Abstract
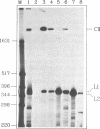
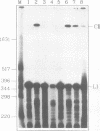
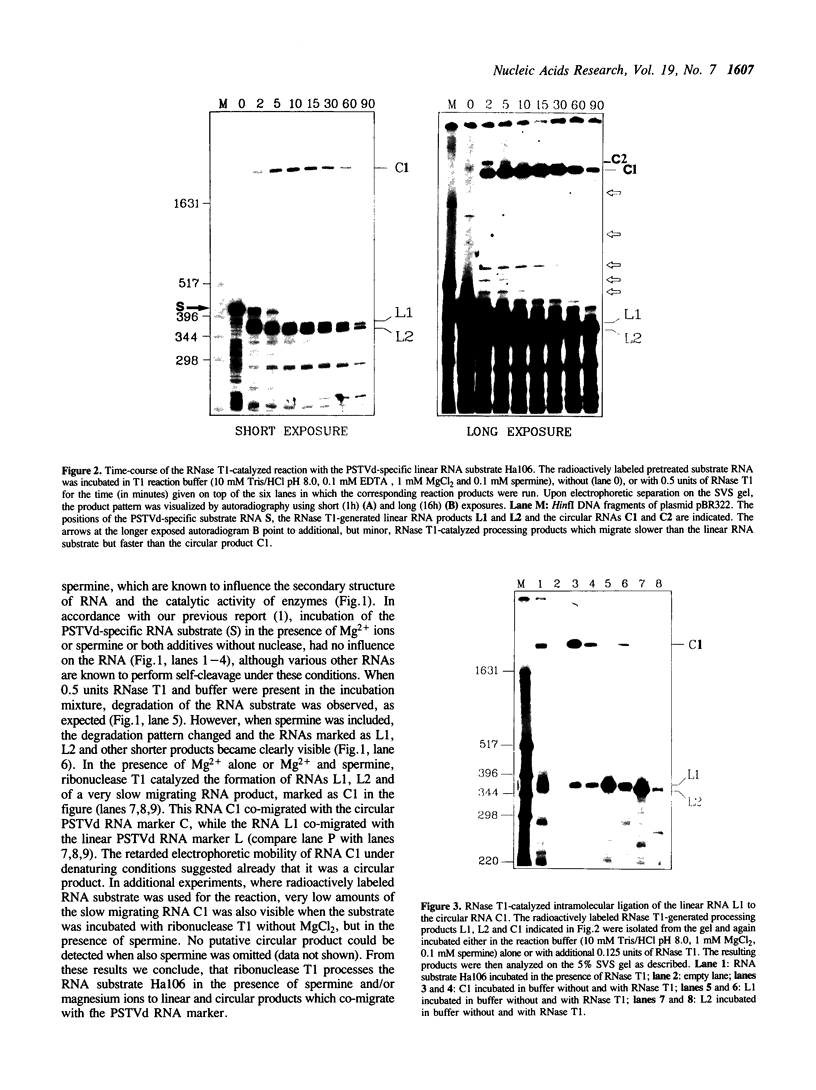
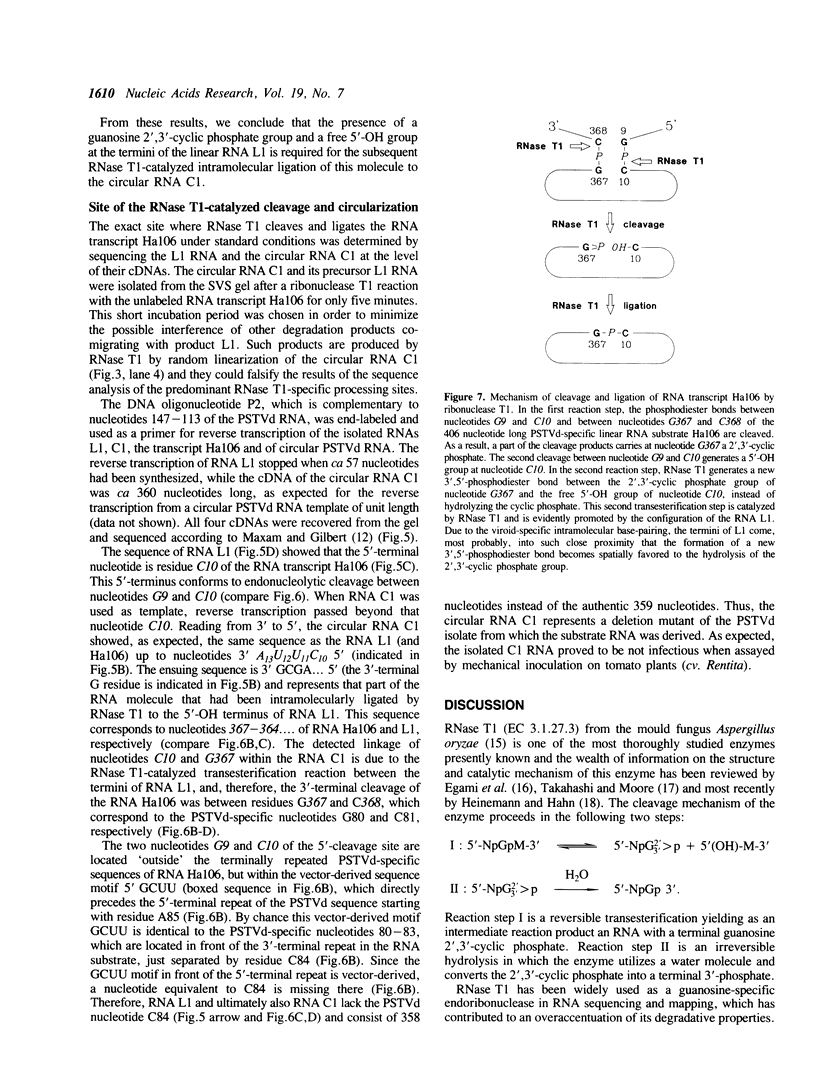
A 406 nucleotide long potato spindle tuber viroid (PSTVd)-specific linear RNA transcript was synthesized in vitro and subjected to limited digestion with ribonuclease (RNase) T1. Under certain conditions this guanosine-specific endoribonuclease proved to be capable of processing the longer-than-unit-length, precursor-like viroid RNA transcript by cleaving out a linear 358 nucleotide long product and ligating that to a circular RNA molecule. The new finding that RNase T1 acts as an RNA processing enzyme and, in particular, as an RNA 'circulase' can be explained by the unique structural preconditions inherent in the viroid-specific substrate and by the well characterized two-step cleavage mechanism of the enzyme. These in vitro potentials of RNase T1 suggest that also in vivo procaryotic and eucaryotic RNases with a similar reaction mechanism might not only be involved in RNA degradation and trimming, but also in processing, ligation and recombination of RNA.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Davies D. A., Pope A. M. Mycolase, a new kind of systemic antimycotic. Nature. 1978 May 18;273(5659):235–236. doi: 10.1038/273235a0. [DOI] [PubMed] [Google Scholar]
- Deutscher M. P. E. coli RNases: making sense of alphabet soup. Cell. 1985 Apr;40(4):731–732. doi: 10.1016/0092-8674(85)90330-7. [DOI] [PubMed] [Google Scholar]
- Diener T. O. Circular RNAs: relics of precellular evolution? Proc Natl Acad Sci U S A. 1989 Dec;86(23):9370–9374. doi: 10.1073/pnas.86.23.9370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Egami F., Oshima T., Uchida T. Specific interaction of base-specific nucleases with nucleosides and nucleotides. Mol Biol Biochem Biophys. 1980;32:250–277. doi: 10.1007/978-3-642-81503-4_21. [DOI] [PubMed] [Google Scholar]
- Hammond R., Smith D. R., Diener T. O. Nucleotide sequence and proposed secondary structure of Columnea latent viroid: a natural mosaic of viroid sequences. Nucleic Acids Res. 1989 Dec 11;17(23):10083–10094. doi: 10.1093/nar/17.23.10083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keese P., Symons R. H. Domains in viroids: evidence of intermolecular RNA rearrangements and their contribution to viroid evolution. Proc Natl Acad Sci U S A. 1985 Jul;82(14):4582–4586. doi: 10.1073/pnas.82.14.4582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kikuchi Y., Tyc K., Filipowicz W., Sänger H. L., Gross H. J. Circularization of linear viroid RNA via 2'-phosphomonoester, 3', 5'-phosphodiester bonds by a novel type of RNA ligase from wheat germ and Chlamydomonas. Nucleic Acids Res. 1982 Dec 11;10(23):7521–7529. doi: 10.1093/nar/10.23.7521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koltunow A. M., Rezaian M. A. Grapevine viroid 1B, a new member of the apple scar skin viroid group contains the left terminal region of tomato planta macho viroid. Virology. 1989 Jun;170(2):575–578. doi: 10.1016/0042-6822(89)90450-9. [DOI] [PubMed] [Google Scholar]
- Maxam A. M., Gilbert W. Sequencing end-labeled DNA with base-specific chemical cleavages. Methods Enzymol. 1980;65(1):499–560. doi: 10.1016/s0076-6879(80)65059-9. [DOI] [PubMed] [Google Scholar]
- Mohr S. C., Thach R. E. Application of ribonuclease T1 to the synthesis of oligoribonucleotides of defined base sequence. J Biol Chem. 1969 Dec 25;244(24):6566–6576. [PubMed] [Google Scholar]
- Podder S. K. Synthetic action of ribonuclease T1. Biochim Biophys Acta. 1970;209(2):455–462. doi: 10.1016/0005-2787(70)90742-2. [DOI] [PubMed] [Google Scholar]
- Rezaian M. A. Australian grapevine viroid--evidence for extensive recombination between viroids. Nucleic Acids Res. 1990 Apr 11;18(7):1813–1818. doi: 10.1093/nar/18.7.1813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SATO-ASANO K., EGAMI F. Réactions synthétiques par la ribonucléase T1. Biochim Biophys Acta. 1958 Sep;29(3):655–656. doi: 10.1016/0006-3002(58)90032-5. [DOI] [PubMed] [Google Scholar]
- Schnölzer M., Haas B., Raam K., Hofmann H., Sänger H. L. Correlation between structure and pathogenicity of potato spindle tuber viroid (PSTV). EMBO J. 1985 Sep;4(9):2181–2190. doi: 10.1002/j.1460-2075.1985.tb03913.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Symons R. H. Self-cleavage of RNA in the replication of small pathogens of plants and animals. Trends Biochem Sci. 1989 Nov;14(11):445–450. doi: 10.1016/0968-0004(89)90103-5. [DOI] [PubMed] [Google Scholar]
- Tabler M., Günther I., Kern R., Sänger H. L. A microscale procedure for isolating and sequencing the viroid RNA present in one gram of infected leaf tissue. J Virol Methods. 1989 Feb;23(2):111–126. doi: 10.1016/0166-0934(89)90125-0. [DOI] [PubMed] [Google Scholar]
- Tabler M., Sänger H. L. Infectivity studies on different potato spindle tuber viroid (PSTV) RNAs synthesized in vitro with the SP6 transcription system. EMBO J. 1985 Sep;4(9):2191–2199. doi: 10.1002/j.1460-2075.1985.tb03914.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsagris M., Tabler M., Mühlbach H. P., Sänger H. L. Linear oligomeric potato spindle tuber viroid (PSTV) RNAs are accurately processed in vitro to the monomeric circular viroid proper when incubated with a nuclear extract from healthy potato cells. EMBO J. 1987 Aug;6(8):2173–2183. doi: 10.1002/j.1460-2075.1987.tb02488.x. [DOI] [PMC free article] [PubMed] [Google Scholar]