnosis of gastrointestinal stromal tumors in fine needle aspiration specimens. Cancer. 2005;105:165–70. doi: 10.1002/cncr.21064. [DOI] [PubMed] [Google Scholar]
- 23.Kaur G, Manucha V, Verma K. Gastrointestinal stromal tumors: Cytomorphologic spectrum in fine needle aspiration smears. Indian J Pathol Microbiol. 2010;53:271–5. doi: 10.4103/0377-4929.64338. [DOI] [PubMed] [Google Scholar]
- 24.Wildi SM, Fickling WE, Day TA, Cunningham CD, 3rd, Schmulewitz N, Varadarajulu S, et al. Endoscopic ultrasonography in the diagnosis and staging of neoplasms of the head and neck. Endoscopy. 2004;36:624–30. doi: 10.1055/s-2004-814521. [DOI] [PubMed] [Google Scholar]
- 25.Vu DH, Erickson RA. Endoscopic ultrasound-guided fine-needle aspiration with aspirate assay to diagnose suspected mediastinal parathyroid adenomas. Endocr Pract. 2010;16:437–40. doi: 10.4158/EP09220.CR. [DOI] [PubMed] [Google Scholar]
- 26.Crowe DR, Eloubeidi MA, Chhieng DC, Jhala NC, Jhala D, Eltoum IA. Fine-needle aspiration biopsy of hepatic lesions: Computerized tomographic-guided versus endoscopic ultrasound-guided FNA. Cancer. 2006;108:180–5. doi: 10.1002/cncr.21912. [DOI] [PubMed] [Google Scholar]
- 27.Nguryen P, Feng JC, Chang KJ. Endoscopic ultrasound (EUS) and EUS-guided fine-needle aspiration (FNA) of liver lesions. Gastrointest Endosc. 1999;50:357–61. doi: 10.1053/ge.1999.v50.97208. [DOI] [PubMed] [Google Scholar]
- 28.von Bartheld MB, Veseliç-Charvat M, Rabe KF, Annema JT. Endoscopic ultrasound-guided fine-needle aspiration for the diagnosis of sarcoidosis. Endoscopy. 2010;42:213–7. doi: 10.1055/s-0029-1243890. [DOI] [PubMed] [Google Scholar]
- 29.Peng HQ, Darwin P, Papadimitriou JC, Drachenberg CB. Liver metastases of pancreatic acinar cell carcinoma with marked nuclear atypia and pleomorphism diagnosed by EUS FNA cytology: A case report with emphasis on FNA cytological findings. Cytojournal. 2006;3:29. doi: 10.1186/1742-6413-3-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Khan AS, Crowe DR, Trevino JM, Eloubeidi MA. Multiple metastases to the pancreas from primary maxillary osteosarcoma: Diagnosis with EUS-guided FNA. Gastrointest Endosc. 2011;73:1320–2. doi: 10.1016/j.gie.2010.09.024. [DOI] [PubMed] [Google Scholar]
- 31.Chhieng DC, Benson E, Eltoum I, Eloubeidi MA, Jhala N, Jhala D, et al. MUC1 and MUC2 expression in pancreatic ductal carcinoma obtained by fine-needle aspiration. Cancer. 2003;99:365–71. doi: 10.1002/cncr.11857. [DOI] [PubMed] [Google Scholar]
- 32.Jhala D, Eloubeidi M, Chhieng DC, Frost A, Eltoum IA, Roberson J, et al. Fine needle aspiration biopsy of the islet cell tumor of pancreas: A comparison between computerized axial tomography and endoscopic ultrasound-guided fine needle aspiration biopsy. Ann Diagn Pathol. 2002;6:106–12. doi: 10.1053/adpa.2002.30613. [DOI] [PubMed] [Google Scholar]


