Abstract
Glutaraldehyde-stabilized bovine pericardium is used for clinical application since 1970s because of its desirable features such as less immunogenicity and acceptable durability. However, a propensity for calcification is reported on account of glutaraldehyde treatment. In this study, commercially available glutaraldehyde cross-linked bovine pericardium was evaluated for its in vitro cytotoxic effect, macrophage activation, and in vivo toxic response in comparison to decellularized bovine pericardium. Glutaraldehyde-treated bovine pericardium and its extract were observed to be cytotoxic and it also caused significant inflammatory cytokine release from activated macrophages. Significant antibody response, calcification response, necrotic, and inflammatory response were noticed in glutaraldehyde-treated bovine pericardium in comparison to decellularized bovine pericardium in a rat subcutaneous implantation model. Glutaraldehyde-treated bovine pericardium also failed in acute systemic toxicity testing and intracutaneous irritation testing as per ISO 10993. With respect to healing and implant remodeling, total lack of host tissue incorporation and angiogenesis was noticed in glutaraldehyde-treated bovine pericardium compared to excellent host fibroblast incorporation and angiogenesis within the implant in decellularized bovine pericardium. In conclusion, using in vitro and in vivo techniques, this study has demonstrated that glutaraldehyde-treated bovine pericardium elicits toxic response compared to decellularized bovine pericardium which is not congenial for long-term implant performance.
Keywords: Calcification, decellularized bovine pericardium, glutaraldehyde-treated bovine pericardium, immunogenicity, toxic response, tissue incorporation
INTRODUCTION
Glutaraldehyde-stabilized bovine pericardium is commercially available and used for medical application in the form of patches and bioprosthetic valves on account of its low immunogenicity and excellent durability.[1] Glutaraldehyde treatment produces stable cross-links in cellular and extra-cellular matrix proteins which substantially reduced graft immunogenicity.[2] However, it was also shown that such tissue had altered mechanical property, early mechanical failure, cytotoxicity, and incomplete suppression of immunological recognition.[2–4] Besides this severe calcification was noticed in glutaraldehyde-treated bovine pericardium in pediatric patients.[4] An emerging alternative to glutaraldehyde treatment for reducing xenograft immunogenicity is decellularization.[5,6] Use of decellularized xenograft is an approach where acellular tissue matrices are produced by selective removal of cellular components that are believed to promote immunogenicity and calcification. These acellular matrices promote induced regeneration through remodeling of the prosthesis by neo-vascularization, re-cellularization, and laying of new extracellular matrix by the host. The remodeled decellularized xenografts may functionally and structurally integrate into the host tissue.[6]
In this study, the in vitro and in vivo toxic effects of glutaraldehyde-treated bovine pericardium in comparison to decellularized bovine pericardium are reported. The in vivo effects of this toxic response such as inflammatory potential, immunogenicity, calcification potential, and lack of tissue incorporation are discussed as this may have effect on the long-term implant performance in clinical situation.
MATERIALS AND METHODS
Glutaraldehyde-treated bovine pericardium
Commercially available glutaraldehyde-treated bovine pericardium (Glut BP) approved for clinical use in India was used in this study. This treatment group was named as Glut BP.
Decellularized bovine pericardium
Decellularization of bovine pericardium (Dcl BP) was achieved using a proprietary process based on a nondetergent method. Decellularization is confirmed by demonstrating the absence of nuclear remnants using routine HE staining as well as by the use of specific nuclear stain such as Hoechst 33258 on 5 μm paraffin embedded sections (n = 10). Besides this, residual DNA was extracted from 100 mg decellularized BP using DNA-XPress Kit (Hi-Media) and concentration was estimated spectrophotometrically (n = 3). This was followed by demonstration of extractable DNA by 1% agarose gel electrophoresis. The structural content was evaluated using histochemical staining of 5 μm paraffin embedded sections using Movats Pentachrome staining (n = 10). Decellularized bovine pericardium was sterilized by a proprietary process, and sterility was confirmed by routine sterility testing.
Collagenase susceptibility
This study was conducted to assess the ability of the graft to undergo in vivo degradation which will pave the way for laying the host extracellular matrix. In this test, the susceptibility of the test material to Collagenase II activity (from Clostridium histolyticum, 125 μm/mg, Sigma Aldrich) was studied. Approximately 50 mg (wet weight in triplicates) of glutaraldehyde-treated bovine pericardium (Glut BP) and decellularized bovine pericardium (Dcl BP) were weighed. To the weighed samples, 0.5 mL of 0.1 M Tris chloride (pH 7.4) containing 0.005 M calcium chloride and 0.05 mg/mL sodium azide was added. The samples were incubated at 37°C for 1 h with constant shaking. Collagenase enzyme was prepared in 0.1 M Tris chloride (pH 7.4) at 37°C and added in to the vials so as to make the final concentration to 2 U/mg of tissue. The vials were incubated at 37°C for 24 h, 72 h, and 7 days, at the end of which, the samples were centrifuged at 12,000 rpm for 20 min at 4°C and the remaining tissue samples are blotted in filter papers for 5 min to dry them. They were weighed using Afcoset Electronic Weighing Balance ER120A. The weight loss was determined by paired comparison before and after treatments.
In vitro response
xi toxic responses of Glut BP and Dcl BP were assessed by cytotoxicity studies using L929 fibroblast cells by a direct contact method as well as by studying the effect of the extract on L929 fibroblast metabolism by MTT assay. In vitro inflammatory response was studied by macrophage activation studies.
Cytotoxicity testing: a direct contact method
Cytotoxic potential of Glut BP and Dcl BP groups on account of their direct contact or due to leachables was evaluated by a direct contact method (ISO 10993-5). The test samples were rinsed thrice with normal saline before cytotoxicity evaluation. Test samples, negative control (high density poly ethylene, USP), and positive control (copper) in triplicate were placed on a subconfluent monolayer of L-929 mouse fibroblast cells. After incubation of cells with test samples at 37 ± 2°C for 24 ± 1 h, the cell culture was examined microscopically for cellular response around the samples. Cellular responses were expressed as noncytotoxic, mildly cytotoxic, moderately cytotoxic, and severely cytotoxic.
Cytotoxicity testing on extract
In vitro MTT assay on extract of Glut BP and Dcl BP in physiological saline was performed to measure the effect of extract on the metabolic activity of L929 fibroblast cells. The ability of the fibroblast cells to metabolize yellow-colored tetrazolium salt 3-(4,5-dimethyl thiazol-2-yl)-2,5-diphenyltetrazolium bromide to purple-colored formazan was measured as an indication of its activity. Different dilutions (50% and 25%) of extracts of Glut BP sample, Dcl BP, positive control (dilute phenol), negative control (high density poly ethylene (USP)), and reagent control in triplicate were placed on the subconfluent monolayer of L929 cells. After incubation of cells with the extract at 37 ± 2°C for 2 h, extract and control medium were replaced with 50 μL MTT solution (1 mg/mL in the medium without supplements) wrapped with aluminum foils and were incubated at 37 ± 2°C for 2 h. After discarding the MTT solution, 100 μL of isopropanol was added to all wells and swayed the plates. The color developed was quantified by measuring absorbance at 540 nm using a microplate reader. The data obtained were compared with the negative control.
Macrophage activation
Macrophage activation was studied by estimating inflammatory cytokine released by adhered macrophages on contact with materials belonging to different groups. THP-1 (human acute monocytic leukemia cell lines) cells were grown in RPMI 1640 (Sigma) containing 10 % v/v fetal bovine serum (Gibco), 160 U/mL benzylpenicillin, and 100 U/mL streptomycin (Sigma). The cells were maintained at 37°C in an atmosphere of 95% air and 5% CO2 at 90% relative humidity. The differentiation into macrophages was induced by treating THP-1 cells in a 24-well plate for 24 h with RPMI-1640 containing 20 ng/mL phorbol 12-myristate 13- acetate (PMA). Thereafter, a fresh medium was added and cells were grown for another 24 h under similar conditions. Release of proinflammatory cytokines (IL-1b, IL-6, and TNF-a) from THP 1 cells upon contact with triplicate samples of Glut BP and Dcl BP and positive control (bacterial lipopolysaccharide) at 20 pg were carried using ELISA. The concentration of cytokines in the culture supernatant was quantified by a specific ELISA kit (U-CyTech biosciences, Netherlands) as per manufacturer instructions.
In vivo response
In vivo toxic response was studied by conducting an acute systemic toxicity testing (ISO 10993-11) and an intracutaneous (intradermal) reactivity test (ISO 10993-10). In vivo response such as immune response, calcification response, and tissue response was studied by juvenile rat subcutaneous implantation for 60 days. The animal test protocols were approved by Institutional Animal Ethics Committee and animal care and maintenance were done as per CPCSEA guidelines.
Acute systemic toxicity testing (ISO 10993-11)
This test was done to evaluate the systemic response of mice following intraperitoneal and intravenous injection of cotton seed oil and physiological saline extract of the test samples, respectively. The test samples were prepared by washing the samples in sterile normal saline for three wash cycles, with each cycle lasting for 10 min. The samples were dried at 37°C for 24 h. Two grams each of Glut BP and Dcl BP samples were extracted with 10 mL each of physiological saline and cotton seed oil for 72 h at 50°C. Then, 50 mL/kg body weight of the above extracts were injected through intravenous (n = 5) and intraperitoneal routes (n = 5), respectively, in separate groups of randomly selected health active mice. Injection of plain extraction media in another set of animals served as control. The animals were observed immediately and after 4, 24, 48, and 72 h for evidence of abnormalities such as convulsions, prostrations, loss of body weight more than 2 g and death.
Intracutaneous (intradermal) reactivity test (ISO 10993-10)
This test was done to assess the potential of the material to produce irritation following intradermal injection of the material extract. The test samples were prepared by washing the samples in sterile normal saline for three wash cycles, with each cycle lasting for 10 min. The samples were dried at 37°C for 24 h. Two grams each of Glut BP and Dcl BP samples were extracted with 10 mL each of physiological saline and cotton seed oil for 72 h at 50°C. Then, 0.2 mL (per each site) of the extract was injected into five sites with appropriate controls in randomly selected healthy, active New Zealand white rabbits. The animals were observed for erythema and edema which was scored as 0 (nil), 1 (very slight), 2 (well-defined edema/erythema), 3 (moderate), and 4 (severe). The requirement of the test is met if the difference between the test sample mean score and control sample mean score is 1.0 or less. All the procedures were done aseptically.
Implantation study
This study was done to evaluate calcification potential, immunogenic potential, and tissue response by subcutaneous implantation for 60 days in 3-week-old male Wistar rats. Healthy animals were randomly assigned into Glut BP and Dcl BP groups with each group consisting of five animals. The animals were subcutaneously implanted under the dorsal skin with evenly placed six implants of the same treatment group. The animals were observed daily for 60 days and at the end of which they were killed. The serum was collected for estimating antibody response against bovine pericardial proteins. One set of explants (n = 15) were used for calcium estimation and another set (n = 15) was processed for histopathological evaluation.
Antibody response
The antibody response (IgG, IgM, and IgA) elicited in different groups of animals implanted with Glut BP and Dcl BP samples was assessed using indirect ELISA. For this, extractable protein of fresh bovine pericardium was coated in the 96 well ELISA immuno plate (F96 MAXISORP, NUNC) in PBSN (2 μg protein per well). Serum samples at 1:1000 dilution prepared in blocking buffer from animals belonging to the above groups (n = 5) was used as primary antibody. Serum from animals which is immunized with extractable protein from fresh bovine pericardium was treated as positive control. Rabbit polyclonal to rat IgG + IgM + IgA conjugated with HRP (Abcam ab8521) was used as secondary antibody. After the reaction, the ELISA plates were read with microplate reader Expert Plus (ASYS Hitech GmbH, Austria). OD readings of micro wells without secondary antibody were taken as the negative control. OD values subtracted from the mean negative control values were used for comparison.
Calcification response
Calcium estimations from explanted samples were done as per the procedure reported earlier.[7] In brief, the explanted Glut BP and Dcl BP samples (n = 15, three samples from each animal) were digested in 2 mL of 2 N HCl for 48 h at 60°C. The supernatant was neutralized with 2 N NaOH followed by the estimation of calcium using the colorimetric method of O-Cresolphthalein complexone obtained as a standard kit. OD readings were taken at 578 nm using a Shimadzu UV-1700 spectrophotometer, Japan.
Tissue response
The explanted Glut BP and Dcl BP samples (n = 15, three samples from each animal) were fixed in 10% buffered formalin, embedded in paraffin, and 5 μm sections were made. The sections were stained by hematoxylin and eosin and Von Kosa stain. A qualitative assessment was made for peri-implant necrosis, inflammatory response, peri-implant fibrosis, tissue incorporation, angiogenesis in the implanted material and calcification.
Statistics
All quantitative estimations are expressed in mean ± SD. Significance testing for differences of means between groups is carried out using the unpaired Student-t test for equal or unequal variance depending on the homogeneity of variance tested using the F-test.. Statistically significant difference between means was assumed whenever P-value was less than 0.05.
RESULTS
Confirmation of decellularization
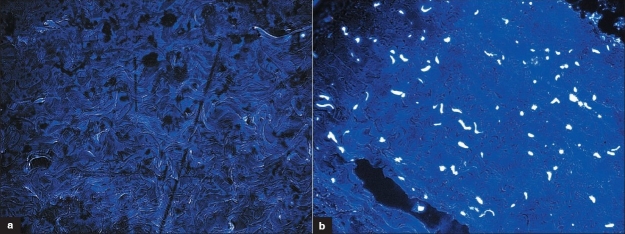
Nuclear remnants could not be demonstrated both by hematoxylin and eosin as well as by Hoechst 33258 staining [Figure 1], indicating satisfactory decellularization. DNA extraction yielded a residual DNA content of 7 ± 3.5 μg/mL in decellularized bovine pericardium compared to 97 ± 2 μg/mL in fresh bovine pericardium (triplicate samples). Extractable DNA from decellularized tissue could not be demonstrated in 1% agarose gel electrophoresis probably because of extensive DNA fragmentation [Figure 2]. Structurally, decellularized pericardium mainly consisted of well-separated collagen bundles with a few elastin fibers as demonstrated by Movats Pentachrome staining.
Figure 1.
Confirmation of decellularization with Hoechst 3328 staining. (a) Decellularized pericardium with no nuclear remnants. (b) Normal pericardium showing intact nucleus (Hoechst 33258, 400×)
Figure 2.
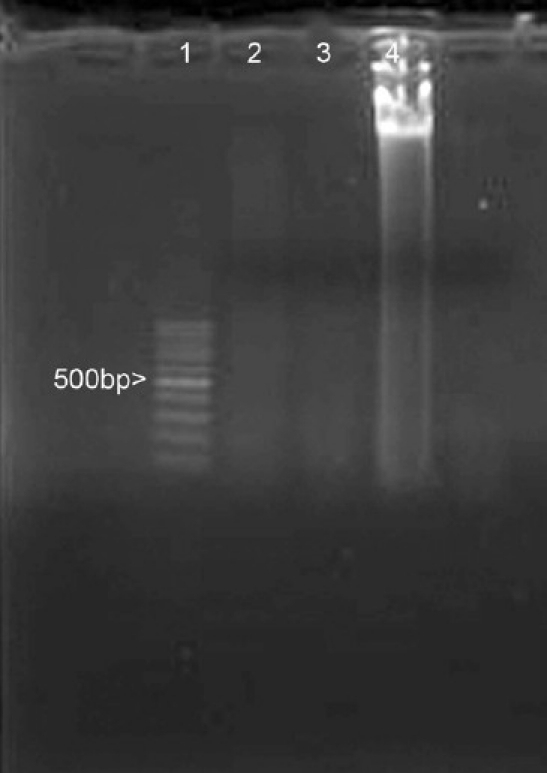
Extractable DNA from decellularized bovine pericardium; first lane: 100 bp DNA ladder, second and third lanes: DNA from decellularized bovine pericardium, fourth lane: DNA from fresh bovine pericardium
Collagenase susceptibility
Data on residual weight of different groups following Collagenase II digestion are given in Table 1 and data on degradation are given in Table 2.
Table 1.
Residual weights in different groups with time following Collagenase type II digestion

Table 2.
Toxic response (in vitro and in vivo) of Glut BP compared to Dcl BP
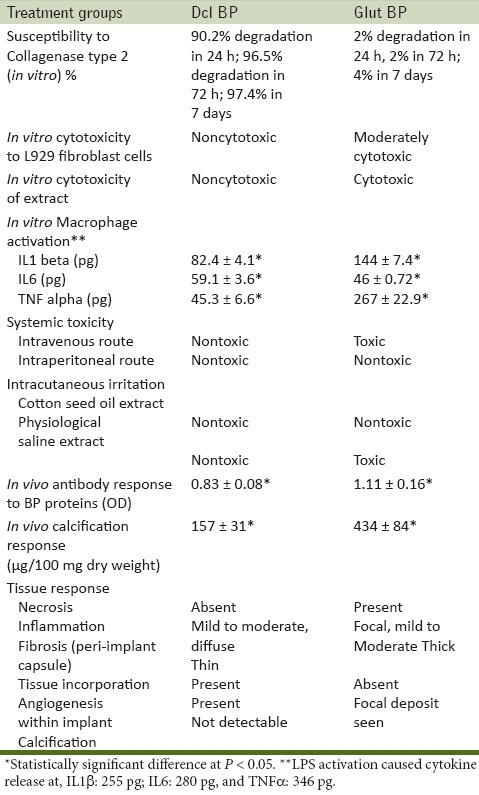
Following Collagenase type II digestion for 24 h, Dcl BP samples had 90.2% degradation with a residual weight of 4.9 ± 0.4 mg. Glut BP group had maximum Collagenase resistance with minimum weight loss of around 1 mg (2%). At 72 h residual weight Dcl BP and Glut BP groups showed a residual weight of 1.8 ± 0.3 mg (96.5% degradation) and 49 ± 1 mg (2% degradation), respectively. At 7 days, the residual weights were 1.3 ± 0.25 mg and 48 ± 1 mg for Dcl BP and Glut BP groups, respectively. At this period, the rate of weight loss has slowed down in the Dcl BP group with only an additional 1% weight loss was noticed in about 7 days. The Glut BP group continued to lose weight at very low rate reaching about 4% at 7 days.
In vitro response
Cytotoxicity: A direct contact
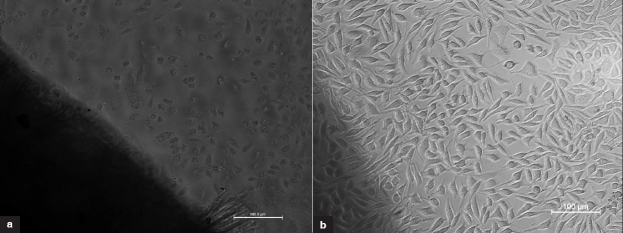
Dcl BP samples were seen noncytotoxic to L929 fibroblasts by a direct contact method [Figure 3]. The Glut BP sample was seen moderately cytotoxic probably because of residual glutaraldehyde. The positive control was severely cytotoxic, and the negative control was noncytotoxic.
Figure 3.
(a) Glut BP sample showing moderate cytotoxicity (b) Dcl BP sample showing noncytotoxic nature
Cytotoxicity testing on extract
The MTT assay of L929 cells after contact with 50% and 25% extracts of Dcl BP showed 96% and 97% metabolic activities, respectively, compared to Glut BP which had 14% and 15.1% metabolic activities, respectively. This clearly showed that the extract of Glut BP samples has significantly affected the metabolic activity of fibroblast cells and hence it is toxic in nature compared to Dcl BP.
Macrophage activation
Cytokine release profile of activated macrophage in response to exposure to different groups is given in Table 2. The positive control group showed the highest cytokine response. With respect to the TNFα and IL1β, highest macrophage activation was seen in the Glut BP group. This was seen statistically significant compared to the Dcl BP group. In the case of IL6, the Dcl BP group showed a higher response when compared to the Glut BP group (P = 0.004).
In vivo response
Acute systemic toxicity testing (ISO 10993-11)
Administration of cotton seed oil extract of the Glut BP sample through an intraperitoneal route did not produce a systemic toxic effect evidenced as loss of body weight more than 2 g, the presence of any abnormalities or death. However, intravenous administration of physiological saline extract of the Glut BP sample produced abnormalities and death immediately after injection in three mice, indicating systemic toxicity of this sample. The control animals did not show any signs of systemic toxicity. The cotton seed oil extract and physiological saline extract of the Dcl BP sample did not produce any abnormalities, loss of weight or death following intraperitoneal and intravenous administration, respectively. This indicated that the Dcl BP extract do not cause systemic toxicity.
Intracutaneous (intradermal) reactivity test (ISO 10993-10)
The physiological saline extract of the Glut BP sample produced an average irritation score of 1.18 which is a positive reactivity for this test. At the same time, the cotton seed oil extract showed 0 score of irritation indicating no intracutaneous irritation. The physiological saline and cotton seed oil extract of Dcl BP samples produced irritation scores of 0.3 and 0.1, respectively, which indicated that the samples are nonirritating as per the set criteria for this test.
Implantation study
Animal implantation was uneventful and all animals in the groups Glut BP and Dcl BP survived the duration of the study uneventfully.
Antibody response
Antibody response against extractable protein from fresh bovine pericardium elicited in animals implanted with different treatment groups is given in Table 2. The Dcl BP group had significantly lower antibody response against fresh bovine pericardial protein compared to the Glut BP group (P < 0.05). This indicated that glutaraldehyde treatment which is done to reduce immune response of xenografts has failed to reduce it in comparison to decellularization.
Calcification response
The result of calcification experiment is given in Table 2. The Dcl BP group explant had significantly lower calcium content of 157 ± 31 μg/100 mg dry weight of tissue in comparison to 434 ± 84 μg/100 mg of dry weight of the Glut BP explant.
Tissue response
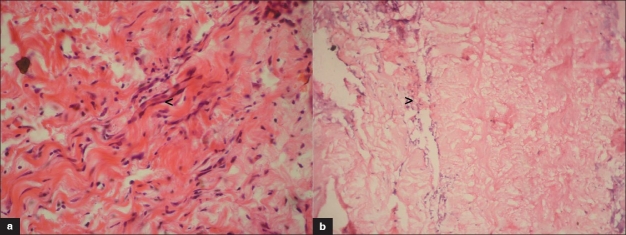
The tissue response studied on hematoxylin and eosin stained paraffin sections is presented in Figure 4. The sections were qualitatively assessed for peri-implant necrosis, inflammatory response, calcification, peri-implant fibrosis, tissue incorporation, and angiogenesis into the implanted material. The Dcl BP implant did not show any peri-implant necrosis. A thin fibrous capsule could be appreciated around the implant. Mild-to-moderate diffuse infiltration of mononuclear cells into the implant is noticed indicating chronic inflammation. Macrophages could be identified at the site of implant degradation. Implant calcification could not be observed. Implant showed excellent ingrowth of cells with fibroblast morphology with evidence of newly laid collagen. Angiogenesis is noticed in the implant as well at the periphery.
Figure 4.
Tissue response to different implants at 60 days in rat subcutaneous implantation. (a) Dcl BP group minimum inflammatory response and uniform host cell incorporation and angiogenesis (arrow) within implant H and E, ×400. (b) Glut BP group, inflammatory response (arrow) in the interphase and remarkably acellular interior (H and E, ×200)
Glut BP explants showed a thick capsule around the implant. Certain areas showed peri-implant necrosis. Angiogenesis was seen only in the periphery of the implant. Focal mild-to-moderate chronic inflammation observed as mononuclear cell infiltration could be seen at the periphery of the implant. The implant interior was remarkably acellular. Calcium deposit could be observed in many sections.
DISCUSSION
In this study, it is attempted to demonstrate that glutaraldehyde treatment of bovine pericardium induces toxic response which will ultimately affect its long-term performance in clinical situation. This is because of its cytotoxicity, persistent inflammation, immunogenicity, calcification, and lack of remodeling. It is reasoned that decellularization of bovine pericardium will bring about positive remodeling of the implant which is brought about by its susceptibility to matrix metalloproteinase which promotes intraimplant host tissue incorporation, new matrix deposition, and angiogenesis besides its low immunogenicity, inflammatory potential, and calcification potential. In this study, a commercially available clinically used glutaraldehyde-treated bovine pericardium was studied and hence it was not further characterized. Decellularized tissue produced by a nondetergent based decellularization method was used for comparison. The decellularization method employed was found adequate as nuclear remnants could not be detected by routine HE staining or by nuclear stain Hoechst 33258. Polyacrylamide gel electrophoresis of residual DNA could not demonstrate DNA bands indicating extensive DNA fragmentation.
The data clearly indicated that the glutaraldehyde-treated bovine pericardium is resistant to Collagenase which is a major enzyme in the body responsible for extracellular matrix remodeling. In the rat subcutaneous implantation model, this was seen as acellular areas in the implants indicating the absence of tissue penetration into the implant. In comparison decellularized bovine pericardium was seen susceptible to Collagenase enzyme and correspondingly the in vivo data showed excellent host tissue incorporation into the implant.
In vitro data such as direct cytotoxicity as well as toxicity of extract clearly suggested cytotoxic nature of the glutaraldehyde-treated bovine pericardium. This is clearly correlated in rat implantation studies where it was observed as areas of peri-implant necrosis around Glut BP samples. Whereas Dcl BP samples or its extracts were seen to be noncytotoxic and subsequently peri-implant necrosis was absent in the rat study. The intracutaneous irritation study and systemic toxicity also showed that the physiological saline extract of the glutaraldehyde-treated bovine pericardium is of toxic nature. Interestingly, only the water-soluble extract was observed as toxic whereas as the cotton seed oil extract was seen nontoxic. Earlier studies have reported on the cytotoxic nature of glutaraldehyde-treated tissue without indicating which fraction is cytotoxic.[8] Macrophages as inflammatory cells as well as antigen-presenting cells have an important role in in vivo response against biomaterials. Hence a macrophage activation study was conducted. This study showed a definite increase in the release of inflammatory cytokines such as TNFα and IL1β in Glut BP samples compared to Dcl BP samples. This clearly indicated that the glutaraldehyde-treated bovine pericardium activates macrophages significantly in comparison to decellularized bovine pericardium. Increased inflammatory response noticed in Glut BP samples of a rat implantation study might be a reflection of what is observed in in vitro. Interestingly, IL6 release was seen higher in the Dcl BP sample compared to Glut BP samples. In contrast to the above observations, a study showed no significant variation between in vitro cytokine release of inflammatory and anti-inflammatory cytokines in response to different polymeric materials.[9]
In vivo data such as antibody response, calcification response, and tissue response demonstrated better biocompatibility for decellularized bovine pericardium compared to glutaraldehyde-treated one. For studying the immune response, focus was given on antibody response elicited against extractable protein from fresh pericardium as it was assumed that in processed bovine pericardium the residual protein is the major immunogen. Since there was complete fragmentation of DNA following decellularization, immunogenicity on this account was not anticipated. DNA fragments less than 300 bp is reported to be nonimmunogenic.[10] Decellularized bovine pericardium had significantly reduced antibody response compared to glutaraldehyde-treated one. This indicated that glutaraldehyde treatment is not necessarily immunosuppressive. A study on antigenicity of glutaraldehyde-treated bovine serum albumin (BSA) reported that glutaraldehyde caused intermolecular cross-linkages forming soluble aggregates; such modified proteins were seen antigenic and produced antibodies against antigenic determinant of BSA as well as against newly acquired groups arising from this modification.[11] The increased antibody response seen in the glutaraldehyde-treated samples might be due to formation of soluble aggregates of BSA. In vivo calcification was seen directly linked to the extent of glutaraldehyde treatment. Glutaraldehyde-treated samples were seen most calcifying as previously reported.[12] Decellularized pericardium had minimum calcification. A previous study has reported direct relation between calcification and antibody response.[13] This relationship was noticed here also. Decellularized pericardium and glutaraldehyde-treated pericardium presented two extreme tissue responses. Glutaraldehyde-treated pericardium showed no host cell incorporation into implant and a thicker fibrous tissue capsule separated the implant from the host tissue. The presence of mild-to-moderate chronic inflammation is noticed in the implant periphery. There is calcification as well as significant antibody response in this group. These conditions may not favor a positive tissue remodeling.[10] Similar observation was previously reported for glutaraldehyde-treated tissue.[8] Decellularized bovine pericardium showed contrasting and desirable tissue response such as thin fibrous capsule, undetectable calcification, implant degradation with laying of new collagen, and mild chronic inflammation. All the above observations point to better implant remodeling.[14] It is assumed that the presence of mononuclear cells in this group should not be of concern since the antibody response elicited in this group was significantly lower compared to the Glut BP group. In contrast the observations noted in the glutaraldehyde-treated group such as thicker fibrous capsule, implant calcification, moderately intense mononuclear cell infiltration in the periphery and angiogenesis restricted to implant periphery and visible lack of host cell incorporation into implant are not conducive for the sustained implant function. Hence the observations made in glutaraldehyde-treated bovine pericardium are not congenial for an acceptable tissue compatibility as well as implant remodeling which is essential for successful long-term clinical performance of a surgical implant.
CONCLUSION
Thus, it is concluded that glutaraldehyde-treated bovine pericardium incites significant toxic, inflammatory, immune, and calcification responses which is not desirable for a long-term function as an implant. Furthermore, glutaraldehyde also prevents tissue remodeling on account of its resistance to matrix metalloproteinase resulting in the implant remaining as a separate entity in the body without physiologically integrating into the host.
ACKNOWLEDGMENTS
This work was supported by a grant from Department of Biotechnology, Government of India. The authors gratefully acknowledge the support of the Director, the Head, and other colleagues in Biomedical Technology Wing, Sree Chitra Tirunal Institute for Medical Sciences and Technology, India.
Footnotes
Source of Support: Department of Biotechnology, Government of India
Conflict of Interest: No conflict of interest.
REFERENCES
- 1.Ionescu MI, Tandon AP. The Ionescu-Shiley Pericardial Xenograft Heart valve. In: Ionescu MI, editor. Tissue Heart Valves. London-Boston: Butterworts; 1979. pp. 203–52. [Google Scholar]
- 2.Jayakrishnan A, Jameela SR. Glutaraldehyde as a fixative in bioprostheses and drug delivery matrices. Biomaterials. 1996;17:471–84. doi: 10.1016/0142-9612(96)82721-9. [DOI] [PubMed] [Google Scholar]
- 3.Shoen FJ, Levy RJ. Calcification of tissue heart valve substitutes: Progress toward understanding and prevention. Ann Thorac Surg. 2005;79:1072–80. doi: 10.1016/j.athoracsur.2004.06.033. [DOI] [PubMed] [Google Scholar]
- 4.Dahm M, Lyman WD, Schwell AB, Factor SM, Frater RW. Immunogenicity of glutaraldehyde-tanned bovine pericardium. J Thorac Cardiovasc Surg. 1990;99:1082–90. [PubMed] [Google Scholar]
- 5.Badylak SF, Freytes DO, Gilbert TW. Extracellular matrix as a biological scaffold material: Structure and function. Acta Biomaterialia. 2009;5:1–13. doi: 10.1016/j.actbio.2008.09.013. [DOI] [PubMed] [Google Scholar]
- 6.Gilbert TW, Sellaro TL, Badylak SF. Decellularization of tissues and organs. Biomaterials. 2006;27:3675–83. doi: 10.1016/j.biomaterials.2006.02.014. [DOI] [PubMed] [Google Scholar]
- 7.Yannas IV. Natural Materials. In: Ratner BD, Hoffman AS, Schoen FJ, Lemons JE, editors. Biomaterial Science. San Diego: Academic Press; 1996. pp. 84–94. [Google Scholar]
- 8.Gendler E, Gendler S, Nimmi ME. Toxic reactions evoked by glutaraldehyde fixed pericardium and cardiac valve tissue bioprostheses. J Biomed Mater Res. 1984;18:727–36. doi: 10.1002/jbm.820180703. [DOI] [PubMed] [Google Scholar]
- 9.Schutte RJ, Xie L, Klitzman B, Reichert WM. In-vivo cytokine-associated response to biomaterials. Biomaterials. 2009;30:160–8. doi: 10.1016/j.biomaterials.2008.09.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Badylak SF, Gilbert TW. Immune response to biologic scaffold materials. Semin Immunol. 2008;20:109–16. doi: 10.1016/j.smim.2007.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Habeeb AF. A study of the antigenicity of formaldehyde and glutaraldehyde treated bovine serum albumin and ovlabumin-bovine serum albumin conjugate. J Immunol. 1969;102:457–65. [PubMed] [Google Scholar]
- 12.Jorge-Herrero E, Fernandez P, Guiterrez M, Castilo-Olivares JL. Study of calcification of bovine pericardium: Analysis of the implications of lipids and proteoglycans. Biomaterials. 1991;12:683–9. doi: 10.1016/0142-9612(91)90117-s. [DOI] [PubMed] [Google Scholar]
- 13.Human P, Zilla P. Characterization of the immune response to valve bioprostheses and its role in primary tissue failure. Ann Thorac Surg. 2001;71:S385–8. doi: 10.1016/s0003-4975(01)02492-4. [DOI] [PubMed] [Google Scholar]
- 14.Liang HC, Chang Y, Hsu CK, Lee MH, Sung HW. Effects of cross linking degree of an acellular biological tissue on it tissue regeneration pattern. Biomaterials. 2004;25:3541–52. doi: 10.1016/j.biomaterials.2003.09.109. [DOI] [PubMed] [Google Scholar]