Abstract
Pulmonary arterial hypertension (PAH) is characterized by pulmonary vascular remodeling leading to right ventricular (RV) hypertrophy and failure. Intralipid®, a source of parenteral nutrition for patients, contains γ-linolenic acid and soy-derived phytoestrogens that are protective for lungs and heart. We therefore investigated the therapeutic potential of Intralipid® in preventing and rescuing monocrotaline-induced PAH and RV dysfunction. PAH was induced in male rats with monocrotaline (60mg/kg). Rats then received daily Intralipid® (1mL of 20% Intralipid®/day, intraperitoneal) from day 1 to day 30 for prevention protocol or from day 21 to day 30 for rescue protocol. Other monocrotaline-injected rats were left untreated to develop severe PAH by day 21 or RV failure (RVF) by ~day 30. Saline or Intralipid®-treated rats served as controls. Significant increase in RV-pressure and decrease in RV-ejection fraction in RVF group resulted in high mortality. Therapy with Intralipid® resulted in 100% survival and prevented PAH-induced RVF by preserving RV-pressure and RV-ejection fraction, and preventing RV-hypertrophy and lung remodeling. In pre-existing severe PAH, Intralipid® attenuated most lung and RV abnormalities. The beneficial effects of Intralipid® in PAH seem to result from interplay of various factors among which preservation and/or stimulation of angiogenesis, suppression and/or reversal of inflammation, fibrosis and hypertrophy in both lung and RV appear to be major contributors. In conclusion, Intralipid® not only prevents the development of PAH and RV failure, but also rescues pre-existing severe PAH.
Keywords: pulmonary arterial hypertension, Intralipid®, inflammation, angiogenesis, hypertrophy
Introduction
Category I pulmonary hypertension, also known as pulmonary arterial hypertension (PAH), includes idiopathic, familial, and acquired PAH1. PAH is characterized by arterial obstruction resulting from excessive proliferation of pulmonary artery smooth muscle cells and endothelial cells, endothelial dysfunction and inflammation2. Progressive increase in pulmonary artery pressure eventually leads to right ventricular (RV) hypertrophy, RV failure and sudden cardiac death. Currently available therapies for PAH only improve the symptoms and delay the progression of the disease. A definitive therapy for PAH is still lacking3.
Lipids and in particular polyunsaturated fatty acids have recently received special cardiovascular research attention. Intralipid® (ILP) is the first safe emulsion of fatty acids for human use. ILP has been widely used as a source of parenteral nutrition for patients for more than 4 decades. ILP 20% is an emulsion of soybean oil (20%), egg-yolk phospholipids (1.2%) and glycerol (2.2%). Gamma-linolenic acid (GLA), one of the key essential fatty acids in ILP, has been shown to be protective against doxorubicin-induced cardiotoxicity4. A soy-derived phytoestrogen, genistein, has been shown to ameliorate monocrotaline (MCT)-induced PAH5.
As some of the constituents of ILP, like GLA and soy-derived phytoestrogens, are protective for lungs and heart, we hypothesized that ILP may prevent and rescue the development of PAH and RV failure. We report that ILP prevents MCT-induced pulmonary and cardiac dysfunction by preserving both lung and RV structure. Most importantly, ILP is effective in rescuing severe pre-existing PAH by restoring lung and RV structure and function.
Methods
For full methodological details please see the supplement at http://hyper.ahajournals.org.
Animals and treatments
Male Sprague-Dawley rats ~3–4 months old (350–400 g) were treated with a single subcutaneous dose (60 mg/kg) of monocrotaline (MCT) at day-0 to induce PAH by day-21 and RVF by day-30. Some MCT-treated rats received daily Intralipid® (1mL of 20% Intralipid®/day, intraperitoneal) from day 1 to day 30 for prevention protocol or from day 21 to day 30 for rescue protocol. Rats treated with a single dose of PBS, or daily injection of ILP for 30 days served as controls (Fig. S1,A,B). Protocols received institutional review and committee approval.
Cardiac and pulmonary hemodynamics
B-mode, M-mode and pulmonary pulsed-wave Doppler echocardiography were performed (VisualSonics Vevo 770, 30-MHz linear transducer) to accurately monitor the stage of disease. Direct cardiac catheterisation was performed to record RV pressure just before sacrifice6.
Gross histologic evaluation
The lungs, RV wall, left ventricular (LV) wall and interventricular septum (IVS) were dissected. The wet lung weight and RV hypertrophy index [RV/(LV+IVS)] were determined.
Real time PCR
Total RNA from lungs and RV were isolated using Trizol (Invitrogen) and reverse transcribed with oligo-dt using the Omniscript RT kit (Qiagen). Controls were: (1) the reaction without reverse transcriptase; and (2) H2O instead of cDNA.
Western blot analysis, immunohistochemistry and imaging
Standard Western blot analysis was performed using whole lung and RV lysates. Tissue sections of hearts and lungs were stained with immunofluorescence, immunoperoxidase, standard hematoxylin-eosin or Masson trichrome staining. The images were acquired using light microscopes (Zeiss Axiovert 135, and Nikon Eclipse E400) or with a high-resolution laser scanning confocal microscope (Olympus).
Statistical Analysis
One-way ANOVA tests were used to compare between groups using SPSS13.0 for Windows. When significant differences were detected, individual mean values were compared by post-hoc tests (Bonferroni) that allowed for multiple comparisons. P<0.05 were considered statistically significant. Values are expressed as mean±SE.
Results
Improvement of cardiopulmonary function with ILP therapy
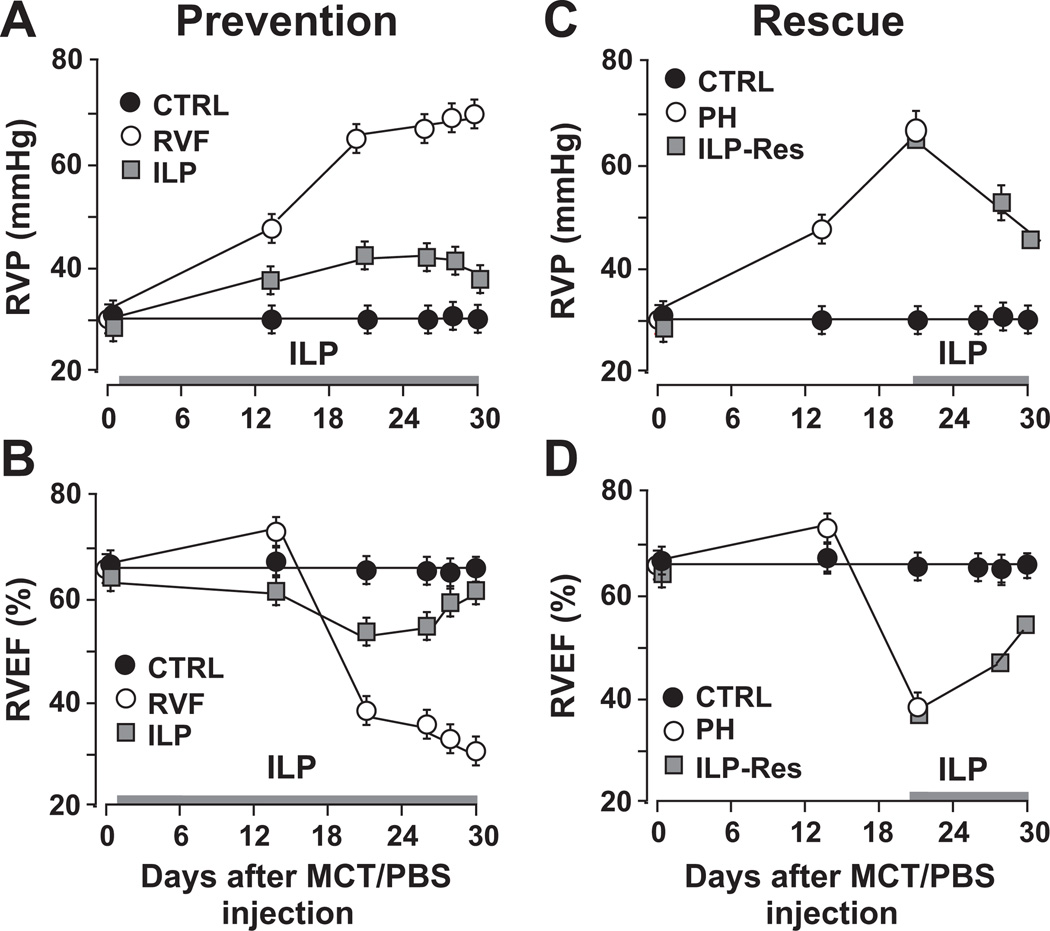
A single injection of MCT induced RV failure secondary to PAH in ~30 days (Fig. S1). Peak systolic RV pressure (RVP) increased sharply to 68±1mmHg at day 21 and further to 70±2mmHg at ~day 30 (Fig. 1A). RV ejection fraction (RVEF) was initially increased to 72±2% at day 14, a stage known as compensated hypertrophy, but later declined rapidly to 39±1% at day 21 and further to 31±3% at day 30, a clear indication of RVF (Fig. 1B). ILP also prevented the sharp increase in RVP and the development of RVF by maintaining RVEF above 50% throughout the course of therapy (Fig. 1A,B). Both RVP (34±2 in ILP vs. 30±2mmHg in CTRL) and RVEF (62.8±1.5% in ILP vs. 66±1% in CTRL) did not differ significantly between ILP and CTRL at day 30. In RVF group mortality started as early as day 24 and reached 100% by day 32 (Fig. S2). Daily ILP therapy from day-1 to 30 resulted in 100% survival of rats and significantly improved body weight gain (Fig. S2).
Figure 1. ILP prevents and rescues severe PAH.
A, C. Right ventricular pressure (RVP) measured by echocardiography throughout the course of experiment except at the end of the experiment measured by direct RV catheterization (day-30 for all groups and day-21 in PAH) and B, D. RV ejection fraction (RVEF), measured by echocardiography, as a function of time in prevention and rescue protocol (n=7–8 rats per group).
Since PAH is not always diagnosed early, we explored whether ILP could also rescue pre-existing severe PAH. As severe PAH was already evident at day 21 (Figs. 1,2), we started ILP therapy at day 21 until day 30 (Fig. S1). We found that even 10 days of ILP therapy was sufficient to rescue pre-existing severe PAH as RVP (44.7±1.1mmHg) and RVEF (53.6±0.4%) were rescued to a great extent (Fig. 1C,D). These data suggest that ILP not only prevents PAH but also rescues pre-existing severe PAH.
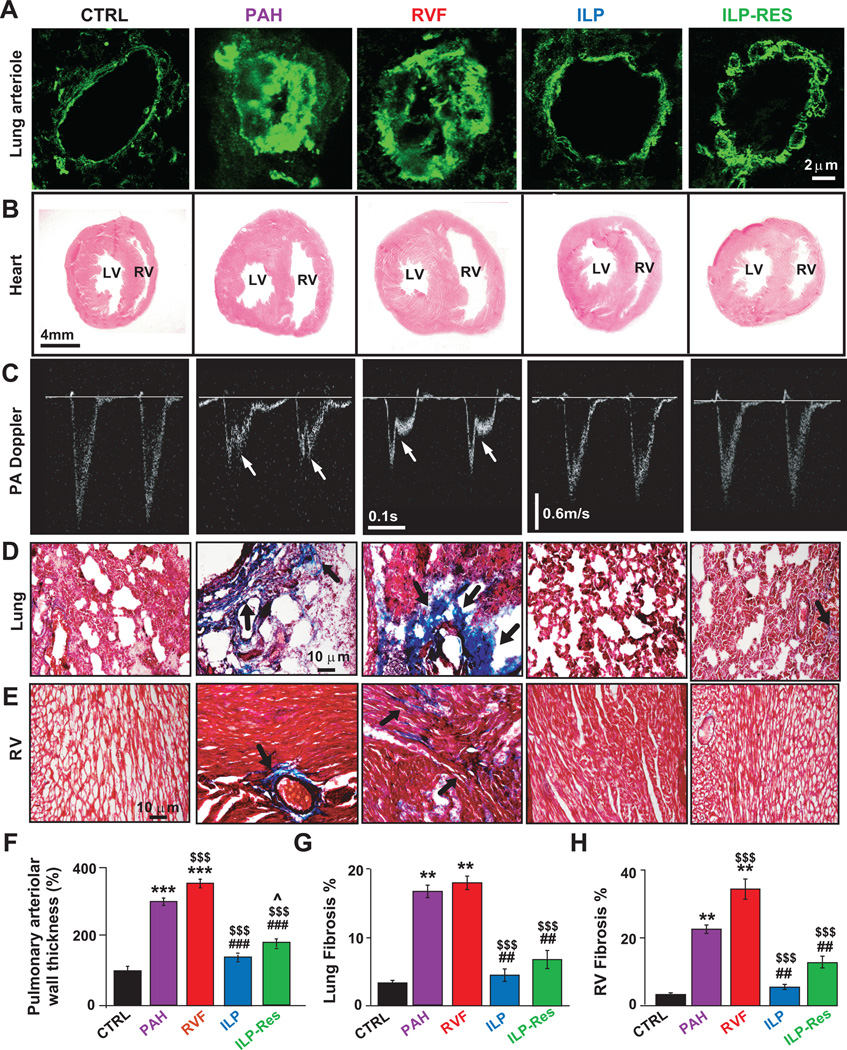
Figure 2. ILP preserves and reverses the cardiopulmonary abnormalities induced by PAH.
A. Immunofluorescence labelling of pulmonary arterioles stained with anti-α-smooth muscle actin antibody (green). B. Hematoxylin-eosin staining of heart cross-sections. C. Echocardiographic images of pulse-waved Doppler (taken at day 30 except for PAH-group at day 21) show mid-systolic notch (arrows) present in PA flow in PAH and RVF groups only. Masson trichrome staining of lung (D) and RV sections (E), arrows point to fibrosis (blue). Quantification of pulmonary arteriolar medial wall thickness (F), lung fibrosis (G) and RV fibrosis (H), please see http://hyper.ahajournals.org for quantification details. **P<0.01 vs. CTRL; ***P<0.001 vs. CTRL; $$$P<0.001 vs. PAH; ##P<0.01 vs. RVF; ###P<0.001 vs. RVF; ^P<0.05 vs. ILP (n=3–4 animals/group).
Preservation and reversal of cardiopulmonary structure with ILP therapy
PAH also led to structural changes in the lungs and RV. ILP prevented the increase in lung weight (1.8±0.06g in ILP vs. 2.38±0.2g in RVF; 1.36±0.1g in CTRL, Fig. S1C), pulmonary arteriolar medial hypertrophy (Fig. 2A,F), RV hypertrophy (RV/(LV+IVS)=0.33±0.04 in ILP vs. 0.68±0.1 in RVF; 0.24±0.06 in CTRL, Fig. S1D), RV dilatation (Fig. 2B) and the increase in RV end-diastolic diameter (Fig. S2A). ILP was also able to even reverse pre-existing severe lung and RV remodeling (lung weight: 2.01±0.05g in ILP-Res vs. 2.39±0.15g in PAH, and RV hypertrophy: 0.39±0.02 in ILP-Res vs. 0.65±0.08 in PAH, Figs. S1C,D). ILP also prevented and reversed both the increase in pulmonary artery (PA) acceleration time and appearance of mid-systolic notching on PA flow (Fig. 2C).
ILP ameliorates and/or reverses cardiopulmonary fibrosis and inflammation associated with PAH-induced RVF
ILP therapy was associated with significantly lower total fibrosis in the lungs (4.46±0.92% in ILP vs. 18±1% in RVF; 3.35±0.35% in CTRL, Fig. 2D,G, S3) and RV (5.53±0.67 in ILP vs. 34.3±2.9% in RVF; 3.33±0.43% in CTRL, Fig. 2E,H, S3). Interestingly ILP was also able to reverse the pre-existing fibrosis in both lung (5.80±0.48% in ILP-Res vs. 17.33±2% in PAH) and RV (7.99±1.05% in ILP-Res vs. 21.3±0.66% in PAH).
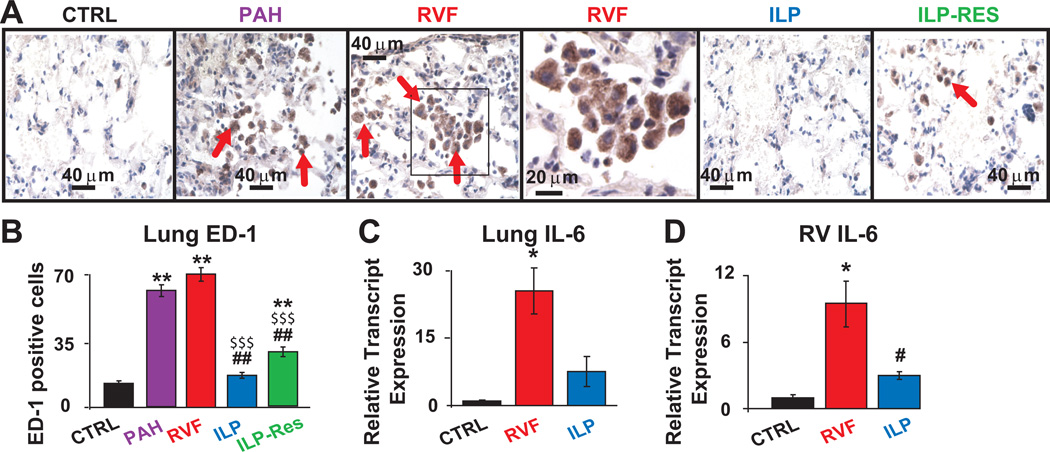
In addition to fibrosis, ILP not only prevented but also reversed the significant increase in ED-1 positive inflammatory cells in PAH and RVF (cells per field: 19±3 in ILP vs. 70±4 in RVF, 14±2 in CTRL; 28±3 in ILP-Res vs. 64±2 in PAH, Fig. 3A,B). In addition, in the prevention protocol, ILP therapy was associated with significantly lower levels of IL-6 transcript (one of the known pro-inflammatory markers of PAH, Fig. 3C,D) and cleaved caspase-3 levels in both tissues (Fig. S4). These data suggest that suppression and/or reversal of lung macrophages, cardiopulmonary fibrosis as well as restoration of IL-6 and caspase-3 (in the prevention protocol) by ILP could be the mechanisms contributing to improved cardiopulmonary function.
Figure 3. ILP therapy was associated with suppression and even reversal of pulmonary macrophage recruitment as well as prevention of cardiopulmonary IL-6 upregulation.
A. Lung sections stained for ED1, a marker for macrophage/monocyte inflammatory cells. Red arrows indicate the ED1-positive cells (brown). The box in RVF is also shown at higher magnification. B. Quantification of ED1-positive cells per field **P<0.01 vs. CTRL; $$$P<0.001 vs. PAH; ##P<0.01 vs. RVF (n=3–4 animals per group) please see http://hyper.ahajournals.org for quantification details. Relative transcript expressions of lung IL-6 (C) and RV IL-6 (D) *P<0.05 vs. CTRL; #P<0.05 vs. RVF (n=5 animals per group).
ILP prevents and rescues loss of capillaries in RV
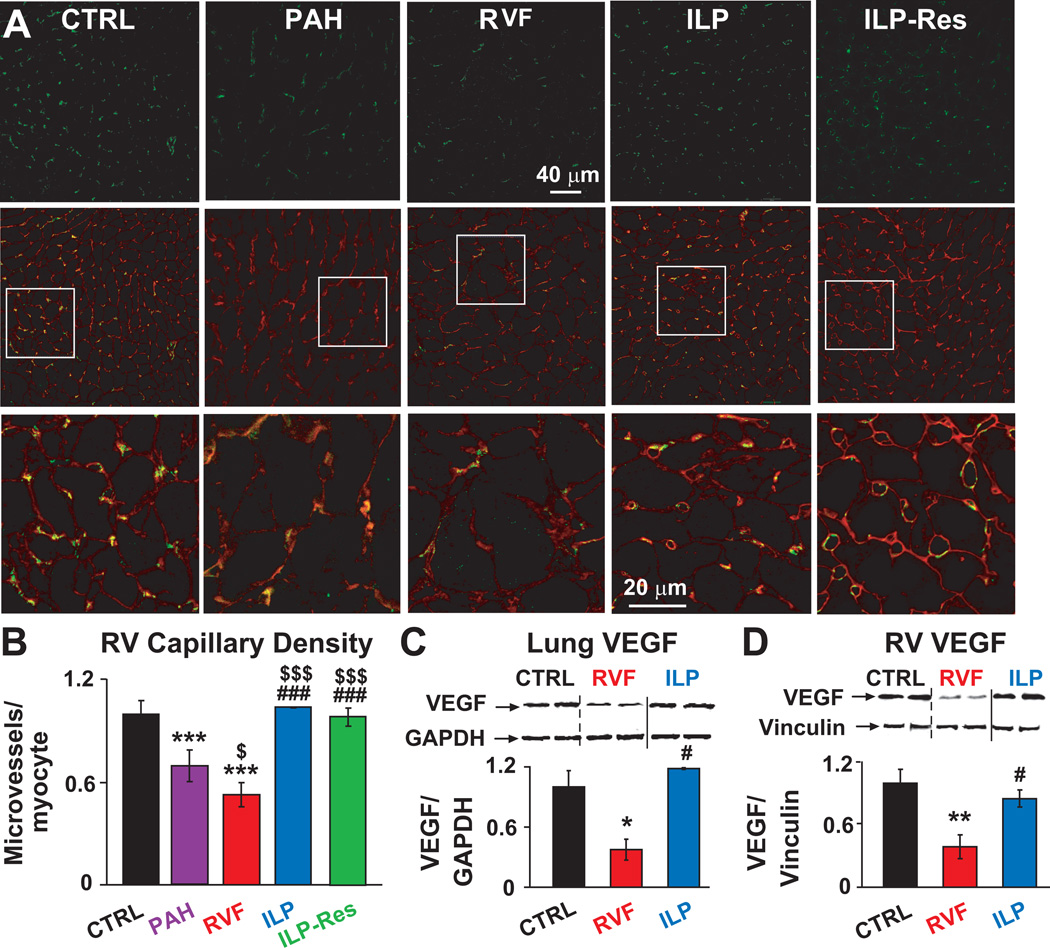
We examined whether preservation or stimulation of cardiac capillary growth participated in ILP-induced prevention and/or rescue of PAH. ILP therapy not only prevented the loss of blood vessels observed in RVF (microvessels/cardiomyocytes normalized to CTRL: 1.03±0.001 in ILP vs. 0.52±0.07 in RVF), it even stimulated capillary growth by fully restoring the loss of capillaries in RV (0.98±0.002 in ILP-Res vs. 0.69±0.07 in PAH, Fig. 4A,B). Concomitant with decline of capillary density, there was a significant reduction of pro-angiogenic vascular endothelial growth factor (VEGF) protein levels in lungs and RV of RVF which was fully restored by ILP prevention therapy in both lungs and RV (Fig. 4C,D).
Figure 4. Preservation and even stimulation of cardiac capillary growth as well as preservation of cardiopulmonary VEGF expression by ILP.
A. Single confocal images of RV sections immunostained for CD31 (green, upper panel), overlay of CD31 (green) and wheat germagglutinin (red, middle panel) and at higher display magnification of the respective squares (lower panel). B. Quantification of RV capillary density as microvessels/cardiomyocyte. Representative immunoblots of lung (C) and RV (D) lysates labelled with anti-VEGF and GAPDH or vinculin antibodies together with Western blot analyses normalized to GAPDH or vinculin. *P<0.05 vs. CTRL; **P<0.01 vs. CTRL; ***P<0.001 vs. CTRL; $P<0.05 vs. PAH; $$$P<0.001 vs. PAH; #P<0.05 vs. RVF; ###P<0.05 vs. RVF (n=3 animals per group).
Discussion
Here we show that Intralipid® (ILP), the first clinically safe lipid emulsion for parenteral nutrition in humans, not only prevents the progression of PAH to RV failure but also effectively rescues pre-existing severe PAH by reversing cardiopulmonary dysfunction in rats. ILP therapy resulted in 100% survival, whereas mortality in untreated rats reached 100% by day 32 (Fig. S2). The complete disappearance of the characteristic mid-systolic notching on PA flow in PAH and RVF rats was another very strong indicator of the effective prevention and rescue action of PAH by ILP (Fig. 2C). ILP was not only able to prevent the onset of PAH-induced RVF, but to also reverse the deleterious effects of pre-existing PAH. We demonstrated that preservation and/or stimulation of angiogenesis, suppression and/or reversal of hypertrophy, inflammation and fibrosis are pivotal events associated with the therapeutic efficacy of ILP (Fig. S5).
ILP therapy preserves and rescues lung and heart structure and function
Our results clearly demonstrate that ILP prevents the development of fatal PAH and rescues even established pre-existing PAH. Since ILP has been shown to protect the heart (against ischemia/reperfusion injury7 and local anesthetic-induced cardiac arrest8) and lungs (adult dogs9 and newborn rats10), we speculate that the beneficial actions of ILP are mediated through its combined protective effects on the lungs and on the heart. One of the key essential fatty acids in ILP, γ-linolenic acid (GLA), is a precursor of prostacyclin, a potent pulmonary vasodilator and platelet aggregation inhibitor11 that is effective in the treatment of PAH12. GLA improves lung microvascular permeability, oxygenation, and cardiopulmonary function and reduces pro-inflammatory eicosanoid synthesis and lung inflammation13. Besides GLA, ILP also contains 20% soybean oil. Genistein, a soy-derived phytoestrogen also found in ILP, has previously been shown to ameliorate MCT-induced PAH5. Thus, the combined effects of the soy and GLA, along with other components found in ILP, offer even more optimal and global cardiopulmonary benefits against PAH.
ILP ameliorates lung and RV inflammation, fibrosis and apoptosis associated with PAH
We have observed severe lung and RV remodeling in PAH and RVF as previously reported6, as well as pulmonary and RV fibrosis (Figs. 1,2). ILP not only prevented but also reversed these cardiopulmonary abnormalities observed in PAH-induced RVF. Inflammation plays an important role in the progression of PAH in animal models as well as in PAH patients, most notably in the lungs14 and the RV15. IL-6 is an established pro-inflammatory cytokine in PAH16, and its serum levels are elevated in PAH patients17. In fact, IL-6 serum levels could predict patient survival, as these levels were correlated with the severity of the disease18. Here we show that ILP therapy prevented the upregulation of IL-6 both in the lungs and the RV of PAH rats. Furthermore, ILP not only prevented but also rescued the accumulation of macrophages/monocytes in the lungs (Fig. 3). RV cardiomyocyte apoptosis has recently been shown to be a key mechanism in the progression of RVF secondary to PAH19. The significant upregulation of cleaved caspase-3 protein in both lung and RV of RVF were effectively prevented by ILP (Fig. S4).
Taken together, increase in lung weight, medial hypertrophy and pulmonary fibrosis observed in the PAH and RVF groups indicate active lung remodelling, while RV hypertrophy, fibrosis, inflammation and apoptosis indicate RV remodelling. ILP very effectively attenuated and remarkably reversed adverse cardiopulmonary remodelling associated with PAH and RVF.
Preservation and/or stimulation of cardiopulmonary angiogenesis by ILP
RV ischemia has been described in hearts of PAH patients with normal coronary arteries20 caused by increased oxygen demand and loss of RV microvessels21. Stimulation of pulmonary neoangiogenesis has been suggested as treatment of PAH-induced RV failure22. Cardiac angiogenesis has also been shown to be a key event in maintaining heart function during adaptive hypertrophy23. We demonstrated that ILP therapy not only preserved the capillary density but also stimulated capillary growth and regeneration in the RV after the onset of PAH. The decrease in RV capillary density has been proposed to be due to an insufficient up-regulation of vascular endothelial growth factor (VEGF)24. We found a significant reduction of VEGF protein in lungs and RV of RVF. ILP was able to prevent VEGF downregulation in both tissues (Fig. 4C,D), which may also contribute to its beneficial effect. Therefore, increased myocardial blood vessels and preservation of VEGF by ILP both in lung and heart may underlie the prevention of RV failure and decrease in PH severity, thus leading to marked improvements in cardiopulmonary structure and function.
Conclusion
In conclusion, ILP prevents and rescues the development of MCT-induced PAH, RV hypertrophy and RV failure by preserving and even reversing the abnormalities in lung and heart structure and function. Suppression and/or reversal of inflammation, fibrosis, and hypertrophy as well as preservation and/or stimulation of angiogenesis are vital mechanisms in ILP-induced prevention and rescue of PAH (Fig. S5). Our findings raise the exciting prospect that ILP, a clinically safe lipid emulsion for human use, may be used for prevention/treatment of PAH and consequent RV failure, which most definitely warrants further investigation.
Perspectives
Despite significant advances in cardiopulmonary research, PAH still remains a difficult disease to treat, as therapeutic strategies to simultaneously reduce pulmonary vascular damage and prevent RV dysfunction are lacking. The hallmark of PAH is remodelling of pulmonary vasculature, resulting in RV hypertrophy and failure. As daily administration of Intralipid® 1) protected rats from monocrotaline-induced PAH, 2) attenuated RV dysfunction, and 3) proved to be very effective in rescuing severe pre-existing PAH, Intralipid® promises new clinical applications in patients with PAH.
Acknowledgments
Sources of funding
Supported by HL089876, HL089876S1 and HL088640 (M.E.)
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosures
None
Contributor Information
Soban Umar, Department of Anaesthesiology, Division of Molecular Medicine, David Geffen School of Medicine at UCLA, Los Angeles, CA, USA.
Rangarajan Nadadur, Department of Anaesthesiology, Division of Molecular Medicine, David Geffen School of Medicine at UCLA, Los Angeles, CA, USA.
Jingyuan Li, Department of Anaesthesiology, Division of Molecular Medicine, David Geffen School of Medicine at UCLA, Los Angeles, CA, USA.
Federica Maltese, Department of Anaesthesiology, Division of Molecular Medicine, David Geffen School of Medicine at UCLA, Los Angeles, CA, USA.
Parisa Partownavid, Department of Anaesthesiology, Division of Molecular Medicine, David Geffen School of Medicine at UCLA, Los Angeles, CA, USA.
Arnoud van der Laarse, Department of Anaesthesiology, Division of Molecular Medicine, David Geffen School of Medicine at UCLA, Los Angeles, CA, USA; Department of Cardiology, Leiden University Medical Center, Leiden, the Netherlands.
Mansoureh Eghbali, Department of Anaesthesiology, Division of Molecular Medicine, David Geffen School of Medicine at UCLA, Los Angeles, CA, USA.
References
- 1.Rabinovitch M. Molecular pathogenesis of pulmonary arterial hypertension. J Clin Invest. 2008;118:2372–2379. doi: 10.1172/JCI33452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Farber HW, Loscalzo J. Pulmonary arterial hypertension. N Engl J Med. 2004;351:1655–1665. doi: 10.1056/NEJMra035488. [DOI] [PubMed] [Google Scholar]
- 3.Umar S, Steendijk P, Ypey DL, Atsma DE, van der Wall EE, Schalij MJ, van der LA. Novel approaches to treat experimental pulmonary arterial hypertension: a review. J Biomed Biotechnol. 2010;2010:702–836. doi: 10.1155/2010/702836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chakrabarti KB, Hopewell JW, Wilding D, Plowman PN. Modification of doxorubicin-induced cardiotoxicity: effect of essential fatty acids and ICRF-187(dexrazoxane) Eur J Cancer. 2001;37:1435–1442. doi: 10.1016/s0959-8049(01)00145-9. [DOI] [PubMed] [Google Scholar]
- 5.Homma N, Morio Y, Takahashi H, Yamamoto A, Suzuki T, Sato K, Muramatsu M, Fukuchi Y. Genistein, a Phytoestrogen, Attenuates Monocrotaline-Induced Pulmonary Hypertension. Respiration. 2006;73:105–112. doi: 10.1159/000088946. [DOI] [PubMed] [Google Scholar]
- 6.Umar S, de Visser YP, Steendijk P, Schutte CI, Laghmani EH, Wagenaar GTM, Bax WH, Mantikou E, Pijnappels DA, Atsma DE, Schalij MJ, van der Wall EE, van der Laarse A. Allogenic stem cell therapy improves right ventricular function by improving lung pathology in rats with pulmonary hypertension. Am J Physiol Heart Circ Physiol. 2009;297:H1606–H1616. doi: 10.1152/ajpheart.00590.2009. [DOI] [PubMed] [Google Scholar]
- 7.Liu SL, Wang Y, Wang RR, Chai YF, Wu W, Huang H, Liu J. Protective effect of intralipid on myocardial ischemia/reperfusion injury in isolated rat heart. Zhongguo Wei Zhong Bing Ji Jiu Yi Xue. 2008;20:227–230. [PubMed] [Google Scholar]
- 8.Weinberg GL, Di GG, Ripper R, Kelly K, Massad M, Edelman L, Schwartz D, Shah N, Zheng S, Feinstein DL. Resuscitation with lipid versus epinephrine in a rat model of bupivacaine overdose. Anesthesiology. 2008;108:907–913. doi: 10.1097/ALN.0b013e31816d91d2. [DOI] [PubMed] [Google Scholar]
- 9.Ali J, Wood LD. The acute effects of intralipid on lung function. J Surg Res. 1985;38:599–605. doi: 10.1016/0022-4804(85)90081-2. [DOI] [PubMed] [Google Scholar]
- 10.Sosenko IR, Innis SM, Frank L. Intralipid increases lung polyunsaturated fatty acids and protects newborn rats from oxygen toxicity. Pediatr Res. 1991;30:413–417. doi: 10.1203/00006450-199111000-00004. [DOI] [PubMed] [Google Scholar]
- 11.Guivernau M, Meza N, Barja P, Roman O. Clinical and experimental study on the long-term effect of dietary gamma-linolenic acid on plasma lipids, platelet aggregation, thromboxane formation, and prostacyclin production. Prostaglandins Leukot Essent Fatty Acids. 1994;51:311–316. doi: 10.1016/0952-3278(94)90002-7. [DOI] [PubMed] [Google Scholar]
- 12.Tawara S, Fukumoto Y, Shimokawa H. Effects of combined therapy with a Rho-kinase inhibitor and prostacyclin on monocrotaline-induced pulmonary hypertension in rats. J Cardiovasc Pharmacol. 2007;50:195–200. doi: 10.1097/FJC.0b013e31806befe6. [DOI] [PubMed] [Google Scholar]
- 13.Gadek JE, DeMichele SJ, Karlstad MD, Pacht ER, Donahoe M, Albertson TE, Van HC, Wennberg AK, Nelson JL, Noursalehi M. Effect of enteral feeding with eicosapentaenoic acid, gamma-linolenic acid, and antioxidants in patients with acute respiratory distress syndrome. Enteral Nutrition in ARDS Study Group. Crit Care Med. 1999;27:1409–1420. doi: 10.1097/00003246-199908000-00001. [DOI] [PubMed] [Google Scholar]
- 14.Dorfmuller P, Perros F, Balabanian K, Humbert M. Inflammation in pulmonary arterial hypertension. Eur Respir J. 2003;22:358–363. doi: 10.1183/09031936.03.00038903. [DOI] [PubMed] [Google Scholar]
- 15.Broderick TL, Wang Y, Gutkowska J, Wang D, Jankowski M. Downregulation of oxytocin receptors in right ventricle of rats with monocrotaline-induced pulmonary hypertension. Acta Physiol.(Oxf) 2010;200:147–158. doi: 10.1111/j.1748-1716.2010.02134.x. [DOI] [PubMed] [Google Scholar]
- 16.Humbert M, Monti G, Brenot F, Sitbon O, Portier A, Grangeot-Keros L, Duroux P, Galanaud P, Simonneau G, Emilie D. Increased interleukin-1 and interleukin-6 serum concentrations in severe primary pulmonary hypertension. Am J Respir Crit Care Med. 1995;151:1628–1631. doi: 10.1164/ajrccm.151.5.7735624. [DOI] [PubMed] [Google Scholar]
- 17.Junhui Z, Xingxiang W, Guosheng F, Yunpeng S, Furong Z, Junzhu C. Reduced number and activity of circulating endothelial progenitor cells in patients with idiopathic pulmonary arterial hypertension. Respir Med. 2008;102:1073–1079. doi: 10.1016/j.rmed.2007.12.030. [DOI] [PubMed] [Google Scholar]
- 18.Soon E, Holmes AM, Treacy CM, Doughty NJ, Southgate L, Machado RD, Trembath RC, Jennings S, Barker L, Nicklin P, Walker C, Budd DC, Pepke-Zaba J, Morrell NW. Elevated levels of inflammatory cytokines predict survival in idiopathic and familial pulmonary arterial hypertension. Circulation. 2010;122:920–927. doi: 10.1161/CIRCULATIONAHA.109.933762. [DOI] [PubMed] [Google Scholar]
- 19.Campian ME, Verberne HJ, Hardziyenka M, de BK, Selwaness M, van den Hoff MJ, Ruijter JM, van Eck-Smit BL, de Bakker JM, Tan HL. Serial noninvasive assessment of apoptosis during right ventricular disease progression in rats. J Nucl Med. 2009;50:1371–1377. doi: 10.2967/jnumed.108.061366. [DOI] [PubMed] [Google Scholar]
- 20.Gomez A, Bialostozky D, Zajarias A, Santos E, Palomar A, Martinez ML, Sandoval J. Right ventricular ischemia in patients with primary pulmonary hypertension. J Am Coll Cardiol. 2001;38:1137–1142. doi: 10.1016/s0735-1097(01)01496-6. [DOI] [PubMed] [Google Scholar]
- 21.Partovian C, Adnot S, Eddahibi S, Teiger E, Levame M, Dreyfus P, Raffestin B, Frelin C. Heart and lung VEGF mRNA expression in rats with monocrotaline- or hypoxia-induced pulmonary hypertension. Am J Physiol Heart Circ Physiol. 1998;275:H1948–H1956. doi: 10.1152/ajpheart.1998.275.6.H1948. [DOI] [PubMed] [Google Scholar]
- 22.Campbell AIM, Zhao Y, Sandhu R, Stewart DJ. Cell-Based Gene Transfer of Vascular Endothelial Growth Factor Attenuates Monocrotaline-Induced Pulmonary Hypertension. Circulation. 2001;104:2242–2248. doi: 10.1161/hc4201.097838. [DOI] [PubMed] [Google Scholar]
- 23.Sano M, Minamino T, Toko H, Miyauchi H, Orimo M, Qin Y, Akazawa H, Tateno K, Kayama Y, Harada M, Shimizu I, Asahara T, Hamada H, Tomita S, Molkentin JD, Zou Y, Komuro I. p53-induced inhibition of Hif-1 causes cardiac dysfunction during pressure overload. Nature. 2007;446:444–448. doi: 10.1038/nature05602. [DOI] [PubMed] [Google Scholar]
- 24.Bogaard HJ, Natarajan R, Henderson SC, Long CS, Kraskauskas D, Smithson L, Ockaili R, McCord JM, Voelkel NF. Chronic pulmonary artery pressure elevation is insufficient to explain right heart failure. Circulation. 2009;120:1951–1960. doi: 10.1161/CIRCULATIONAHA.109.883843. [DOI] [PubMed] [Google Scholar]