Abstract
Male wistar rats (weighting 160-180 g) were divided into six groups of six animals per group. Groups A and F served as control. Groups B, C, D, and E received acrylamide at 20 mg/kg body weight for 28 days and groups C and E received additionally vitamin E (50 IU/kg body weight) for 1 to 28 days and 29 to 42 days of experiment, respectively. The animals from groups A, B, and C were sacrificed on day 28 of experiment and from groups D, E, and F on day 42 of experiment, respectively. The FOB (Functional Observational Battery) and histopathological changes were investigated at the end of 4th week and 6th week. FOB at the end of 4th week, of rats given acrylamide alone, or in combination with vitamin E, revealed a significant change in CNS, neuromuscular, and autonomic domains. A marked decrease in grip strength was recorded. A significant increase in foot splay, reduction in width and angle of sequential stride was noticed. Degenerative changes, necrosis, congestion, and kupffer cell proliferation in liver while vacuolar degenerative changes in tubular epithelium, coagulative necrosis, and hemorrhages in kidney were constant findings in acrylamide intoxicated rats. Neuronal degeneration, severe gliosis, congestion were found in brain. Spinal cord revealed demyelination. Acute microscopic softening of lumbar cord, bilateral necrosis with malacia and liquefaction of white matter, and loss of myelin from grey matter were seen. In the recovery period, vitamin E-treated rats revealed improvement in remyelination of spinal cord. In brain mild gliosis was seen. Thus, it appears that vitamin E is not able to protect them from acrylamide toxicity during active feeding, but after cessation of acrylamide feeding treatment with vitamin E revealed faster recovery as compared to the non-treated group.
Keywords: Acrylamide toxicity, functional observational battery, neurotoxicity, protective effect of vitamin E
INTRODUCTION
Acrylamide (CH2=CHCONH2) is a widely used industrial chemical. Acrylamide is a white, odorless, crystalline solid at room temperature with a molecular formula of C3H5NO.[1] Acrylamide has been reported to be present in plant material like potatoes, carrots, radish, lettuce, Chinese cabbage, parsley, onions, spinach and rice paddy,[2] in sugar[3] and olives.[4] Acrylamide is found in carbohydrate-rich food prepared at high temperatures such as French fries and potato chips which are consumed by humans. Consumption of these foods may result in significant human exposure to acrylamide. The formation of acrylamide is associated with the high temperature (higher than 200°C) cooking process in certain carbohydrate-rich foods, especially when asparagines react with sugar.[5] Furthermore, acrylamide can undergo oxidative biotransformation by cytochrome P450 (CYP) 2E1.[6] The resulting metabolite is an epoxide derivative, i.e. glycidamide, which is more reactive toward DNA and proteins than the parent compound, acrylamide.[7] The biological consequences of acrylamide exposure have chiefly centered on neurotoxicity ever since this effect was observed in humans occupationally exposed to this compound.[8] Subsequently, experimental exposure of rodents to acrylamide has also revealed a carcinogenic mode of action for this chemical.[4] Acrylamide also crosses placenta and passes in a significant concentration to developing fetus leading to direct prenatal and postnatal changes in rodent off- springs.[9]
Vitamin E can protect critical cellular structures against damage both from free radicals such as peroxy radical, hydroxyl radical, and super oxide, and from oxidation products such as manoldialdehyde and hydroxynonenal.[10] Vitamin E, as an important antioxidant, plays a role in inhibition of mutagen formation and repair of membranes and DNA. Therefore, it has been suggested that vitamin E may be useful in cancer prevention.[11] Vitamin E supplementation in cancer patients showed that vitamin E has an important neuroprotective effect.[12] Vitamin E has the ability to protect neuronal tissue in several neurodegenerative disorders including Alzheimer's disease.[13] The aim of this study was to determine the effect of acrylamide exposure on brain and spinal cord of rats and to assess whether these effects can be ameliorated by co-treatment with vitamin E for 4 weeks or for 2 weeks during the recovery period.
MATERIALS AND METHODS
Chemicals
Acrylamide (99.50%) and vitamin E pure were purchased from Hi Media Laboratories Pvt. Limited, Mumbai. All other required chemicals were used of extra pure grade.
Maintenance of animals
Male wistar rats weighing approximately 160-180 g were procured. The animals were acclimatized for 7 days prior to experiment. The institutional ethics committee approved the experimental protocols. All the animals used in this study were placed in cages in an air conditioned room maintained at a temperature of 25°C±30°C and 12 h light and dark schedule. Throughout the experiment, the animals were provided standard food pellet (Ashirwad industries, Nagpur) and water ad libitum. Essential cleanliness conditions were also maintained.
The LD50 for a single oral dose in rats, guinea pigs, and rabbits about 150-180 mg/kg B.W.[8] based on the results of study conducted earlier in our laboratory to study to the neuroprotective effect of vitamin E during sub acute toxicity the dose of acrylamide was decide to be 20 mg/kg body weight.[14]
Experimental protocol
Experiment was designed for the duration of 28 days to study sub-acute toxicity of acrylamide as per OECD guidelines.
Wistar rats were divided into six groups of six animals per group and the groups were as follows:
Group A – Control animals (received vehicle, no treatment 28 days).
Group B – Acrylamide (20 mg/kg body weight for 28 days).
Group C – Acrylamide (20 mg/kg body weight) + vitamin E (50 IU/kg body weight) for 28 days.
Group D – Acrylamide (20 mg/kg body weight for 28 days, 29 days onward stop treatment).
Group E – Acrylamide (20 mg/kg body weight for 28 days) and 29 days onward vitamin E (50 IU/kg body weight) up to 42 days.
Group F – Control animals (received vehicle, no treatment 42 days).
All the above groups were treated once daily. Blood samples were collected for the serum biochemical assay from retro- orbital plexus at days 28 and 42 of experiment from groups A, B, C and D, E, F, respectively. All the animals from groups A, B, C and D, E, F were sacrificed on days 28 and 42, respectively. Brain, spinal cord, liver, and kidney samples were collected and fixed in neutral formal saline for histopathological examinations.[15]
Neurobehavioral functional observational battery
The FOB battery was developed as a simple and objective way to screen for preliminary disturbances in the neurobehavioral make up of animals. The FOB parameters were studied as per protocols laid down earlier.[16] The FOB consisted of side cage observation (30 sec); open field observation (2 min); evaluation of handling, reflexes, gait scoring and grip strength of the fore limbs and hind limbs. Temperature and body weight were measured. Gait analysis was based on the measurements of the distances and angles between the sequential foot strides after walking in a corridor. Landing foot splay was the distance between the inner surfaces of the fourth digits of each foot after the animals were dropped from a 30 cm height.
Statistical analysis
Data generated are expressed as the mean ± SE. Statistical analysis was done using one-way ANOVA.[17] The level of significance was set at P < 0.05.
RESULTS AND DISCUSSION
Toxic manifestation
All the experimental animals were observed for toxic manifestations if any, daily morning and evening from the starting day of experiment till last day. On day 12, two animals from groups B and C, three and two animals from groups D and E respectively started showing splaying of hind limbs. Two days later, along with this weakness loss of pain sensation was noticed in these groups. On day 19, dragging of hind limbs was seen in animals from groups B, D, and E and on the next day animals from these groups rested on hind limbs and were unable to walk. On day 25, animals from groups B and C showed splaying of hind limbs, weakness of hind limbs along with paralytic signs, and dragging of hind limbs. On day 36, two animals from groups D and E showed improvement and well coordination. On day 38, four animals from group D and five animals from group E showed improvement and well coordination in walking and splaying hind limbs. Similar signs were seen on the last day of experiment. The weakness, splaying, dragging of hind limbs and in coordination of movements encountered in the present study were also reported earlier.[18,19] However, symptoms like tremors, bleeding, spots of blood, and cuts on skin along with general hair loss from the body, especially on the face region in the mice of 60 day study was reported earlier.[18] In the recovery period of study, rats showed improvement in dragging of hind limbs and incoordination of movement in group E animals receiving vitamin E. Attenuation of demyelination and neuroprotection with vitamin E was reported in aluminum neurotoxicity and ethidium bromide toxicity in rats.[20,21] We could also report a similar protective effect of vitamin E during acrylamide induced toxicity in brain and spinal cord tissue.
Simple neurobehavioral functional observational battery and gait analysis
Observations of the central nervous system domain from all the treatment groups in both periods of observations are presented [Table 1]. Observations of neuromuscular domain from all treatment groups at both periods of observations are presented [Table 2]. To study autonomic domain urine pools (number of times of urination by each animal from each treatment group) and fecal pellets (number of pellets passed by each animal from each treatment group) during FOB, rectal temperature was recorded at days 28 and 42 of experiment (data not shown).
Table 1.
Mean values of CNS domains during FOB in rats exposed to acrylamide and vitamin E for 28 and 42 days
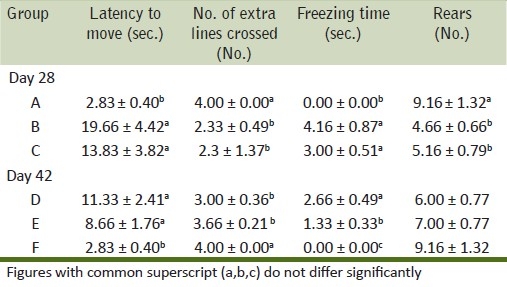
Table 2.
Mean values of neuromuscular domains in rats exposed to acrylamide and vitamin E for days 28 and 42
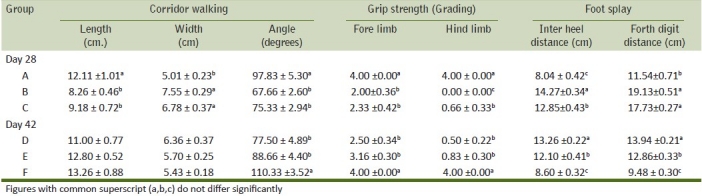
Increase in latency to move and freezing time was noticed in toxicated rats during the present study. A decrease in number of squares, extra lines crossed, and rears made by rats during FOB was recorded. An increase in freezing time, a decrease in the number of squares crossed, and a decrease in the angle of stride in a dose-dependent manner were in correlation with the findings made[16] but differ in length and width of stride from his findings. Landing foot splay showed changes in acrylamide administered to rats. An increase in inter heel and fourth digits distance responsible for ascertaining the degree of landing foot splay and gait disturbances and a significant decrease in fore and hind limb grip strength recorded in the present study were similar to the earlier observations.[16] Gait abnormalities recorded in the present study confirm the earlier observations.[21] Manifestation of neurological effects may be due to degeneration in spinal cord as observed in the present study. During simultaneous dosing of acrylamide and vitamin E, the ameliorative effect was not noticed prominently on the FOB parameters studied. In the recovery period of study, rat of group E showed improvement in simple neurobehavioral FOB and gait analysis which received vitamin E additionally. This might be the result of the neuroprotective effect of vitamin E as also observed earlier in aluminum neurotoxicity and ethidium bromide toxicity in rats.[20,21]
Histopathological findings
Liver
Sections of liver from control group A (day 28) and group F (day 42) revealed normal orientation of hepatic parenchyma. Sections of liver from group B revealed vacuolar and granular degenerative changes [Figure 1]. Liver sections from group C showed similar changes as that of groups B; additionally, congestion and Kupffer cell proliferation were noticed in liver [Figure 2]. Sections of liver from group D showed vacuolar and granular degenerative changes [Figure 3]. Liver sections from group E showed proliferation of Kupffer cells [Figure 4] and severity of degenerative changes was comparatively milder than group D. Reduced glutathione (reduced cytochrome P 450 concentration) in hepatocytes after acrylamide treatment is responsible for liver damage.[22] During the recovery period of observations, vitamin E-treated rats exhibited normal arrangement of hepatocyte and better tissue integrity than rats received none. Protection of liver tissue by vitamin E during malathion induced toxicity in rats is documented earlier.[23,24]
Figure 1.
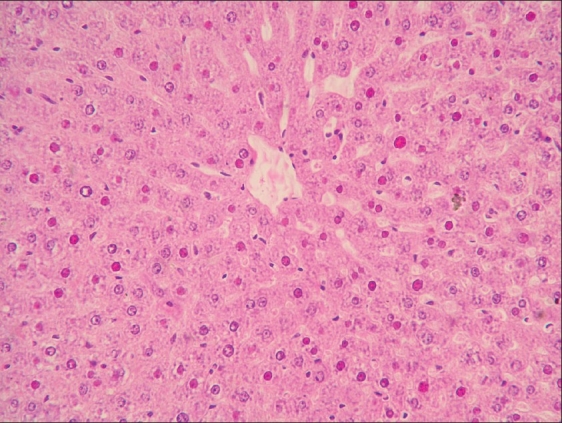
Focal necrosis, hemorrhages, and Kupffer cell proliferation in liver of rat from group B (H and E, ×200)
Figure 2.
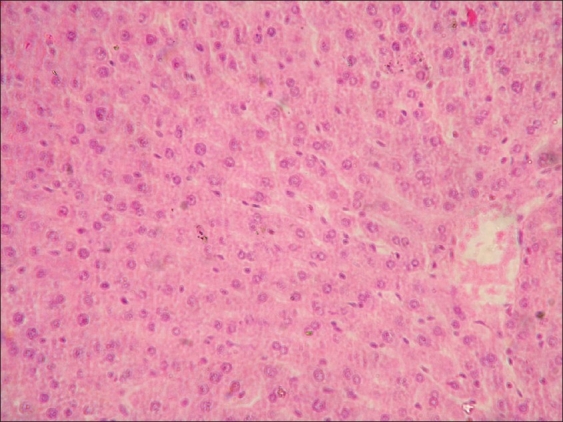
Focal necrosis, in liver of rat from group C (H and E, ×200)
Figure 3.
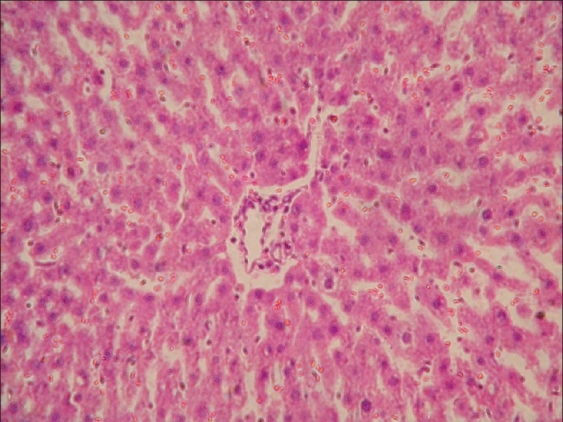
Focal degenerative changes and mild hemorrhages in liver of rat from group D (H and E, ×200)
Figure 4.
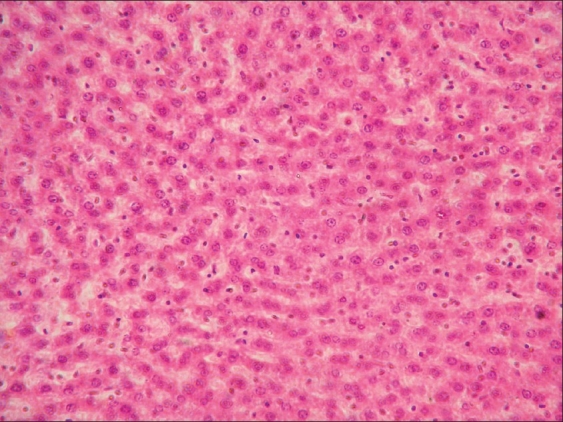
Focal degenerative changes in liver of rat from group E (H and E, ×200)
Kidney
Microscopically, the lesions in the kidney from group B had swelling of tubular epithelium with coagulative necrosis, vacuolation, and congestion of glomeruli [Figure 5]. Degenerative changes observed were mainly restricted to proximal convoluted tubules. Similar but less extensive lesions were observed in group C. Hemorrhages were seen in the sections from group C [Figure 6]. Similar observations were recorded 14 days after cessation of feeding of acrylamide in group D. The lesions were less intense in sections from group E which received vitamin E during the recovery period. Degeneration of the convoluted tubular epithelium observed during the present study confirms the earlier observations[8] who reported glomerular degeneration at higher dose rate (200 mg/kg B.W.) of acrylamide in guinea pig.
Figure 5.
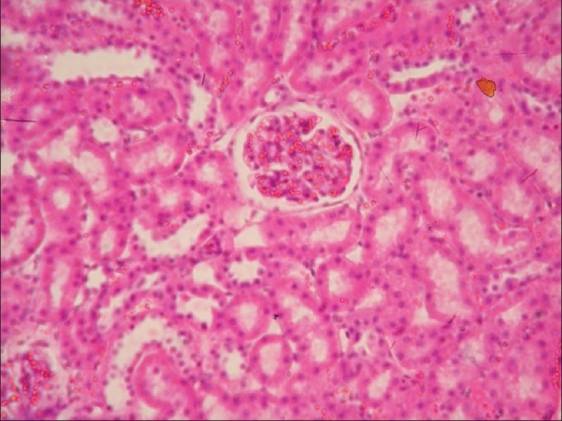
Glomeruli congestion and necrosis of tubular epithelial cells in kidney of rat from group B (H and E, ×200)
Figure 6.
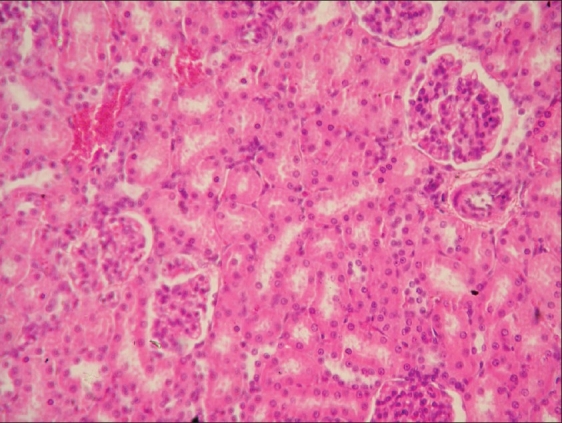
Necrosis of tubular epithelial cells in kidney of rat from group C (H and E, ×200)
Brain and spinal cord
Histopathological examination of brain from group B revealed necrosis, foamy appearance of cytoplasm, and severe gliosis, neuronal degeneration, demyelination, separation of nerve fibers, aggregation of glial cells and necrosis [Figure 7] and group C [Figure 8]. The lesions were continued further and 14 days after cessation of feeding of acrylamide similar observations were seen in brain sections of group D [Figure 9]. Changes were comparatively less severe in group E [Figure 10] as compared to groups D. Sections of spinal cord from group B [Figure 11] and C [Figure 12] revealed degenerative process and demyelination of nerve fibers at day 28 of experiment. However, gray matter was relatively intact. The spinal cords from group D lesions from these groups were suggestive of poliomalacia. During the later period of observation, spinal cord revealed acute microscopic softening of lumbar cord and bilateral necrosis with malacia and liquefaction of white matter. Gray matter also revealed loss of myelin. In D group, bilateral demyelination and softening mainly of white matter was observed [Figure 13]. There was malacia and liquefaction leading to complete necrosis of white matter of spinal cord. Group E showed normal orientation of gray matter of spinal cord [Figure 14] and less severity of white matter lesions as compared to group D. There was no inflammatory reaction and hemorrhages in spinal cord of animals from treatment group E.
Figure 7.
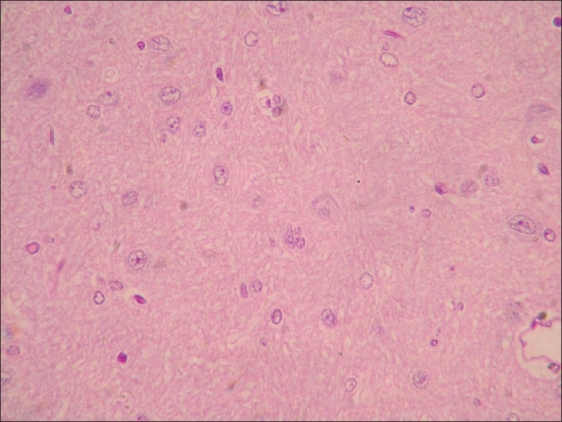
Neuronal degeneration in brain of rat from group B (H and E, ×200)
Figure 8.
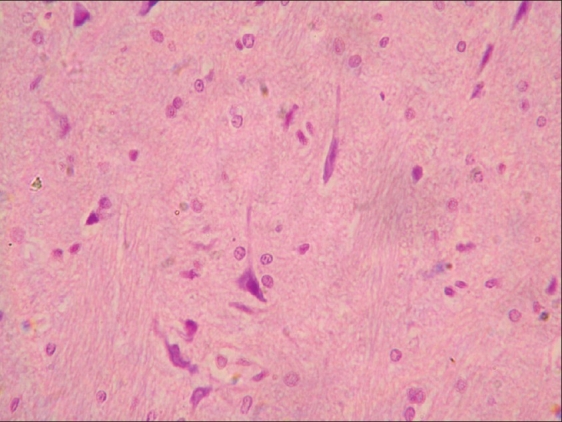
Neuronal degeneration and gliosis in brain of rat from group C (H and E, ×200)
Figure 9.
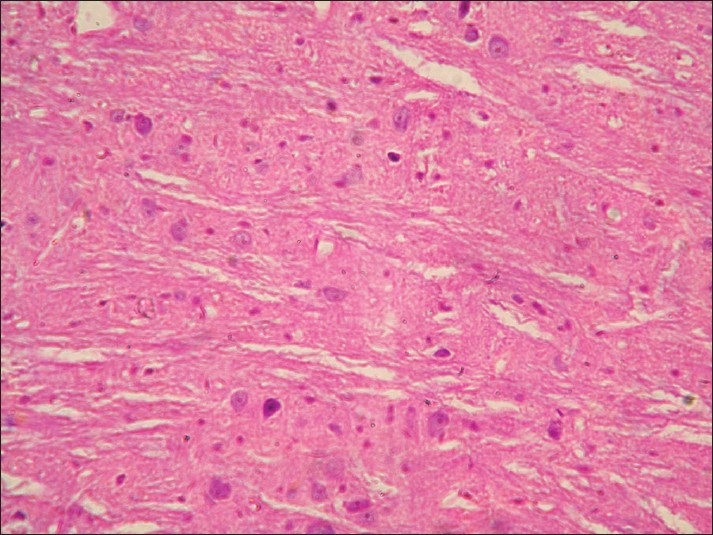
Neuronal degeneration and necrosis of neurons in brain of rat from group D (H and E, ×200)
Figure 10.
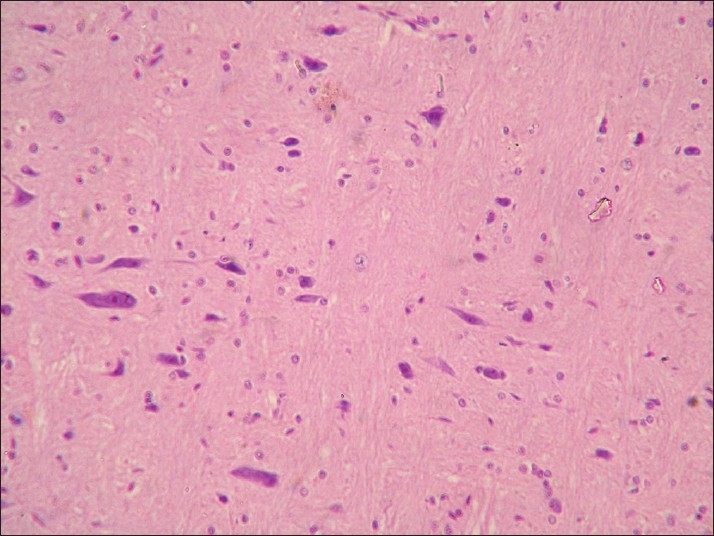
Neuronal degeneration and glial cell proliferation in brain of rat from group E (H and E, ×200)
Figure 11.
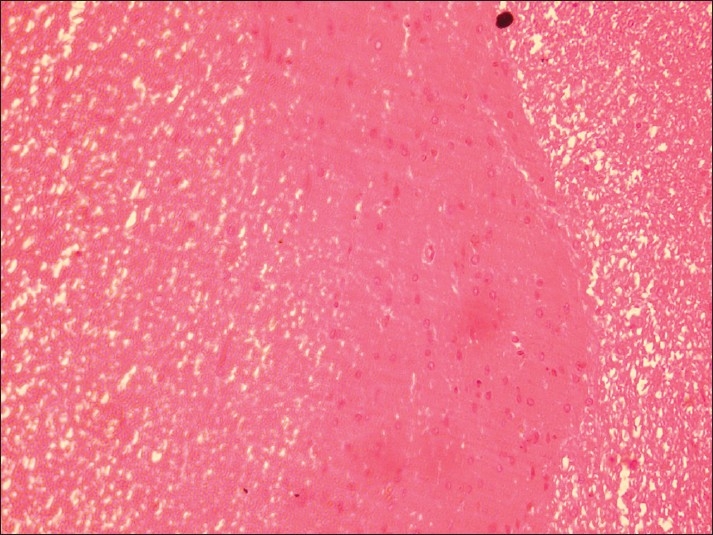
Spinal cord of rat from group B showing demyelination of gray matter and liquefaction of white matter (H and E, ×200)
Figure 12.
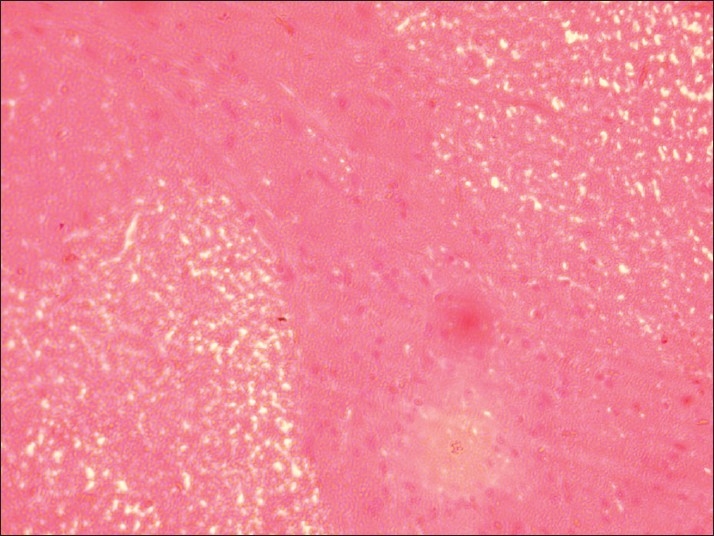
Spinal cord of rat from group C showing necrosis and demyelination of gray matter (H and E, ×200)
Figure 13.
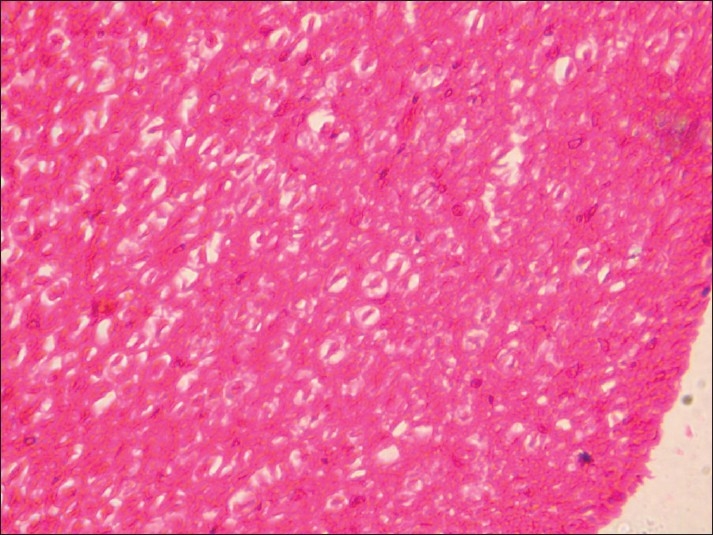
Spinal cord of rat from group D showing necrosis, liquefaction, and demyelination of white matter (H and E, ×200)
Figure 14.
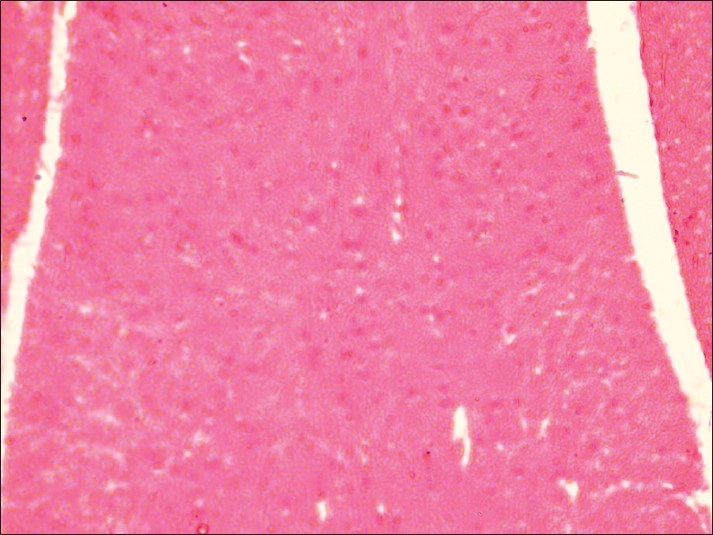
Spinal cord of rat from group E showing demyelination of gray matter (H and E, ×200)
Neuronal degeneration in acrylamide intoxicated rats was reported earlier. Better tissue integrity was seen in group E receiving vitamin E as compared to group D. Similar changes in the sections of brain[21] and spinal cord[22,25,26] in rats intoxicated with acrylamide were also reported earlier. However, no histological abnormalities were reported in the spinal cord in rats receiving acrylamide on alternate days.[20,27] This demonstrated that vitamin E inhibits aluminum-induced activation of glial cell and pro inflammatory cytokine expression. The finding of the recovery period suggests that a group treated with vitamin E exhibited remyelination of spinal cord in gray matter but not in brain. Significant increased myelin secretion in the rats treated with vitamin E after ethdium bromide-induced demyelination.[21] Vitamin E reduced the level of caspase-3 which is responsible for demyelination. Protection of nervous tissue by vitamin E at higher dose rate of 150 mg/kg/day during chlorpyrifos-induced toxicity within 7 days was reported earlier.[28] During the present experiment we used lower dose of vitamin E which probably was not sufficient to completely protect the nervous tissue from the damage caused by acrylamide in rats in spite of 14 days feeding during recovery period.
In conclusion, the study revealed that acrylamide induces lesions in spinal cord and liver. In turn there is paralytic signs and splaying and dragging of hind limbs. Vitamin E in not able to protect spinal cord and liver from acrylamide toxicity during active feeding, but after cessation of acrylamide feeding treatment with vitamin E revealed faster recovery as compared to the non-treated group.
ACKNOWLEDGMENT
Research presented in this manuscript was supported by grant from Department of Veterinary Pathology, Nagpur Veterinary College, Nagpur, India.
Footnotes
Source of Support: Department of Veterinary Pathology, Nagpur Veterinary College, Nagpur, India.
Conflict of Interest: None declared.
REFERENCES
- 1.Grivas S, Jagerstad M, Lingnert H, Skog K, Tornqvist M, Aman P. Sweden: Swedish National Food Administration; 2002. Acrylamide in food mechanisms of formation and influencing factors during heating of foods. [Google Scholar]
- 2.Arikawa A, Shiga M. Determination of trace acrylamide in the crops by gas chromatography. Bunseki Kagaku. 1980;29:33–9. Chem. Abstr., 93: 202742. [Google Scholar]
- 3.Schultzova J, Tekel J. Acrylamide monomer occurrence in sugar.Dtsch Lebensm -Rundsch. 1996;92:281–2. Chem Abstr, 125: 326762. [Google Scholar]
- 4.Friedman M. Chemistry, biochemistry, and safety of acrylamide:A review. J Agric Food Chem. 2003;51:4504–26. doi: 10.1021/jf030204+. [DOI] [PubMed] [Google Scholar]
- 5.Rydberg P, Eriksson S, Tareke E, Karlsson P, Ehrenberg L, Törnqvist M. Investigations of factors that influnce the acrylamide content of heated foodstuffs. J Agric Food Chem. 2003;51:7012–8. doi: 10.1021/jf034649+. [DOI] [PubMed] [Google Scholar]
- 6.Sumner SC, Fennell TR, Moore TA, Chanas B, Gonzalez F, Ghanayem BI. Role of cytochrome P450 2E1 in the metabolism of acrylamide and acrylonitrile in mice. Chem Res Toxicol. 1999;12:1110–6. doi: 10.1021/tx990040k. [DOI] [PubMed] [Google Scholar]
- 7.Dearfield KL, Douglas GR, Ehling UH, Moore MM, Sega GA, Brusick DJ. Acrylamide: a review of its genotoxicity and an assessment of heritable genetic risk. Mutat Res. 1995;330:71–99. doi: 10.1016/0027-5107(95)00037-j. [DOI] [PubMed] [Google Scholar]
- 8.Mccollister DD, Oyen F, Rowe VK. Toxicology of acrylamide. Ther Ggw. 1964;103:172–81. doi: 10.1016/0041-008x(64)90103-6. [DOI] [PubMed] [Google Scholar]
- 9.Ewards PM. The insensitivity of the developing rat fetus to the toxic effects of acrylamide. Chem Biol Interact. 1976;12:13–8. doi: 10.1016/0009-2797(76)90062-4. [DOI] [PubMed] [Google Scholar]
- 10.Erin AN, Spirin MM, Tabidze LV, Kagan VE. Formation of alpha-tocopherol complexes with fatty acids.A hypothetical mechanism of stabilization of biomembranes by vitamin E. Biochim Biophys Acta. 1984;774:96–102. doi: 10.1016/0005-2736(84)90279-7. [DOI] [PubMed] [Google Scholar]
- 11.London RS, Murphy L, Kitlowski KE. Breast cancer prevention by supplemental vitamin E. J Am Coll Nutr, 1985;4:559–64. doi: 10.1080/07315724.1985.10720098. [DOI] [PubMed] [Google Scholar]
- 12.Pace A, Savarese A, Picardo M, Maresca V, Pacetti U, Del Monte G, et al. Neuroprotecteve effect of Vitamin E supplementation in patiens treated with cisplantin chemotherapy. J Clin Oncol. 2003;21:927–31. doi: 10.1200/JCO.2003.05.139. [DOI] [PubMed] [Google Scholar]
- 13.Sano M, Ernesto C, Thomas RG, Klauber MR, Schafer K, Grundman M, et al. A controlled trial of selegiline, alpha-tocopherol, or both as treatment for Alzheimer's disease.The Alzheimer's Disease Cooperative Study. N Engl J Med. 1997;336:1216–22. doi: 10.1056/NEJM199704243361704. [DOI] [PubMed] [Google Scholar]
- 14.Jangir B. Nagpur: Maharastra Animal and Fishery Sciences University; 2009. Toxicopathological and genotoxicity effect of acrylamide toxicity in Wistar rats. M.V.Sc thesis. [Google Scholar]
- 15.Luna AG. 3rd ed. London: Mc Graw Hill Book Co; 1968. Manaul of histological staining methods of the Armed Force Institute of Pathology; pp. 124–5. [Google Scholar]
- 16.Youssef AF, Santi BW. Simple neurobehavioural functional observational battery and objective gait analysis validation by the use of acrylamide and methanol with a built-in recovery period. Environ Res. 1997;73:52–62. doi: 10.1006/enrs.1997.3718. [DOI] [PubMed] [Google Scholar]
- 17.Snecdecor GW, Cochron WG. 6th ed. Oxford: Oxford and IBH; 1994. Statistical methods. [Google Scholar]
- 18.Yousef MI, El-Demerdash FM. Acrylamide-induced oxidative stress and biochemical perturbation in rats. Toxicology. 2006;219:133–41. doi: 10.1016/j.tox.2005.11.008. [DOI] [PubMed] [Google Scholar]
- 19.Sharma A, Sharma R, Jain J. Biochemical changes in the liver of Swiss albino mice orally exposed to acrylamide. Maejo International Journal of Science and Technology. 2008;2:542–50. [Google Scholar]
- 20.Nedzvetsky VS, Tuzcu M, Yasar A, Tikhomirov AA, Baydas G. Effects of vitamin E against aluminum neurotoxicity in rats. Biochemistry (Mosc) 2006;71:239–44. doi: 10.1134/s0006297906030023. [DOI] [PubMed] [Google Scholar]
- 21.Goudarzvand M, Javan M, Mirnajafi-Zadeh J, Mozafari S, Tiraihi T. Vitamins E and D3 attenuate demyelination and potentiate remyelination processes of hippocampal formation of rats following local injection of ethidium bromide. Cell Mol Neurobiol. 2010;30:289–99. doi: 10.1007/s10571-009-9451-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lehning EJ, Balaban CD, Ross JF, LoPachi RM. Acrylamide neuropathy. II. Spatiotemporal characteristics of nerve cell damage in brainstem and spinal cord. Neurotoxicology. 2002;23:415–29. doi: 10.1016/s0161-813x(02)00080-3. [DOI] [PubMed] [Google Scholar]
- 23.Awad ME, Abdel-Rahman MS, Hassan SA. Acrylamide toxicity in isolated rat hepatocytes. Toxicol in Vitro. 1998;12:699–704. doi: 10.1016/s0887-2333(98)00051-4. [DOI] [PubMed] [Google Scholar]
- 24.Kalender S, Uzun FG, Durak D, Demir F, Kalender Y. Malathion-induced hepatoxicity in rats: The effects of vitamin C and E. Food Chem Toxicol. 2010;48:633–8. doi: 10.1016/j.fct.2009.11.044. [DOI] [PubMed] [Google Scholar]
- 25.Schulze GE, Boysen BG. A neurotoxicity screening battery for use in safety evaluation: effects of acrylamide and 3’, 3’-iminodipropionitrile. Fundam Appl Toxicol. 1991;16:602–15. doi: 10.1016/0272-0590(91)90099-p. [DOI] [PubMed] [Google Scholar]
- 26.Crofton KM, Padilla S, Tilson HA, Anthony DC, Raymer JH, MacPhail RC. The impact of dose rate on the neurotoxicity of acrylamide: the interaction of administered dose, target tissue concentrations, tissue damage, and functional effects. Toxicol Appl Pharmacol. 1996;139:163–76. doi: 10.1006/taap.1996.0155. [DOI] [PubMed] [Google Scholar]
- 27.Gipon L, Schotman P, Jennekens FG, Gispen WH. Polyneuropathies and CNS protein metabolism.Description of the acrylamide syndrome in rats. Neuropathol Appl Neurobiol. 1977;3:115–23. [Google Scholar]
- 28.Hossary EI GG, Mansour SM, Mohamed AS. Neurotoxic effects of Chloropyrifos and Possible Protective Role of Antioxidant Supplements: an Experimental Study. J Appl Sci Res. 2009;9:1218–22. [Google Scholar]


