Abstract
This study aimed at investigating the mechanism by which sodium arsenite induces brain injury and the role of L-ascorbate. Thirty adult (n=5) Wistar rats weighing between 140 and 160 g were used. Group 1 neither received sodium arsenite nor L-ascorbate (control), group 2 was administered low dose of arsenite only, group 3 received high dose of arsenite only, group 4 was administered L-ascorbate only, group 5 was administered low dose of arsenite and L-ascorbate, and group 6 received high dose of arsenite and L-ascorbate. M0 alon dialdehyde, MDA, levels were significantly increased in rats treated with high dose of arsenite when compared with those treated with low dose of arsenite. However, all treated groups except those treated with L-ascorbate only showed significant increase in MDA levels when compared with the control group. Rats treated with high dose of arsenite and L-ascorbate showed a significantly higher MDA level than those treated with low dose of arsenite and L-ascorbate. However, catalase activity, body weight gain, brain weight and mean food consumption were comparable across all groups. Brain tissue total protein was similar in all groups except in both groups treated with high dose of arsenite, where they were significantly reduced when compared with the control group. I0 n conclusion, sodium arsenite treatment induces brain injury via a mechanism associated with lipid peroxidation, but not catalase-dependent. However, L-ascorbate ameliorates arsenite-induced oxidative injury in the brain. L-ascorbate antioxidative potential in alleviating arsenite-induced brain injury is dependent on the concentration of arsenite.
Keywords: Arsenite, brain, lipid peroxidation, L-ascorbate, weight
INTRODUCTION
Arsenical compounds are environmental toxins with multiple effects in animals and humans.[1,2] They are commonly used as herbicides, fungicides and rodenticides and may cause air, soil and water pollution.[3–5] The main source of environmental arsenic exposure is oral consumption of contaminated water, food and drugs.[6,7]
Exposure to arsenic has been associated with decreased birth weight and an increased rate of spontaneous abortion, tumors in a variety of tissues including skin, lung, liver and bladder,[2,8,9] inhibition of ovarian steroidogenic function and gonadotrophins secretion,[10] severe metabolic disorders such as diabetes,[11] gastrointestinal tract disorders,[12] sperm toxicity[2,13,14] and brain injury.[15,16]
On the other hand, L-ascorbate (Vitamin C) has been reported to have antioxidative properties,[17,18] but its role in brain injury has not been documented.
Hence, this study aimed at investigating the mechanism by which sodium arsenite induces brain injury and the role of L-ascorbate.
MATERIALS AND METHODS
Animals and treatment
Thirty adult Wistar rats weighing between 140 and 160 g were maintained in a 12h light and 12h dark cycle at 25°C±2. They were fed standard rat chow and tap water ad libitum. Rats were allowed to acclimatize for 2 weeks and then divided into six groups (n=5). Sodium arsenite and L-ascorbate were dissolved in water to achieve specific doses and orally administered for 21 days.
The doses were as follows:
Group 1 : Rats given neither sodium arsenite nor L-ascorbate; control
Group 2 : Rats administered low dose of arsenite only (0.5 mg/100 kgBW/rat day); LDA
Group 3 : Rats administered high dose of arsenite only (1 mg/100 kgBW/rat day); HDA
Group 4 : Rats administered L-ascorbate only (25 mg/100 gBW/rat day); Vit.C
Group 5 : Rats administered low dose of arsenite and L-ascorbate; LDAV
Group 6 : Rats administered high dose of arsenite and L-ascorbate; HDAV
The NIH Guide for the Care and Use of Laboratory Animals (NIH publication No. 85-23 revised 1985 : U0 S Department of Health, Education and Welfare, Bethesda, MD, USA) was followed throughout the experimental duration. The experimental protocol also met the American Physiological Society's “Guiding Principles for Research Involving Animals”.
Amount of food consumed were recorded daily throughout the experiment and the mean food consumption in g/rat/day was calculated.
After the experimental period, rats were fasted overnight and sacrificed the following day.
Body and organ weight
The body weights were recorded on the first day of treatment (initial weight) and on the day of sacrifice (final weight). Brain of each rat was dissected out and weighed. The brain was homogenized and malon dialdehyde level (MDA), and catalase activity (CAT) were assayed using the homogenate as described by Varshney and Kate,[19] and Beers and Sizer,[20] respectively. Total brain tissue protein was assayed using a standard kit (Randox).
Statistical analysis
Results of the experiment are expressed as mean±standard error of mean. The differences between the mean values were evaluated by ANOVA followed by multiple Student's t-test. The values P<0.05 were considered significant.
RESULTS
In all treated groups, the body weight gain was not significantly different from that of the control. Similarly, the brain weight was also comparable across all the groups. No differences in the mean food consumption were seen in all the groups during the experimental period [Table 1 and Figure 1].
Table 1.
Weight changes and lipid peroxidation index in brain tissue following administration of low and high doses of arsenite and L-ascorbate (Vitamin C) in rats
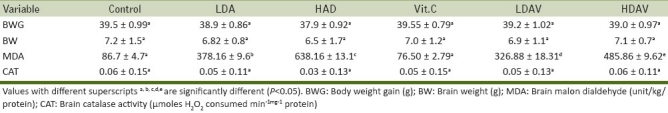
Figure 1.
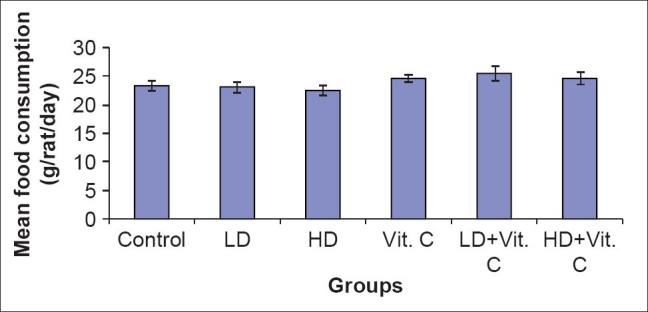
Mean food consumption following administration of low and high doses of sodium arsenite and L-Ascorbate (Vitamin C) in rats
Brain tissue total protein was similar in all groups except in HDA and HDAV, where they were significantly reduced when compared with the control group [Figure 2].
Figure 2.
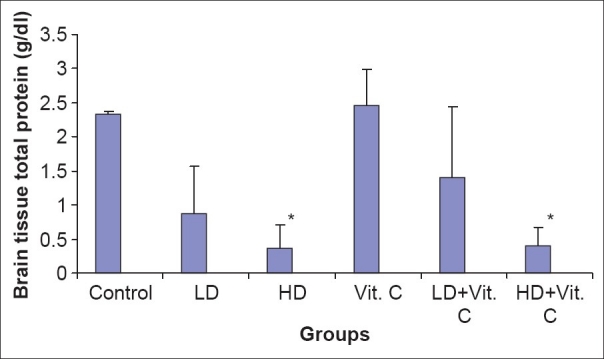
Total protein level in brain tissue following administration of low and high doses sodium arsenite and L-ascorbite (Vitamin C) in rats *P <0.05 vs control
MDA levels were significantly increased in rats treated with HDAV and HDA when compared with those treated with LDAV and LDA, though HDAV and LDAV showed a significantly lower MDA levels when compared with HDA and LDA, respectively. However, all treated groups except those treated with L-ascorbate only showed significant increase in MDA levels when compared with the control group. CAT activity was comparable in all groups.
DISCUSSION
This study reveals that the administration of sodium arsenite, in low and high doses, and L-ascorbate does not affect food consumption. This agrees with the study of Kuladip et al.,[21] which showed comparable values of food consumption in arsenite-treated and control rats. This suggests that arsenite exposure does not affect the appetite center in anyway.
This study also reveals that sodium arsenite and L-ascorbate administration does not affect body weight gain and brain weight. This does not agree with previous studies that reported though no significant differences in body weight, but significant decrease in both male and female reproductive organ weight following arsenite administration.[10,13,21] This variation might be due to higher doses and longer period of exposure of rats to arsenite in previous studies.
Previous study of Akhigbe et al.,[22] has reported the effect of food intake on body weight gain. High food intake is known to help in tissue repair and other anabolic processes with consequent increase weight gain besides the production of energy for daily activities. The comparable body weight gain and brain weight seen across all groups in this study could be associated with the similar food consumption also seen across all the groups. Since all groups consumed comparable amount of food throughout the experimental schedule, they consequently have similar body weight gain and brain weight.
Arsenite, like other heavy metals, has been associated with various tissue damage. Study of Lin et al.,[15] revealed that arsenite induces oxidative injury in the nigrostriatal dopaminergic system of rats’ brain. They also documented that arsenite induces apoptosis in the central nervous system of rat. Similar arsenite-induced oxidative injury in rats’ brain was also reported by Su-Feng et al.[16] This study shows the mechanism by which arsenite induces brain injury and the possible role of L-ascorbate in alleviating arsenite-induced brain injury.
This study shows that CAT activity was comparable in all groups. On the other hand, LDAV and LDA-treated rats showed significant increase in MDA levels when compared with the control and L-ascorbate-treated group, while HDAV and HDA-treated rats showed significant increase in MDA levels when compared with all other groups. This is consistent with previous studies[15,16] which reported increased lipid peroxidation following arsenite treatment. This study shows that L-ascorbate enhances lipid peroxidation status in rats exposed to arsenite as seen in LDAV and HDAV groups, which showed a significantly lower MDA levels when compared with LDA and HDA groups, respectively.
Lipid peroxidation, usually measured by the level of MDA, causes degenerative changes of brain cell membrane.[23] The free radicals released in association with lipid peroxidation oxidize polyunsaturated fatty acid, a main component of the cell membrane, thereby destroying the spatial arrangement of membrane and removing the biological activities impairing the functions of the bound enzymes like Na+/K+ ATPase which plays an important role in the functional activity of nerve cells.[24–27]
Administration of L-ascorbate alleviated arsenite-induced raise in MDA level in both LDAV and HDAV-treated rats, though HDAV-treated rats showed a significantly higher level of MDA when compared with the LDAV-treated rats. This result suggests that L-ascorbate protective potential on lipid peroxidation status is dependent on the concentration of arsenite.
L-ascorbate is a free radical scavenger and functions as an antioxidant. However, Frei et al.[28] reported that L-ascorbate disappears faster than other antioxidants when plasma is exposed to reactive oxygen species. This might also explains why L-ascorbate has a better antioxidative potential in low dose of arsenite than in high dose.
CONCLUSIONS
From the present study, sodium arsenite treatment induces brain injury via a mechanism associated with lipid peroxidation, but not catalase-dependent. L-ascorbate antioxidative potentials in alleviating arsenite-induced brain injury is, however, dependent on the concentration of arsenite.
Footnotes
Source of Support: Nil.
Conflict of Interest: None declared.
REFERENCES
- 1.Liu SX, Athar M, Lippai I, Waldren C, Hei TK. Induction of oxyradicals by arsenic: Implication for mechanism of genotoxicity. Proc Natl Acad Sci USA. 2001;98:1643–8. doi: 10.1073/pnas.031482998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Waalkes MP, Ward JM, Liu J, Diwan BA. Transplacental carcniongenility of inorganic arsenic in the drinking water: Induction of hepatic ovarian, pulmonary and adrenal tumors in mice. Toxicol Appl Pharmacol. 2003;186:7–17. doi: 10.1016/s0041-008x(02)00022-4. [DOI] [PubMed] [Google Scholar]
- 3.Borzsonyl A, Bereczky A, Rudnai P, Csanady M, Horvath A. Epidemiological studies on human subjects exposed to arsenic in drinking water in southern Hungary. Arch Toxicol. 1992;66:77–8. doi: 10.1007/BF02307274. [DOI] [PubMed] [Google Scholar]
- 4.Chatterjee A, Das D, Chatterjee D. The study of ground water contamination by arsenic in the residential area of Behala, Calcutta, due to industrial pollution. Environ Pollut. 1993;80:52–65. doi: 10.1016/0269-7491(93)90010-l. [DOI] [PubMed] [Google Scholar]
- 5.Nickson R, McArthur J, Burges W, Ahmed KM, Ravenser P, Rahman M. Arsenic poisoning of Bangladesh ground water. Nature. 1998;395:338. doi: 10.1038/26387. [DOI] [PubMed] [Google Scholar]
- 6.Bates MN, Smith AH, Hopenhayn-Rich C. Arsenic ingestion and internal cancers: A review. Am J Epidemiol. 1992;335:462–76. doi: 10.1093/oxfordjournals.aje.a116313. [DOI] [PubMed] [Google Scholar]
- 7.Pott WA, Bengamin SA, Yang RS. Pharmacokinetics, metabolism and Carcinogenicity of arsenic. Rev Environ contain Toxicol. 2009;169:165–214. doi: 10.1007/978-1-4613-0107-3_3. [DOI] [PubMed] [Google Scholar]
- 8.Washington, DC: National Academy Press; 1999. NRC. Arsenic in the drinking water; pp. 1–310. [Google Scholar]
- 9.Waalkes MP, Keefer LK, Diwan BA. Induction of proliferate lesions of the uterus, testes, and liver in swiss mice given repeated injections of sodium arsenate: Possible estrogenic mode of action. Toxical Appl Pharmacol. 2000;166:24–35. doi: 10.1006/taap.2000.8963. [DOI] [PubMed] [Google Scholar]
- 10.Chattopadyay S, Ghosh S, Chaki S, Debnath J, Ghost D. Effect of sodium arsenite on plasma levels of gonadotropins and ovarian steroidogenesis in mature albino rats : D0 uration dependent response. J Toxicol Sci. 1999;24:425–31. doi: 10.2131/jts.24.5_425. [DOI] [PubMed] [Google Scholar]
- 11.Tseng CH, Tseng CP, Chiou HY, Hsueh YM, Chong CK, Chen CJ. Epidemiologic evidence of diabetogenic effect of arsenic. Toxical Lett. 2002;133:69–76. doi: 10.1016/s0378-4274(02)00085-1. [DOI] [PubMed] [Google Scholar]
- 12.Goebl HH, Schmidt PF, Bohl J, Teltenborn B, Kramer G, Gutman L. Polyneuropathy due to arsenic intoxication biopsy studies. J Neurol. 1990;49:137–49. doi: 10.1097/00005072-199003000-00006. [DOI] [PubMed] [Google Scholar]
- 13.Sarkar M, Ray Chudhuri G, Chattopadhyay A, Biswas NM. Effect of sodium arsenite on spermatogenesis, plasma ginadotrophins and testosterone in rats. Asian J Androl. 2003;1:27–31. [PubMed] [Google Scholar]
- 14.Pant N, Murty RC, Srivasta SP. Male reproductive toxicity of sodium arsenite in mice. Hum Exp Toxicol. 2004;23:399–403. doi: 10.1191/0960327104ht467oa. [DOI] [PubMed] [Google Scholar]
- 15.Lin AM, Fang SF, Chao PL, Yang CH. Melatonin attenuates arsenite- induced apoptosis in rat brain : I0 nvolvement aggregation of alpha-synuctein. J Pincal Res. 2007;43:163–71. doi: 10.1111/j.1600-079X.2007.00456.x. [DOI] [PubMed] [Google Scholar]
- 16.Fan SF, Chao PL, Lin AM. Arsenite induces oxidative injury in rat brain. Ann N Y Acad Sci. 2010;1199:27–35. doi: 10.1111/j.1749-6632.2009.05170.x. [DOI] [PubMed] [Google Scholar]
- 17.Chattopadyhay S, Ghosh S, Debnath J, Ghosh D. Protection of sodium arsenite-induced ovarian toxicity by administration of L-ascorbate (Vitamin C) in mature Wistar strain rat. Arch Environ Contam Toxicol. 2001;41:83–9. doi: 10.1007/s002440010223. [DOI] [PubMed] [Google Scholar]
- 18.Owu DU, Antai AB, Udofia KH, Obembe AO, Obasi KO, Eteng MU. Vitamin C improves basal metabolic rate and lipid profile in alloxan induced diabetes mellitus in rats. J Biosci. 2006;1:575–9. doi: 10.1007/BF02708409. [DOI] [PubMed] [Google Scholar]
- 19.Varshney R, Kale RK. Effect of calmodulin antagonists on radiation induced lipid peroxidation in microsomes. Int J Radiat Biol. 1990;58:733–743. doi: 10.1080/09553009014552121. [DOI] [PubMed] [Google Scholar]
- 20.Beers RF, JR, Sizer IW. A spectrophotonetric method for measuring the breakdown of hydrogen peroxide by catalase. J Biol Chem. 1952;195:133–40. [PubMed] [Google Scholar]
- 21.Jana K, Jana S, Samanta PK. Effects of chronic exposure to sodium arsente on hypothalamo pituitary testicular activities in adult rats: Possible an estrogenic mode of action. Reprod Biol Endocrinol. 2006;4:9. doi: 10.1186/1477-7827-4-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Akhigbe RE, Olatunji LA, Soladoye AO, Oyeyipo IP. Effect of angiotensin I-converting enzyme inhibitor, captopril, on body weight and food and water consumption in oral contraceptive-treated rats. Am J Biochem Mol Biol. 2011;1:95–100. [Google Scholar]
- 23.Stefanello FM, Chiarani F, Kurek AG, Wannmacher CMD, Wajner M, Wyse ATS. Methionine alters Na+, K+ -ATPase activity, lipid peroxidation and non enzymatic antioxidant defenses in rat hippocampus. Int. J. Devel Neurosci. 2005;23:651–656. doi: 10.1016/j.ijdevneu.2005.06.003. [DOI] [PubMed] [Google Scholar]
- 24.Kurella E, Kukley M, Tyulina O, Dobrota D, Matejovicova M, Mezesova V, et al. Kinetic Parameters of Na+/K+ ATPase modified by radicals in vitro and in vivo. Ann NY Acad Sci. 1997;834:661–5. doi: 10.1111/j.1749-6632.1997.tb52344.x. [DOI] [PubMed] [Google Scholar]
- 25.Yousef MI, El-Hendy HA, El-Demerdash FM, Elagamy EI. Dietary zinc deficiency induced- changes in the activity of enzymes and the levels of free radical, lipid and protein electrophoretic behaviour in growing rats. Toxcology. 2002;175:223–34. doi: 10.1016/s0300-483x(02)00049-5. [DOI] [PubMed] [Google Scholar]
- 26.Hong JH, Kim MJ, Park MR, Kwag OG, Lee IS, Byun BH, et al. Effect of vitamin E of oxidative stress and membrane fluidity in brain of streptozotocin-induced diabetic rats. Clin Chim Acta. 2004;340:107–15. doi: 10.1016/j.cccn.2003.10.003. [DOI] [PubMed] [Google Scholar]
- 27.de Assis DR, Maria RC, Ferreira GC, Schuck PF, Latini A, Dutra-Filho CS, et al. Na+/K+ ATPase activity is markedly reduced by cis-4-decenoic acid in synaptic plasma membranes from cerebral cortex of rats. Exp Neurol. 2006;197:143–9. doi: 10.1016/j.expneurol.2005.09.002. [DOI] [PubMed] [Google Scholar]
- 28.Frei B, England L, Ames BN. Ascorbate is and outstanding antioxidant in human blood plasm. Proc Natl Acad Sci USA. 1989;86:6377–81. doi: 10.1073/pnas.86.16.6377. [DOI] [PMC free article] [PubMed] [Google Scholar]


