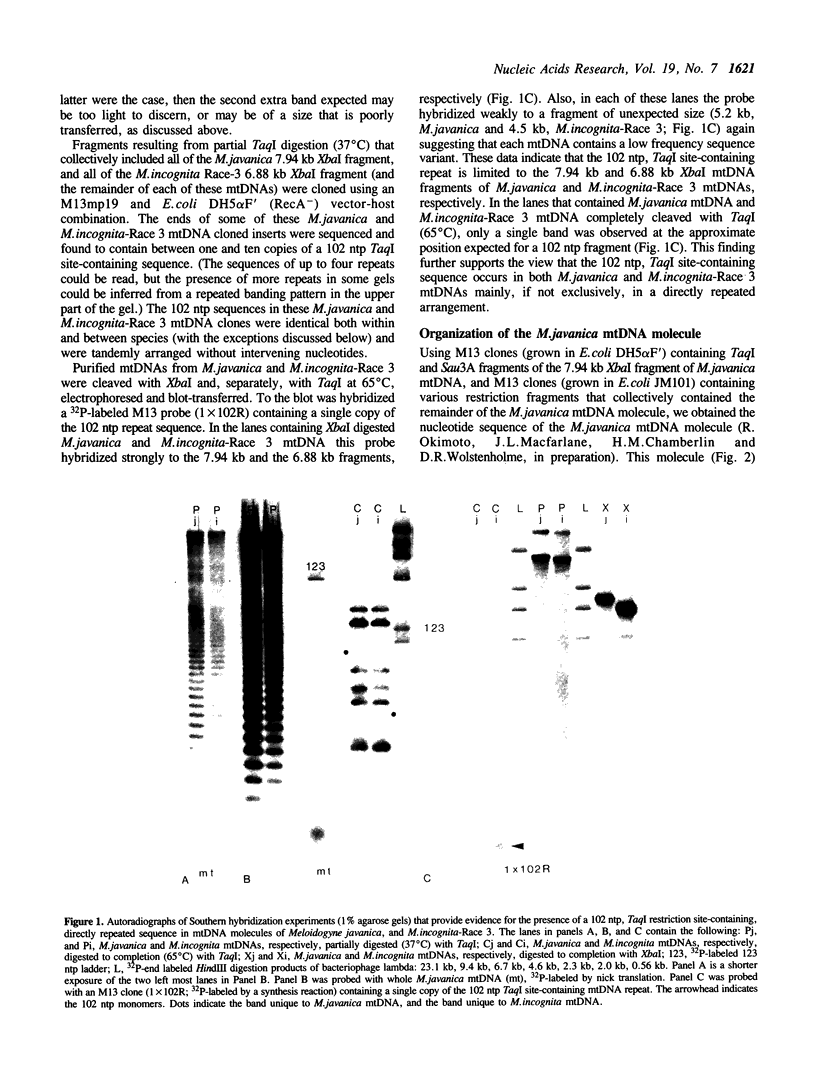
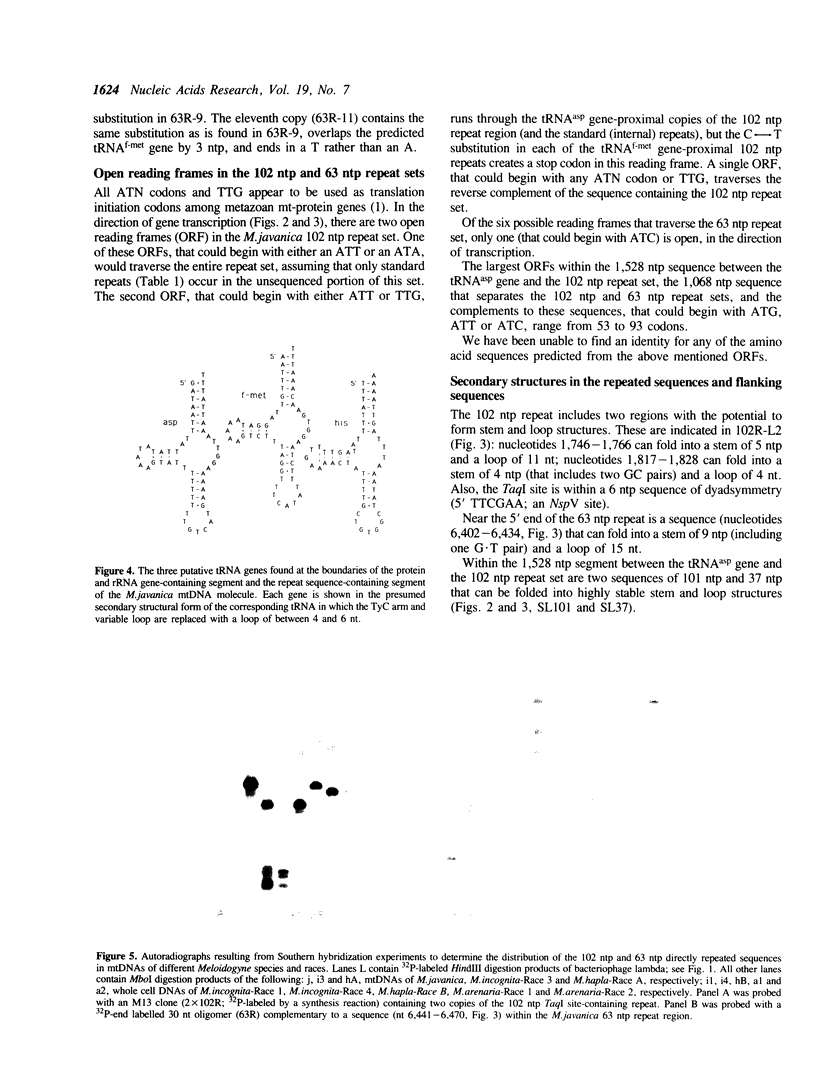
Abstract
Within a 7 kb segment of the mtDNA molecule of the root knot nematode, Meloidogyne javanica, that lacks standard mitochondrial genes, are three sets of strictly tandemly arranged, direct repeat sequences: approximately 36 copies of a 102 ntp sequence that contains a TaqI site; 11 copies of a 63 ntp sequence, and 5 copies of an 8 ntp sequence. The 7 kb repeat-containing segment is bounded by putative tRNAasp and tRNAf-met genes and the arrangement of sequences within this segment is: the tRNAasp gene; a unique 1,528 ntp segment that contains two highly stable hairpin-forming sequences; the 102 ntp repeat set; the 8 ntp repeat set; a unique 1,068 ntp segment; the 63 ntp repeat set; and the tRNAf-met gene. The nucleotide sequences of the 102 ntp copies and the 63 ntp copies have been conserved among the species examined. Data from Southern hybridization experiments indicate that 102 ntp and 63 ntp repeats occur in the mtDNAs of three, two and two races of M.incognita, M.hapla and M.arenaria, respectively. Nucleotide sequences of the M.incognita Race-3 102 ntp repeat were found to be either identical or highly similar to those of the M.javanica 102 ntp repeat. Differences in migration distance and number of 102 ntp repeat-containing bands seen in Southern hybridization autoradiographs of restriction-digested mtDNAs of M.javanica and the different host races of M.incognita, M.hapla and M.arenaria are sufficient to distinguish the different host races of each species.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bentzen P., Leggett W. C., Brown G. G. Length and restriction site heteroplasmy in the mitochondrial DNA of american shad (alosa sapidissima). Genetics. 1988 Mar;118(3):509–518. doi: 10.1093/genetics/118.3.509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyce T. M., Zwick M. E., Aquadro C. F. Mitochondrial DNA in the bark weevils: size, structure and heteroplasmy. Genetics. 1989 Dec;123(4):825–836. doi: 10.1093/genetics/123.4.825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buroker N. E., Brown J. R., Gilbert T. A., O'Hara P. J., Beckenbach A. T., Thomas W. K., Smith M. J. Length heteroplasmy of sturgeon mitochondrial DNA: an illegitimate elongation model. Genetics. 1990 Jan;124(1):157–163. doi: 10.1093/genetics/124.1.157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clayton D. A. Replication of animal mitochondrial DNA. Cell. 1982 Apr;28(4):693–705. doi: 10.1016/0092-8674(82)90049-6. [DOI] [PubMed] [Google Scholar]
- Clayton D. A. Transcription of the mammalian mitochondrial genome. Annu Rev Biochem. 1984;53:573–594. doi: 10.1146/annurev.bi.53.070184.003041. [DOI] [PubMed] [Google Scholar]
- Dale R. M., McClure B. A., Houchins J. P. A rapid single-stranded cloning strategy for producing a sequential series of overlapping clones for use in DNA sequencing: application to sequencing the corn mitochondrial 18 S rDNA. Plasmid. 1985 Jan;13(1):31–40. doi: 10.1016/0147-619x(85)90053-8. [DOI] [PubMed] [Google Scholar]
- Densmore L. D., Wright J. W., Brown W. M. Length variation and heteroplasmy are frequent in mitochondrial DNA from parthenogenetic and bisexual lizards (genus Cnemidophorus). Genetics. 1985 Aug;110(4):689–707. doi: 10.1093/genetics/110.4.689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desjardins P., Morais R. Sequence and gene organization of the chicken mitochondrial genome. A novel gene order in higher vertebrates. J Mol Biol. 1990 Apr 20;212(4):599–634. doi: 10.1016/0022-2836(90)90225-B. [DOI] [PubMed] [Google Scholar]
- Fauron C. M., Wolstenholme D. R. Structural heterogeneity of mitochondrial DNA molecules within the genus Drosophila. Proc Natl Acad Sci U S A. 1976 Oct;73(10):3623–3627. doi: 10.1073/pnas.73.10.3623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hyman B. C., Beck J. L., Weiss K. C. Sequence amplification and gene rearrangement in parasitic nematode mitochondrial DNA. Genetics. 1988 Nov;120(3):707–712. doi: 10.1093/genetics/120.3.707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobs H. T., Elliott D. J., Math V. B., Farquharson A. Nucleotide sequence and gene organization of sea urchin mitochondrial DNA. J Mol Biol. 1988 Jul 20;202(2):185–217. doi: 10.1016/0022-2836(88)90452-4. [DOI] [PubMed] [Google Scholar]
- La Roche J., Snyder M., Cook D. I., Fuller K., Zouros E. Molecular characterization of a repeat element causing large-scale size variation in the mitochondrial DNA of the sea scallop Placopecten magellanicus. Mol Biol Evol. 1990 Jan;7(1):45–64. doi: 10.1093/oxfordjournals.molbev.a040586. [DOI] [PubMed] [Google Scholar]
- Moritz C., Brown W. M. Tandem duplication of D-loop and ribosomal RNA sequences in lizard mitochondrial DNA. Science. 1986 Sep 26;233(4771):1425–1427. doi: 10.1126/science.3018925. [DOI] [PubMed] [Google Scholar]
- Moritz C., Brown W. M. Tandem duplications in animal mitochondrial DNAs: variation in incidence and gene content among lizards. Proc Natl Acad Sci U S A. 1987 Oct;84(20):7183–7187. doi: 10.1073/pnas.84.20.7183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okimoto R., Macfarlane J. L., Wolstenholme D. R. Evidence for the frequent use of TTG as the translation initiation codon of mitochondrial protein genes in the nematodes, Ascaris suum and Caenorhabditis elegans. Nucleic Acids Res. 1990 Oct 25;18(20):6113–6118. doi: 10.1093/nar/18.20.6113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okimoto R., Wolstenholme D. R. A set of tRNAs that lack either the T psi C arm or the dihydrouridine arm: towards a minimal tRNA adaptor. EMBO J. 1990 Oct;9(10):3405–3411. doi: 10.1002/j.1460-2075.1990.tb07542.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rand D. M., Harrison R. G. Molecular population genetics of mtDNA size variation in crickets. Genetics. 1989 Mar;121(3):551–569. doi: 10.1093/genetics/121.3.551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roe B. A., Ma D. P., Wilson R. K., Wong J. F. The complete nucleotide sequence of the Xenopus laevis mitochondrial genome. J Biol Chem. 1985 Aug 15;260(17):9759–9774. [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wahleithner J. A., Wolstenholme D. R. Mitochondrial plasmid DNAs of broad bean: nucleotide sequences, complex secondary structures, and transcription. Curr Genet. 1987;12(1):55–67. doi: 10.1007/BF00420728. [DOI] [PubMed] [Google Scholar]
- Wallis G. P. Mitochondrial DNA insertion polymorphism and germ line heteroplasmy in the Triturus cristatus complex. Heredity (Edinb) 1987 Apr;58(Pt 2):229–238. doi: 10.1038/hdy.1987.37. [DOI] [PubMed] [Google Scholar]
- Wolstenholme D. R., Fauron C. M. A partial map of the circular mitochondrial genome of Drosophila melanogaster. Location of EcoRI-sensitive sites and the adenine-thymine-rich region. J Cell Biol. 1976 Nov;71(2):434–448. doi: 10.1083/jcb.71.2.434. [DOI] [PMC free article] [PubMed] [Google Scholar]