Abstract
Herbal and natural products have been used in folk medicine for centuries throughout the world. There has been renewed interest in screening higher plants for novel biologically active compounds, particularly those that effectively intervene in human ailments in the field of chronic diseases. The present study has been taken up to evaluate the free radical scavenging activity and tumor cell suppression potential of Premna serratifolia leaf in various in vitro model systems. The methanolic extract of P. serratifolia leaf was obtained by soxhlet extraction method. The superoxide radical scavenging activity, nitric oxide radical, hydroxyl radical, DPPH radical and ABTS radical scavenging activity and lipid peroxidation were determined. The tumor cell suppression cell potential was determined in three different cancer cell lines MCF7 (breast cancer), HepG2 (liver cancer) and A549 (lung cancer) by SRB assay. The study showed that the methanolic extract of P. serratifolia was having free radical scavenging activity against superoxide radical, nitric oxide radical, hydroxyl radical, DPPH radical, ABTS radical and inhibition of lipid peroxidation. The IC50 value showed the efficacy was dose dependent. The test extract showed cytotoxic activity against MCF7, HepG2 and A549 cells. The GI50, TGI and LC50 values were determined against each cell line and compared with standard drug Adriamycin. The present study proved the free radical scavenging activity and tumor cell suppression potential of P. serratifolia leaf in the selective in vitro model systems. The further study has to be carried out in the aspects of isolation of functional molecules of the extract.
Keywords: Antioxidants, cytotoxicity, tumor, Premna serratifolia, tumor cell
INTRODUCTION
Cancer is a major disease affecting mankind, which is responsible for millions of deaths each year worldwide. Chemotherapy is an essential strategy for the treatment of disseminated cancers. Intervention with chemopreventive agents at the early stage of carcinogenesis is theoretically more rational than attempting to eradicate fully developed tumors with chemotherapeutic drugs. However, most cancer chemotheraputants severely affect the host normal cells. Hence, the use of natural products now has been contemplated of exceptional value in the control of cancer and its eradication program.[1]
The reactive oxygen species (ROS) including superoxide radical, hydroxyl radical cause destructive and irreversible damage to the components of cell, such as lipids, proteins and DNA.[2–5] Although normal cells possess antioxidant systems against ROS, the continuous accumulation of damage to the cells induces diseases such as cancer and aging. The continuous antioxidant dose plays a preventive role against these diseases by removing the ROS in biological systems.[6] The inhibition of free radical generation can serve as facile model for evaluating the activity of anticancer agents.
Premna serratifolia Linn (family: Verbenaceae) is a large shrub or a small tree up to 9 m in height with yellowish lenticeolate bark, spinous large branches and yellowish brown woody aromatic root, leaves simple, opposite, sometimes whorled, elliptic-ovate, membranous, primary lateral nerves 4-6 pairs, flowers small. The plant parts are used in neuralgia, inflammations, cardiac disorders, hepatopathy, cough, asthma, bronchitis, flatulence, tumors, hemorrhoids and general debility.[7] Based on the usage of plant in the traditional medicine system, the present study has been designed to evaluate the antioxidant and tumor cell suppression potential of methanolic extract of P. serratifolia leaf in different in vitro model systems.
MATERIALS AND METHODS
Collection of plant and extraction
The plant material was collected from the Western Ghat region and authenticated by the Pharmacy Division of NRIP, Cheruthuruthy. Leaf of P. serratifolia was cut in to small pieces and shade dried for three days. The coarse powdered material (50 gm/ thimble) were extracted with methanol using Soxhlet apparatus for 8–10 hr. The methanol extract was completely evaporated at 40°C under vacuum using a rotary evaporator. The residue, thus obtained was stored in sterile vials in refrigerator for the further use.
Chemicals and reagents
All chemicals and solvents were of analytical grade and were obtained from HiMedia Chemicals, Mumbai, India. 2,2-azinobis(3-ethylbenzq-thiozoline-6-sulphonate) (ABTS) was obtained from Sigma Chemicals, USA. The other chemical used were 1,1- diphenyl, 2-picryl hydrazyl (DDPH), sodium nitropursside, sulphanilamide, o-phosphoric acid, napthyl ethylene diamine dihydrochloride, ferrous sulfate (FeSO4), thiobarbituric acid (TBA), trichloroacetic acid (TCA), nitroblue tetrazolium (NBT) and ethylene diamine tetra-acetic acid.
DPPH radical activity
DPPH scavenging activity was measured by the spectrophotometric method.[8] To a methanolic solution of DPPH (200 μM), 0.05 ml of the test compounds dissolved in methanol were added at different concentrations (50-250 μg/ml). An equal amount of methanol was added to the control. After 20 min, the decrease in the absorbance of test mixture (due to quenching of DPPH-free radicals) was read at 517 nm and the percentage inhibition calculated by using the formula
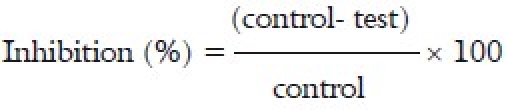
ABTS radical cation decolorization assay
ABTS radical cation was produced as per the method of Sreejayan et al.,(1996).[8] ABTS solution (7 mM) with 2.45 mM ammonium persulfate and the mixture were allowed to stand in dark at room temperature for 12-16 hrs before use. For the study, different concentrations (50-250 μg/ml) of methanolic extract (0.5 ml) were added to 0.3 ml of ABTS solution and the final volume was made up with ethanol to make 1.0 ml. The absorbance was read at 745 nm and the percentage inhibition calculated.
Scavenging of nitric oxide radical
Nitric oxide was generated form sodium nitroprusside and measured by Griess reaction.[9–12] The absorbance of the chromophore formed during diazotization of nitrite with sulphanilamide and its subsequent coupling with napthylene diamine was read at 546 nm. The experiment was repeated in triplicate.
Scavenging of hydroxyl radical
Hydroxyl radical scavenging activity was measured according to the method of Kunchadny and Rao (1990), by studying the competition between deoxyribose and test extract for hydroxyl radical generated by Fenton's reaction.[13]
Scavenging of superoxide radical
The scavenging activity toward the superoxide radical (O2-) was measured in terms of inhibition of generation of O2.[14] The absorbance was read at 530 nm before and after illumination under UV lam for 15 min against a control instead of sample.
In vitro lipid peroxidation assay
Freshly excised rat liver was processed to get 10% homogenate in cold phosphate-buffered saline, pH 7.4 using glass Teflon homogenizer and filtered to get a clear homogenate. The degree of lipid peroxidation was assayed by estimating the TBARS by using the standard method[15] with minor modifications.[16]
Tumor cell suppression potential/cytotoxicity assay
The cytotoxic assay was carried out as per the method of Vanicha vichai et al., 2006 using SRB assay.[17] The three different cancer cell lines MCF7 (breast cancer cell line), HepG2 (liver cancer cell line), and A549 (lung cancer cell line) of human origin.
Statistical analysis
Linear regression analysis was used to calculate the IC50 values.
RESULTS AND DISCUSSION
The methanolic extract of P. serratifolia was found to have a superoxide radical scavenging activity. The extract showed significant superoxide inhibiting activity in dose-dependent manner [Table 1 and Figure 1]. The extract inhibited 50% superoxide anion at the concentration of 165.31 μg/ml (IC50).
Table 1.
Free radical scavenging activity of P. serratifolia

Figure 1.
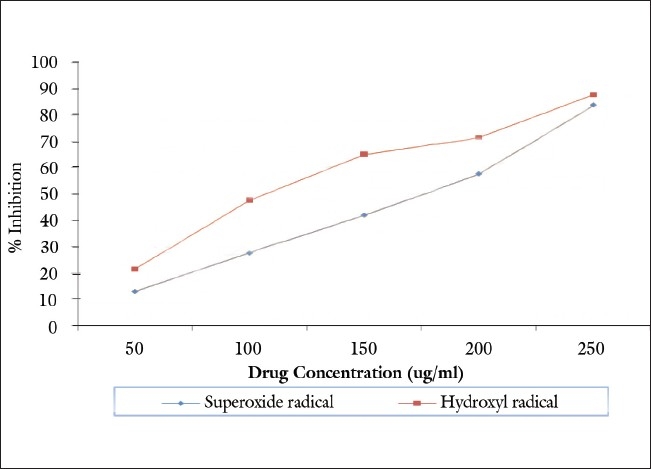
Antioxidant activity of methanolic extract of P. serratifolia
Hydroxyl radicals are the major active oxygen species causing lipid oxidation and enormous biological damage. The effect of methanolic extract of P. serratifolia on hydroxyl radical is shown in Table 1 and Figure 1. The extract exhibited radical scavenging activity by dose-dependent manner. The IC50 value for scavenging the hydroxyl radical is 122.21 μg/ml.
The DPPH radical is considered to be a model of a lipophilic radical. A chain reaction in lipophilic radicals was initiated by lipid autoxidation. The radical scavenging activity of P. serratifolia was determined from the reduction in the optical absorbance at 517 nm. Positive DPPH test suggests that the samples are free radical scavengers. The IC50 value of P. serratifolia on DPPH radical scavenging assays was found to be 101.20 μg/ml.
It is well known that nitric oxide has an important role in various types of inflammatory processes in the animal body. In the present study, crude extract of the stem bark was checked for its inhibitory effect on nitric oxide production. Figure 2 illustrates the percentage inhibition of nitric oxide generation by P. serratifolia. The concentration of P. serratifolia needed for 50% inhibition was found to be 210.21μg/ml.
Figure 2.
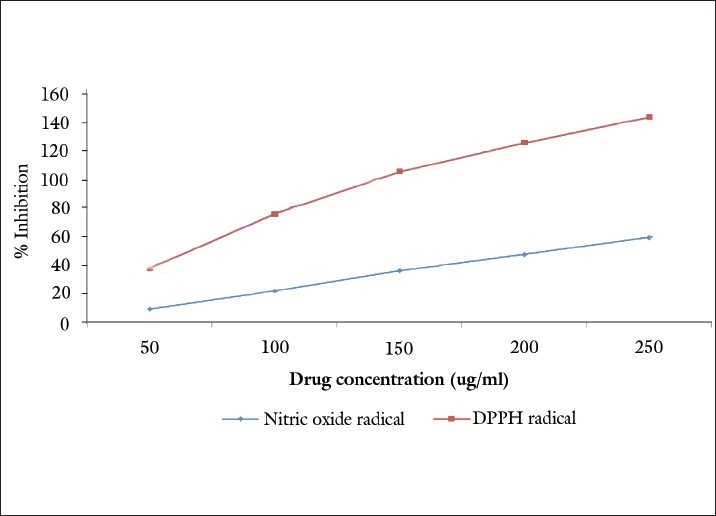
Antioxidant activity of methanolic extract of P. serratifolia
Free radicals induce lipid peroxidation in polyunsaturated lipid rich areas like brain and liver. In this study, in vitro lipid peroxidation was induced to rat liver by using FeSO4 and ascorbic acid. The generation of malondialdehyde and related substances that react with thiobarbituric acid (TBARS) was found to be inhibited by the extract [Figure 3]. This indicated the significant lipid peroxidation inhibiting activity of the extract. The IC50 was found to be 102.12 μg/ml.
Figure 3.
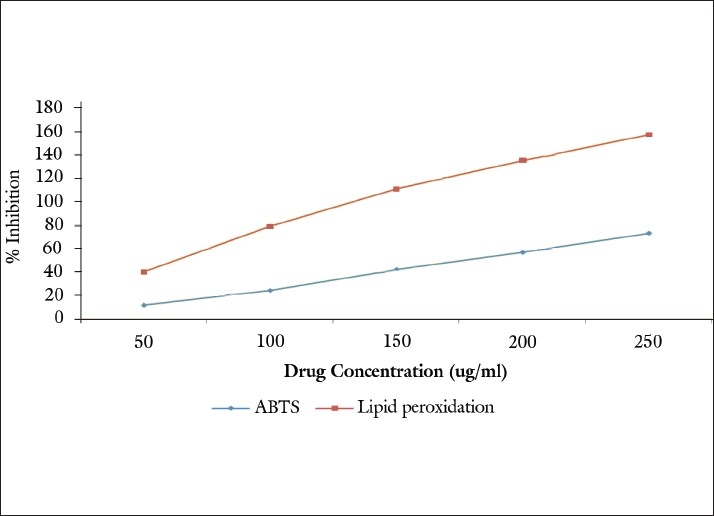
Antioxidant activity of methanolic extract of P. serratifolia
The ABTS assay is based on the inhibition of the absorbance of the radical cation ABTS.+, which has a characteristic long wavelength absorption spectrum. The results imply that the extract of P. serratifolia inhibit or scavenge the ABTS.+ radicals, the IC50 value is found to be 178.26 μg/ml [Figure 3].
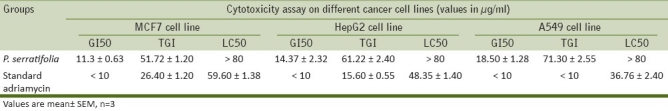
The present study showed the tumor cell suppression potential of P. serratifolia extract on different human cancer cell lines. The cytotoxic study was carried out in MCF7, HEPG2 and A549 cancer cell lines using SRB assay. The growth inhibition of 50% (GI50) of test extract is found to be 11.30, 14.37, and 18.50 μg/ml for the MCF7, HEPG2 and A549 cancer cell lines respectively. The total growth inhibition activity (TGI) of the test extract is 51.72 μg/ml on HepG2 cell line followed by MCF7 cell line (61.22) and A549 cell line (71.30), respectively [Table 2]. The LC50 value, that is, the concentration of drug resulting in a 50% reduction in the measure protein was analyzed and found to be >80 μg/ml in all the cancer cell lines. The results were compared with standard drug Adriamycin. The present study proved the cytotoxic and antioxidant activity of the plant P. serratifolia.
Table 2.
Cytotoxic assay of P. serratifolia on different human cancer cell lines
CONCLUSIONS
Cancer is a dreadful disease and any practical solution in combating this disease is of paramount importance to public health. Therefore, besides the rationalized allopathic drugs, it is worth evaluating the folk medicine- a plant-based therapy which is not a systematized study. An alternative solution to allopathic medicine embodied with severe side effects, is the use of folk medicine plant preparations to arrest the insidious nature of the disease. Many herbs have been evaluated in clinical studies and are currently being investigated phytochemically to understand their anti-tumor actions against various cancers. The present study has proved the antioxidant activity and tumor cell suppression potential of methanolic extract of P. serratifolia leaf on MCF7, HepG2, and A549 human cancer cell lines. The further research is to be focused for the active compound isolation and analyzing its biopotency.
Footnotes
Source of Support: Nil.
Conflict of Interest: None declared.
REFERENCES
- 1.Suffiness M, Pezzuto JM. Vol. 6. New York: Academic Press; 1991. Methods in Plant Biochemistry; p. 71. [Google Scholar]
- 2.Gulcin I, Buyukokuroglu ME, Oktay M, Kufrevioglu OI. On the in vitro antioxidant properties of melatonin. J Pineal Res. 2002;33:167–71. doi: 10.1034/j.1600-079x.2002.20920.x. [DOI] [PubMed] [Google Scholar]
- 3.Halliwell B, Gutteridge JM. Oxford: Oxford University Press; 1999. Free radicals in biology and medicine. [Google Scholar]
- 4.Yildirim A, Mavi A, Oktay M, Kara AA, Algur OF, Bilaloglu V. Comparison of antioxidant and antimicrobial activities of tilia (Tilia argenta Desf Ex DC), sage (Salvia triloba L.) and black tea (Camellia sinensis) extracts. J Agric Food Chem. 2000;48:5030–4. doi: 10.1021/jf000590k. [DOI] [PubMed] [Google Scholar]
- 5.Lopaczyski W, Zeisel SH. Antioxidants, programmed cell death and cancer. Nutr Res. 2001;21:295–307. [Google Scholar]
- 6.Sgambato A, Ardito R, Faraglia B, Boninsegna A, Wolf FI, Cittadini A. Resveratrol, a natural phenolic compound, inhibits cell proliferation and prevents oxidative DNA damage. Mutat Res. 2001;496:171–80. doi: 10.1016/s1383-5718(01)00232-7. [DOI] [PubMed] [Google Scholar]
- 7.Warrier PK, Nambiar VP, Ramankutty C. Vol. 4. Andhra Pradesh, India: Orient Longman Publications; 1995. Indian Medicinal Plants. A compendium of 500 species; pp. 348–52. [Google Scholar]
- 8.Sreejayan N, Rao MN. Free radical scavenging activity by curcuminouids. Arzneimittelforschung. 1996;46:169–71. [PubMed] [Google Scholar]
- 9.Sreejayan M, Rao MN. Nitric oxide scavenging activity by curcuminoids. J Pharm Pharmacol. 1997;47:105. doi: 10.1111/j.2042-7158.1997.tb06761.x. [DOI] [PubMed] [Google Scholar]
- 10.Shirwaikar A, Somashekar AP. Antiinflammatory activity and free radical scavenging studies of Aristoluchia bractoelata Lam. Indian J Pharm Sci. 2003;65:68. [Google Scholar]
- 11.Green LC, Wagner DA, Glogowoski J, Skipper PL, Wishnok JS, Tannenbavm SR. Analysis of nitrate and 15N in biological fluids. Anal Biochem. 1982;126:131–8. doi: 10.1016/0003-2697(82)90118-x. [DOI] [PubMed] [Google Scholar]
- 12.Marcocci L, Maguire JJ, Droy-Lefaix MT, Packer L. The nitric oxide scavenging property of Ginko biloba extract EGB 761. Biochim Biophys Res Commun. 1994;201:748–55. doi: 10.1006/bbrc.1994.1764. [DOI] [PubMed] [Google Scholar]
- 13.Kunchandy E, Rado MN. Oxygen radical scavenging activity of curcuminoid. Int J Pharmacogn. 1990;58:237. [Google Scholar]
- 14.Sanchez-Moreno C. Methods used to evaluate the free radical scavenging activity in foods and biological systems. Food Sci Tech Int. 2002;8:122. [Google Scholar]
- 15.Ohkawa H, Ohishi N, Yagi K. Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal Biochem. 1979;95:351. doi: 10.1016/0003-2697(79)90738-3. [DOI] [PubMed] [Google Scholar]
- 16.Prasanth Kumar V, Shasidhara S, Kumar MM, Sridhara BY. Effect of Luffa echinata on lipid peroxidation and free radical scavenging activity. J Pharm Pharmacol. 2000;52:891. doi: 10.1211/0022357001774589. [DOI] [PubMed] [Google Scholar]
- 17.Vichai V, Kirtikara K. Sulforhodamine B colorimetric assay for cytotoxicity screening. Nat Protoc. 2006;1:1112–6. doi: 10.1038/nprot.2006.179. [DOI] [PubMed] [Google Scholar]


