Abstract
The uricosuric diuretic agent tienilic acid (TA) is a thiophene-containing compound that is metabolized by P450 2C9 to 5-OH-TA. A reactive metabolite of TA also forms a covalent adduct to P450 2C9 that inactivates the enzyme and initiates immune-mediated hepatic injury in humans, purportedly through a thiophene-S-oxide intermediate. The 3-thenoyl regioisomer of TA, tienilic acid isomer (TAI), is chemically very similar and is reported to be oxidized by P450 2C9 to a thiophene-S-oxide, yet it is not a mechanism-based inactivator (MBI) of P450 2C9 and is reported to be an intrinsic hepatotoxin in rats. The goal of the work presented in this manuscript was to identify the reactive metabolites of TA and TAI by the characterization of products derived from P450 2C9-mediated oxidation. In addition, in silico approaches were used to better understand both the mechanisms of oxidation of TA and TAI and/or the structural rearrangements of oxidized thiophene compounds. Incubation of TA with P450 2C9 and NADPH yielded the well-characterized 5-OH-TA metabolite as the major product. However, contrary to previous reports, it was found that TAI was oxidized to two different types of reactive intermediates that ultimately lead to two types of products, a pair of hydroxythiophene/thiolactone tautomers and an S-oxide dimer. Both TA and TAI incorporated 18O from 18O2 into their respective hydroxythiophene/thiolactone metabolites indicating that these products are derived from an arene oxide pathway. Intrinsic reaction coordinate calculations of the rearrangement reactions of the model compound 2-acetylthiophene-S-oxide showed that a 1,5-oxygen migration mechanism is energetically unfavorable and does not yield the 5-OH product, but instead yields a six-membered oxathiine ring. Therefore, arene oxide formation and subsequent NIH-shift rearrangement remains the favored mechanism for formation of 5-OH-TA. This also implicates the arene oxide as the initiating factor in TA induced liver injury via covalent modification of P450 2C9. Finally, in silico modeling of P450 2C9 active site ligand interactions with TA using the catalytically active iron-oxo species revealed significant differences in the orientations of TA and TAI in the active site which correlated well with experimental results showing that TA was oxidized only to a ring carbon hydroxylated product, whereas TAI formed both ring carbon hydroxylated products and an S-oxide.
Introduction
Thiophene is a five-membered heteroaromatic ring that contains one sulfur atom. Although thiophene is quite stable and the sulfur is relatively inert, oxidation can be achieved using hydrogen peroxide. The primary products isolated are mixed S-oxide Diels-Alder dimers known as “sesquioxides”. In addition to the dimer product, a sulfone is formed, and both of these products are derived from a thiophene-S-oxide intermediate.1 Thiophene-S-oxides in general are not stable except in cases where bulky substituents are present at the 2- and 5- positions.2
The electron rich pi-system explains why thiophene, in highly acidic conditions, undergoes peracid oxygenation at the 2 position of the ring to form 2-oxothiophene or thiophene-2-one. This product was proposed to be formed in a pathway that involves protonation of the thiophene ring prior to arene oxidation to generate a thiophene epoxide intermediate.3 Presumably, the thiophene epoxide undergoes an NIH shift rearrangement like other arene oxides to form a thiolactone, and subsequently a phenol, due to the energy gained from re-aromatization.4 However, unlike phenols, only the thiolactone of thiophene was obtained, and it was formed in small quantities relative to the S-oxide dimer suggesting that in peracid conditions sulfur oxidation is preferred.
The mechanism of cytochrome P450-mediated oxidation of thiophenes is different than peracid oxidation. This is because the species involved in the catalytic oxidation is a highly electron deficient thiolate heme-oxo species dubbed P450 compound I.5 This iron (IV) oxo, or ferryl species with an additional oxidizing equivalent delocalized over the porphyrin and thiolate ligands (hereafter referred to as iron-oxo species), can react in a free radical process with the pi-orbital ring electrons of thiophene to produce arene oxide intermediates.6 This same intermediate can also react with the lone pair electrons on the sulfur atom to form a sulfoxide. Since there is more than one possible type of oxidation event, it is often ambiguous which pathway, arene oxidation vs. S-oxidation, is occurring.
A classic example of the ambiguity thiophene metabolism presents is that of the simplest case, unsubstituted thiophene. Two major metabolites were isolated from the urine of rats treated with thiophene. These metabolites were determined to be two isobaric mercapturic acid metabolites containing the elements of an additional water molecule. These mercapturates were postulated to be formed through an arene oxide intermediate.7 However, they were later re-characterized by 1H NMR and found to be diasteromeric dihydrothiophene-S-oxide mercapurates that were postulated to be derived from a thiophene-S-oxide intermediate.8 Further evidence for the intermediate S-oxide came from the identification of sesquioxides that were isolated from rat liver microsomal (RLM) incubations containing thiophene.1 In terms of characterization of a sulfoxidation pathway, formation of sesquioxides is the most convincing evidence to date.
Although there is substantial evidence for the cytochrome P450-mediated S-oxidation of thiophenes1,8-17 there also is evidence that cytochrome P450s (P450s) will catalyze the arene oxidation of some thiophenes.13,17-20 Due to their instability, the highly electrophilic S-oxide and arene oxide intermediates that are formed from thiophenes have been characterized indirectly as products trapped by nucleophiles, most commonly glutathione (GSH), which is found in high concentrations in most cells.
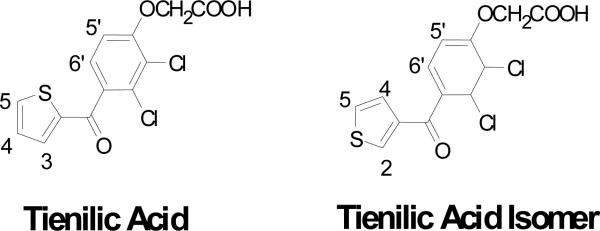
Tienilic acid (TA, [2,3-dichloro-4-(thiophene-2-carbonyl)phenoxy]acetic acid) (Figure 1) is an antihypertensive agent that has both potassium-sparing uricosuric and diuretic activities.21 It was removed shortly after its introduction because of an immune-mediated liver injury that occurred in a small percentage of the patient population.22 TA is primarily metabolized by P450 2C9 and is oxidized at the C-5 position of its thiophene ring. This metabolite, 5-OH-TA, exists in equilibrium with its thiolactone tautomer.23 TA was also found to cause mechanism-based inactivation of P450 2C9 most likely via covalent adduction of a reactive metabolite of TA with P450 2C9.24 A covalent adduct of TA with P450 2C9 is immunologically recognized by sera from patients with TA-induced hepatitis and is thought to act as a neo-antigen.25 The P450 2C9 adduct originally was proposed to arise via a reactive thiophene-S-oxide.10,25 However, an arene oxide also could be the reactive species that inactivates the enzyme.24
Figure 1.
Structures of tienilic acid and its 3-thenoyl regioisomer.
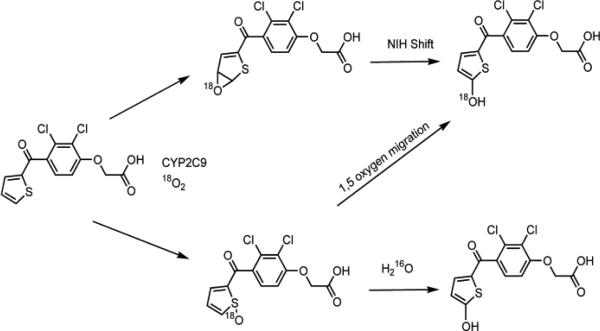
Although there was no direct evidence for its involvement in the mechanism of hydroxylation of TA, a thiophene-S-oxide intermediate was inferred from comparisons with the metabolism of the 3-thenoyl regioisomer of TA that is referred to as tienilic acid isomer (TAI, [2,3 dichloro-4-(thiophene-3-carbonyl)-phenoxy]-acetic acid) (Figure 1). TAI is oxidized by P450 2C9 and the rat orthologue P450 2C11 to an electrophilic thiophene S-oxide based on experiments where the S-oxide was trapped as thiol conjugates.9,25,26 These results led to the hypothesis that 5-OH-TA was formed by 1,5-migration of the oxygen of an initially formed TA S-oxide (see Scheme 1).27 We have investigated the possibility of this reaction theoretically, with the model compound 2-acetylthiophene-S-oxide, using Intrinsic Reaction Coordinate (IRC) calculations. Experimentally, the reaction was attempted using benzothiophene-S-oxide, the only stable thiophene-S-oxide that has a carbon alpha to the S-oxide that is available for oxygen migration. We also re-investigated the metabolism of TA and TAI by P450 2C9, and have constructed models of TA and TAI docked into the active site of the P450 2C9 crystal structure based on our findings that the metabolic profiles of these regioisomers are different.
Scheme 1.
Possible mechanisms for oxygen incorporation into 5-OH-TA.
Materials and Methods
Materials
The synthesis of TA and TAI are described in the Supporting Information and were based on previously described procedures.28,29 Structures and purity were confirmed by LC-UV, LC-MS, and 1H NMR. Trizma base and other reagents were purchased from Sigma-Aldrich (St. Louis, MO). LC solvents were purchased from Fisher Chemical Co. (Fairlawn, NJ) and all were LC-MS OPTIMA grade. P450 2C9 and P450 2C11 Supersomes co-expressing cytochrome b5 and P450 reductase, were purchased from BD Biosciences (San Jose, CA). 18O2 (98%18O2) gas was purchased from Isotech (Champaign, IL). D2O was purchased from Cambridge Isotopes (Andover, MA).
Incubation Conditions and HPLC-qTOF MS Analysis of the Metabolites of TA and TAI
Incubations were performed at 37 °C using a 100 μM solution of either TA or TAI in 50 mM Tris buffer (pH 7.4) or 50 mM kPi which was pre-incubated with Supersomes containing 20 pmols of P450 2C9 (20 μL) for 3 min. This was followed by the addition of 30 μL of a 10 mM solution of NADPH to give a 1 mM NADPH solution. The total reaction volume was 300 μL. Reactions were quenched after 30 min by the addition of 150 μL of ice-cold ACN containing 0.1% (v/v) formic acid. The samples were then placed on ice for 10 min followed by centrifugation at 13,000×g for 10 min.
Liquid chromatography was performed using a Shimadzu SC1 10A HPLC system coupled to a Waters (Milford, MA) Synapt High Resolution Quadrupole TOF mass spectrometer via an ESI interface. For parent ion detection in full scan mode (50-1500 m/z), the cone voltage was 25 eV and the collision and transfer cell energies were both 4 eV. MS expression (MSe) was used for fragmentation analysis. The cone voltage was 35 eV with the collision cell set to 6 eV and trap cell set to 4 eV. Fragmentation in the MSe mode was induced by using a collision cell energy ramp from 15-30 eV with the transfer cell maintained at 10 eV. For both modes, the capillary voltage was 3.5 KeV with a source temperature of 120°C and a desolvation temperature of 350°C. Leucine enkephalin was used as a lockspray reference with a sampling interval of 10 s and scan rate of 0.5 s. The instrument was calibrated with sodium formate over a range of 50-1500 m/z prior to every use. Data were acquired in the centroid mode. The spectra were combined using Masslynx software and also were analyzed by mass defect filtering with the Masslynx software program Metabolynx using the parent mass with a tolerance of 72 mDa. The elemental composition and mass error was calculated using Masslynx. Parent ion and high energy MSe spectra were generated by spectral combination of peaks about their apex using Masslynx.
Separation was achieved using a 2.1×100 mm RX-C8 column from Agilent (Santa Clara, CA) was used. A gradient program consisting of Solvent A (99.9% H2O solution containing 0.1% formic acid (v/v) and Solvent B (99.9% ACN containing 0.1% (v/v) formic acid) was used. Solvent A was held at 90% for 3 min, followed by a linear gradient to 50% B over 17 min. Then solvent B was increased using a linear gradient to 60% B at 25 min then to 95% B at 27 min where it was held for 3 min. The column was pre-equilibrated at 90% A for 5 min prior to each injection.
HPLC-UV Analysis
For HPLC-UV analysis of TA metabolites, a Schimadzu SC1-10A HPLC system with LC-10AD binary pumps was coupled to a Schimadzu SPD-M10A diode array that was set to scan from 190-600 nm over 0.1 s. The solvent flow rate was 1.0 mL/min and a Phenomenex Synergi® Polar-RP column (4.6×150 mm, 5 μm particle size) was used. The gradient solvent system was identical to the LC-ESI-MS analysis except for the flow rate.
For the separation of TAI metabolites, an Agilent XD-C8 column was used (4.6×150 mm, 5 μm particle size). The solvent system consisted of Solvent A (H2O containing 0.1% (v/v) formic acid) and Solvent B (ACN containing 0.1% (v/v) formic acid) at a flow rate of 1 mL/min. Solvent A was held for 3 min at 90% A followed by a linear gradient to 60% B over 20 min followed by a gradient to 95 % B at 25 min which was held for 3 min.
Measurement of 18O Incorporation
Incubations were performed in 1.5 mL polypropylene vials sealed with rubber septa. The vials were purged with N2 gas five times prior to pre-incubation and after adding all of the incubation components except for NADPH. Saturation of the incubation vessels with 18O2 was achieved by purging with 99% 18O2 gas three times via syringe and an oxygen regulator. Between purge cycles the vessels were evacuated using a gastight syringe. Incubations were maintained at a slight positive pressure of 18O2. The samples were then incubated for 3 min at 37 °C prior to the addition of 30 μL (via syringe) of an N2 gas-purged solution of 10 mM NADPH. The final concentration of NADPH was 1 mM. In order to quantify the percentage of 18O relative to 16O in the metabolites, tolbutamide was co-incubated at a final concentration of 100 μM as an internal standard with the concentration of TA or TAI at 10 μM. Liquid chromatography was performed using a Shimadzu SC1 10A HPLC system coupled to a Waters (Milford, MA) Quattro Micro mass spectrometer operating in the negative electrospray ionization (ESI) mode using Single Ion Monitoring (SIM). For parent ion detection in SIM mode the cone voltage was 25 eV with a capillary voltage of 3.0 keV. SIM was conducted by monitoring m/z channels 285, 287, 345 and 347 with a dwell time of 0.2 s for each channel. Calculation of %18O was performed using an equation based on Brauman's least squares method.30
NMR Analysis of TAI-M1 and TAI-M2
An incubation consisting of 100 μM TAI and 500 pmol of P450 2C9 Supersomes in 5 ml of 50 mM kPi (pH 7.4) was incubated at 37°C for 1 hr in the presence of 1 mM NADPH. The incubation mixture was then split into two 2.5 mL aliquots to which there was added 1.25 mL of ACN containing 0.1% v/v formic acid and centrifuged at 3,000×g for 20 min. The metabolites were then extracted using a C18 solid phase extraction cartridge (Bakerbond®, JT Baker) according to the manufacturer's instructions. The metabolites and parent were eluted with MeOH and concentrated to 200 μL. The metabolites were separated from the parent using the conditions mentioned above with D2O replacing H2O as an eluting solvent. A total volume of 300 μL was collected over the peak width of the co-eluting TAI-M1 and TAI-M2. One dimensional 1H NMR analysis of this mixture was performed on a 600MHz Bruker Avance III fitted with a CPTCI cryoprobe and operating at 273K. Data was analyzed using Topspin V2.1 software. A total of 12,288 scans were acquired into 32K data points with solvent suppression at 4.7 ppm and 2.3 ppm.
Hydrogen/Deuterium Exchange
The incubations were carried out as described above. Reaction mixtures were quenched using an equal volume of ACN and centrifuged for 10 min at 13,000×g. The supernatant was removed, diluted with D2O and loaded onto a 6 mL SepPak C18 cartridge from JT Baker (Phillipsburg, NJ) according to the manufacturer's instructions. The C18 cartridge was then washed with 99% D20 (2×2mL) and eluted using 1 mL of ACN containing 0.1% formic acid. The eluent was dried and reconstituted in 150 μL of D2O containing 20% ACN and immediately analyzed by LC-qTOF-MS as described above, but using a solvent mixture of 97% D2O and 0.1% formic acid (v/v) as solvent A.
Density Functional Theory (DFT) Calculations
Quantum-Mechanical (QM) calculations were performed with GAMESS (version number 1 October, 2010 (R1)). Calculations used restricted Hartree-Fock Møller-Plesset, or DFT (B3LYP) methods. GAMESS implemented DFT used a Euler-Maclaurin quadrature with 96 radial points, with theta and phi set to 12 and 24 for the number of angle grids in the Gauss-Legendre quadrature. Initial geometries for model thiophene and thiophene oxides were constructed in Avogadro using Monte Carlo conformational searches and energy minimization with the MMFF94 force-field. Molecular coordinates for QM calculations utilized systematically generated delocalized internal coordinates generated from Cartesian coordinates in the GAMESS input file. Basis sets for geometry optimization, saddle point searches, Hessian (vibrational analysis), and Intrinsic Reaction Coordinate (IRC) calculations were 6-31G+(d,p)//6-31G+(d,p), implemented as spherical harmonics unless indicated otherwise. All geometry minima were validated by a separate Hessian calculation indicating no imaginary frequencies. Transition states were also analyzed and had only one imaginary frequency. IRC calculations in the forward and reverse directions were given the transition-state geometry and Hessian as input, and followed the imaginary vibration mode to stationary points (products) from the starting point (transition state). When implemented, the solvent model used was the Polarizable Continuum Model (PCM), using GAMESS defaults for solvent parameters (H2O). Zero point energy corrections were scaled at 298.15 K. All calculation parameters unless otherwise mentioned used GAMESS default settings. GAMESS output was visualized using MacMolPlot.
Molecular Modeling
Molecular modeling simulations were conducted using a Silicon Graphics Octane workstation with Insight II software (Accelrys, San Diego, CA). The crystal structure of P450 2C9 (Protein Data Bank ID: 1R9O) was used for docking.31 Structures of TA and TAI were constructed using Insight II/Builder module and optimized. Energy minimization and molecular dynamics (MD) simulations were carried out using the Insight II/ Discover module with the consistent valence force field. The parameters for heme and iron-oxo species were as described previously.32, 33 The non-bond cutoff was 16 Å, and all other parameters were set at their default values. Water molecules and flurbiprofen substrate were removed from the CYP2C9 crystal complex (1R9O) prior to docking studies.
Dynamic Docking with T1 NMR Restraints
Initially, TA and TAI were manually placed into the active site of P450 2C9 on the distal side of the heme, in such a way that the distances between the protons of the substrates and the heme iron were close to the distances obtained previously from T1 NMR relaxation studies.34 The TA/TAI-P450 2C9 complex was then minimized by 1000 steps of steepest decent gradient, with heme fixed to prevent structural disturbances. The system was then subjected to 5-ps MD simulations with NMR-based distance restraints imposed. After the MD run, the structure was minimized with the same force of the distance restraints for the 2000 steps of steepest descents and 2000 steps of conjugate gradients minimizations, as described previously.35 In addition, distance restraints (3.13-3.85 Å) were also applied between Arg108 and the carboxylate groups of TA and TAI as a result of ionic salt bridge interactions. The non-bond cutoff was set to 16 Å and a distance-dependent dielectric constant was used to simulate aqueous environment. Residues located with 3 Å from TA or TAI were identified as important for ligand binding.
Results and Discussion
Incubations of TA with P450 2C9-containing Supersomes yielded 5-OH-TA (TA-M1) as the major oxidative metabolite with a protonated molecular ion at m/z 347 consistent with the molecular formula C13H9O5SCl2 which has a calculated m/z of 346.9548. By accurate mass it was found to have an m/z of 346.9550 amu (+0.6 ppm) as determined by high resolution MS in the positive ion mode. Three predominant daughter ions at m/z 247 (4-keto-2,3-dichlorophenoxyacetic acid fragment), m/z 189 (4-keto-2,3-dichlorophenol fragment) and m/z 127 (5-oxo-2-keto-thiophene fragment) were observed in both MS/MS and MSe modes. There were lesser abundant ions at m/z 301 and 287, which are products derived from the cleavage of the acetic acid moiety. A UV spectrum of this metabolite showed an absorbance with a λmax at 381 nm and at 262 nm (pH 2.5) similar to what has been observed by others.36
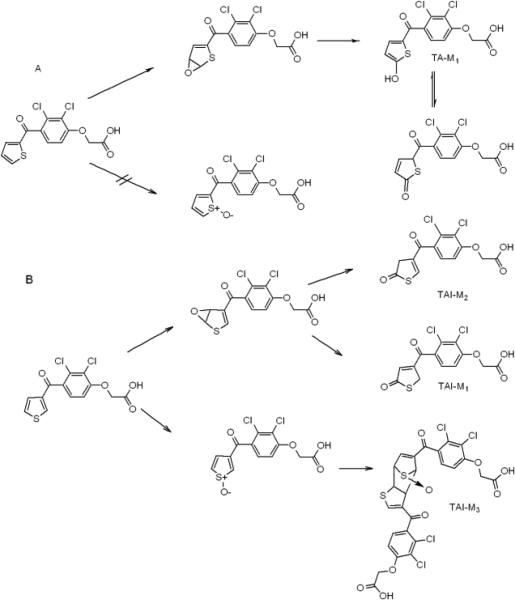
In order to determine the origin of oxygen incorporation into 5-OH-TA, incubations of TA with P450 2C9 Supersomes were conducted in an 18O2 environment. It was observed that 97 ± 2% of the oxygen was incorporated from molecular oxygen, which is consistent with what was observed previously by Belghazi et al.27 In addition, H/D exchange of this metabolite yielded a deuterated parent ion (M+D) at m/z 351. This represents a net exchange of four deuterium atoms for four hydrogen atoms. In addition to the M+D ion, one deuterium is exchanged with the carboxyl hydrogen while the other two deuterium atoms are incorporated by keto/enol tautomerism at the C-4 position of the thiophene ring. This is because 5-OH-TA is in equilibrium with its thiolactone tautomer, with the thiolactone structure favored by 4.5 kcal/mol based on DFT (Density Functional Theory) calculations in the gas phase (B3LYP/6-31+G(d,p)). Previous studies have already shown that the formation of 5-OH-TA is primarily mediated by P450 2C9 although to a minor extent by other P450's, and that this is a major urinary metabolite of TA in humans.23,25 No S-oxide dimers of TA were detected in incubation mixtures with or without the inclusion of GSH. This suggests that the primary mechanism of oxidation of TA is through arene oxidation and thus offers an explanation for the retention of oxygen in the TA-P450 2C9 adduct observed by Koenigs et al.24
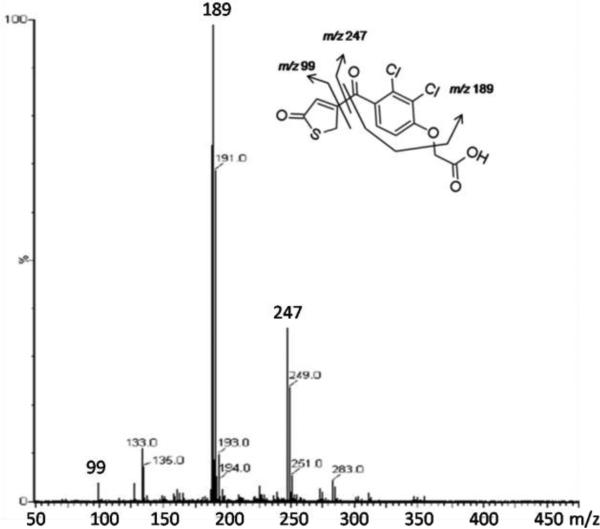
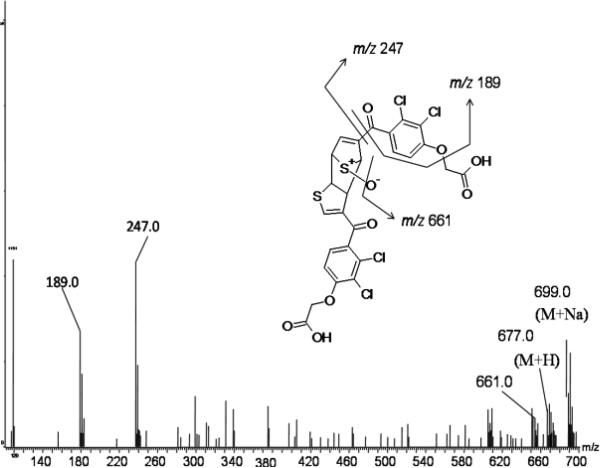
Incubation of TAI with P450 2C9 Supersomes in the presence of NADPH yielded two metabolites (TAI-M1 and TAI-M2) with protonated molecular ions at m/z 347. Further analysis by high resolution MS revealed that TAI-M1 and TAI-M2 had protonated molecular ions at m/z 346.9540 and 346.9538, respectively, that are accurate (-2.3 ppm and -2.8 ppm) for the molecular formula of TAI+O (C13H9Cl2O5S with a calculated m/z of 346.9548). They also produced nearly identical fragmentation patterns with major daughter ions at m/z 247 and m/z 189 as shown in Figure 2, consistent with addition of oxygen to the thiophene ring. A UV spectrum was generated for each of the metabolites at tR 15.3 (TAI-M1) and 16.2 min (TAI-M2) (Figure S1, Panel B, Supporting Information). TAI-M1 had λmax's at 216 nm and at 294 nm, and TAI-M2 had λmax's at 214 nm and 303 nm. A 1H NMR spectrum of TAI-M1 and TAI-M2 in a mixture of D2O/ACN showed resonance absorption for three aromatic protons: two doublets at 7.65 ppm (d, J=8.5 Hz) and 7.35 ppm (d, J=8.5 Hz) corresponding to the dichlorophenoxy ring protons, and one singlet at 7.50 ppm, corresponding to a proton either at the C-2 or C-4 position of the thiophene ring (Figure S2, Supporting Information). Unfortunately, TAI-M1 and TAI-M2 could not be resolved well enough using the chromatographic conditions necessary for NMR analysis of the individual metabolites.
Figure 2.
MSe spectrum of TAI-M1
Incubations with TAI and P450 2C9 in an atmosphere of 18O2 in the presence of NADPH yielded metabolites that incorporated 96 ± 3 % 18O. Both of these metabolites exchanged 4 deuterium atoms, similar to what was observed with 5-OH-TA. Based on these results, we are proposing that the two mono-oxygenated TAI metabolites are closely related isomers. Since under many different LC conditions the two peaks merged and the NMR spectrum indicates only one thiophene ring proton, TAI-M1 and TAI-M2 are probably thiolactone tautomers, one with the double bond in the 3,4 position, or exo isomer, and the other with the double bond in the 2,3 position, or the endo isomer (Scheme 2B). Separation of thiolactone tautomers by LC has been observed with the 2-oxo metabolite of prasugrel.37 The thiolactone structure would also explain the differences in UV spectra that were observed between TAI-M1/M2 and the UV spectrum of TA-M1. Moreover, at equilibrium the exo- (TAI-M1) and endo- (TAI-M2) thiolactones of TAI (see Scheme 2B) are favored over the hydroxythiophene structures by 11.0 and 8.1 kcal/mol, respectively, as determined by DFT calculations in the gas phase (B3LYP/6-31+G(d,p)). The H/D exchange and UV spectra also suggest that TAI-M1 and TAI-M2 cannot be oxygenated at the C-2 position as this would result in the exchange of only 1 deuterium atom on the thiophene ring, and the λmax's of TAI-M1 and TAI-M2 would be shifted to higher wavelength as observed for 5-OH-TA due to conjugation of the anionic form with the ketone as is observed with other 5-hydroxy-2-aroylthiophenes.23,24
Scheme 2.
Proposed mechanisms for the formation of the oxidized metabolites of TA and TAI.
An S-oxide dimer of TAI (TAI-M3) also was identified. It produced a protonated molecular ion consistent with a molecular formula of C26H16Cl4O9S2 which has a calculated m/z of 676.9063 and was found by accurate mass to be m/z 676.9061 (-0.3 ppm). This metabolite was only observed in incubations in the presence of NADPH, and only when TAI was incubated in kPi buffer. The parent ion spectrum of TAI-M3 showed the loss of 16 amu consistent with loss of oxygen presumably from an S-oxide. The filtered daughter ion spectrum from the MSe mode produced fragment ions at m/z 247 and 189 that are common to the TAI substructure (Figure 3). The dimer, TAI-M3, most likely is formed by Diels-Alder coupling of the TAI-S-oxide metabolite with TAI as shown in Scheme 2B. Since the substrate TAI is present throughout the incubation period at much higher concentrations than its S-oxide metabolite, the mono-S-oxide sesquioxide dimer would be anticipated rather than the di-S-oxide dimer. Based on these findings, it appears that TAI is capable of forming both arene oxides as well as thiophene-S-oxides as reactive intermediates, the latter of which is consistent with the thiophene-S-oxide GSH conjugates previously characterized by Valadon et. al.12 Because TAI is a reported intrinsic hepatotoxin in rats, these results suggest that the intrinsic hepatotoxic effects of TAI are likely due to the S-oxide pathway.12 More work is needed with radiolabeled in order to measure the quantity of each pathway for TAI, but these compounds are not commercially available.
Figure 3.
MSe spectrum of TAI-M3
The formation of thiolactone metabolites of TAI with 18O incorporation, although consistent with arene oxide formation and rearrangement, does not exclude the putative 1,5-oxygen migration mechanism previously proposed.27 In order to address this experimentally, benzothiophene-S-oxide was prepared (see Supporting Information) and was incubated with RLMs in the presence and absence of NADPH. The mixture was extracted, concentrated and then dissolved in a mixture of ACN and D2O for analysis by direct injection ESI-MS on an LTQ-FT-ICR mass spectrometer. Direct injection on the LTQ-FT was used because ionization of benzothiophene and its metabolites could not be achieved using non-grounded ionization sources. The anticipated M+H of 151 (M+D, 153) was not observed, but rather a 2M+Na+ cluster ion consistent with the molecular formula C16H12O2S2Na that has a calculated m/z of 323.0165 and was found to have a m/z of 323.0156 (-2.8 ppm). This apparent benzothiophene-S-oxide sodium ion cluster is observed with synthetic benzothiophene-S-oxide as well (see Supporting Information) and is formed in the LTQ-FT-ICR ion source (refer to Supporting Information for additional details). H/D exchange did not occur with benzothiophene-S-oxide on its own or after incubation with rat liver microsomes, which suggests that the rearrangement of this S-oxide to a hydroxythiophene does not occur. Furthermore, this S-oxide did not undergo hydration which is consistent with the lack of incorporation of oxygen from water into metabolites of TA or TAI.
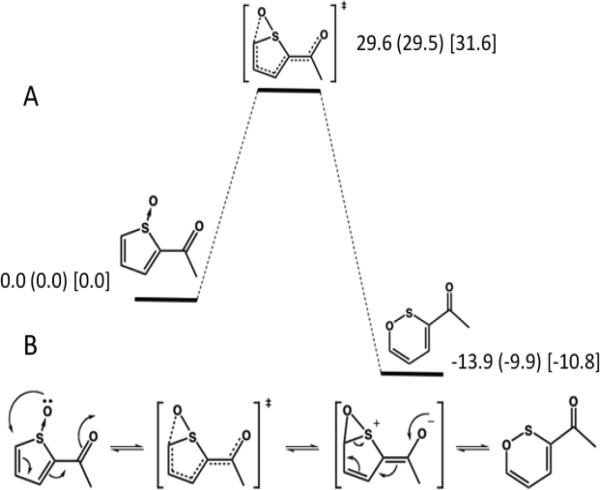
To further address the possibility of a 1,5-migration of oxygen from a thiophene-S-oxide to a hydroxythiophene, IRC calculations were carried out to obtain energy barriers for the reaction. Figure 4A shows a reaction coordinate diagram for the rearrangement of the model thiophene S-oxide, 2-acetylthiophene-S-oxide, to a six-membered oxathiine structure. Saddle-point geometry searches followed by Hessian vibrational analysis calculations at two levels of theory (DFT-B3LYP and MP2) as well as DFT with implicit quantum solvent, give similar results as indicated by the relative energies. We had sought to calculate the energy of the 1,5-oxygen migration reaction, and were at first surprised by this result. However, the rearrangement reaction to an oxathiine is in good agreement with photolytic rearrangements observed with other thiophenes.38 The important conclusion from the calculations on S-oxide rearrangement is that the reaction has a relatively high energy of activation of ~30 kcal/mol (Figure 4A), and the transition state and product at multiple levels of theory are identical. Importantly, a transition state to link the S-oxide to the formation of a hydroxythiophene/-thiolactone product could not be identified; the product identified from the transition state is in each case the six-membered oxathiine ring and not a hydroxythiophene/thiolactone as shown in Figure 4B. From this information we believe that a 1,5-oxygen migration mechanism for the generation of hydroxythiophene/thiolactones from S-oxides does not occur. Based on these results, the thiophene-S-oxide pathway is not possible and leaves only the formation of arene oxide intermediates as a source of 5-OH-TA and by extension, hydroxy thiophene/thiolactone metabolites in general. Thus the formation of an arene oxide intermediate of TA is likely responsible for the idiosyncratic hepatotoxic effects as well as the mechanism-based inactivation observed by others.10,24
Figure 4.
Reaction coordinate for the rearrangement reaction of 2-acetylthiophene-S-oxide Panel A. The product of the rearrangement is an oxathiine and not a hydroxythiophene. Energies are reported in kcal/mol normalized to S-oxide, and are DFT gas phase (DFT PCM water) [MP2 gas phase]. Panel B. A proposed mechanism for the formation of 2-acetyloxathiine from 2-acetylthiophene-S-oxide via the putative 1,5-thioxirane intermediate.
Since P450 2C9 catalyzed the incorporation of oxygen at the C-5 position of the thiophene ring of TA, and at both C-5 and on the sulfur atom of TAI, it seemed likely that the favored orientations of TA and TAI in the active site of P450 2C9 were different. The active site dynamics of TA and TAI in P450 2C9 have been previously modeled using NMR-T1 relaxation data.32 However, the conclusions drawn seemed to disagree somewhat with the experimental findings presented here. In order to better explain our results, the binding orientations of TA and TAI in the active site of the P450 2C9 crystal structure were determined by MD simulations and minimization using the T1 NMR distance constraints from Poli-Scaife et. al.32 in conjunction with the catalytically active iron-oxo species. When the resting-state ferric heme was used, the results indicated that the distances were relatively similar between TA and TAI, as shown in Table 1, and similar to what was observed previously.32
Table 1.
Distances (Å) between the protons and ferric heme found in P450 2C9 after energy minimization using NMR distance constraints.
| Substrate | Proton | Sulfur | |||||
|---|---|---|---|---|---|---|---|
| 2 | 3 | 4 | 5 | 5' | 6' | ||
| Tienilic acid | 5.942 | 5.759 | 5.425 | 6.737 | 5.967 | 5.896 | |
| Tienilic acid isomer | 5.839 | 5.820 | 5.820 | 6.791 | 5.827 | 5.970 | |
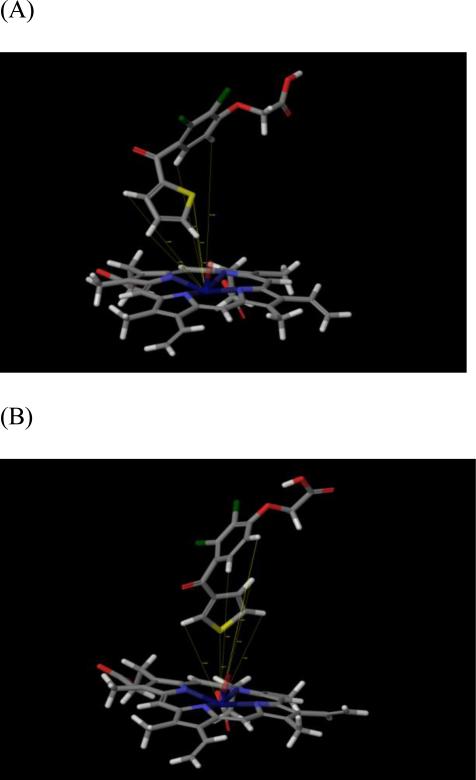
As shown in Table 2, when the active iron-oxo species is used, TA is oriented in such a manner that the iron-oxo species is much closer to the C-4,5 hydrogens of the thiophene ring, which correlates well with our experimental findings indicating that only 5-OH-TA is formed as a metabolite. In fact, the C-5 hydrogen is the closest in proximity to the iron-oxo oxygen with a calculated distance of 3.35 Å. This does provide a possible explanation for the lack of S-oxidation of TA. In contrast, TAI is oriented further away from the oxidizing species, with the closest distances being 6.02 Å and 6.41 Å for the C-2 hydrogen and sulfur atoms of the thiophene ring, respectively (see Table 2). Figure 5 (Panel A for TA, Panel B for TAI) shows that TA and TAI have distinctly different orientations in the active site, and that the oxyacetic acid carboxylic acid groups of both isomers interact with Arg108 forming a possible salt bridge or dipolar interaction. Considering the greater distances away from the P450 2C9 heme iron of the thiophene ring hydrogens of TAI compared to TA, the model raises the possibility that oxidation of TAI is catalyzed by a ferric peroxy complex of P450 2C9. Additional studies with mutants of some of the threonines in the active site of P450 2C9 may help address this possibility
Table 2.
Distances (Å) between the protons and iron-oxo species found in P450 2C9 after energy minimization using NMR distance constraints.
| Substrate | Proton | Sulfur | |||||
|---|---|---|---|---|---|---|---|
| 2 | 3 | 4 | 5 | 5' | 6' | ||
| Tienilic acid | 6.900 | 4.363 | 3.359 | 10.286 | 8.720 | 5.831 | |
| Tienilic acid isomer | 6.049 | 8.162 | 7.709 | 7.807 | 9.924 | 6.417 | |
Figure 5.
Positioning of TA (A) and TAI (B) relative to the iron–oxo heme in the active site of P450 2C9.
Finally, considering the proximity of the C-2 hydrogens of TAI to the P450 2C9 iron-oxo species in our model, it raises the possibility that TAI-M1 and/or TAI-M2 are oxidation products at the C-2 position. Additional work with synthetic standards will be required to confirm this. However, as mentioned earlier and based on previous studies,3 the exo tautomer of thiophene-2-one has only two exchangeable hydrogen atoms, which indicates that it is not in equilibrium with the endo tautomer in acidic medium as this would result in the net exchange of 3 deuteriums. Base-catalyzed rearrangement to the endo tautomer has been observed with other thiolactones such as that of clopidogrel.39 Thus, under our conditions, it would be expected that TAI-M1 and TAI-M2 would exchange 2 deuteriums as is observed.
Conclusion
We conclude that, in terms of cytochrome P450-mediated oxidation, some thiophene derivatives such as TAI are oxidized to thiophene-S-oxides, while others such as TA are oxidized to hydroxythiophene/thiolactones via mechanisms related to the formation and rearrangement of arene oxides.6 Since the orbital energies of the thiophene HOMOs do not differ significantly between the 2- and 3-keto isomers (MP2/6-31+G(d,p), data not shown), our findings suggest that TA and TAI are oxidized at select positions primarily because of their different orientations in the active site of P450 2C9. These conclusions are based on the results of experiments which showed that 1) the oxygen in the product hydroxythiophene/thiolactones of TA and TAI were solely derived from atmospheric molecular oxygen, 2) TAI but not TA formed a thiophene S-oxide dimer, 3) rearrangement of thiophene-S-oxides to hydroxythiophene/thiolactones is neither observed theoretically nor experimentally, and 4) TA and TAI dock in different orientations in the active site of P450 2C9 that are consistent with the formation of 5-OH-TA from TA and not an S-oxide, and with the formation of both hydroxythiophene/thiolactones and an S-oxide from TAI. This may have toxicological importance since it is known that TA is an idiosyncratic immunotoxin in humans and TAI is a reported intrinsic hepatotoxin in rats.9
Supplementary Material
Acknowledgement
We would like to thank Muireann Coen, Olaf Beckonert and Isobelle Grant of Imperial College for their assistance in acquiring the NMR data for the characterization of the metabolites of TAI. Molecular modeling studies were performed at the Computational Chemistry and Molecular Modeling Laboratory, Department of Basic Pharmaceutical Sciences, School of Pharmacy, West Virginia University, Morgantown, WV.
Funding and Support. The authors gratefully acknowledge the financial support of the Drug Metabolism, Transporter, and Pharmacogenomics Research (DMTPR) Consortium and the NIH (GM32165, GM07750 and GM079724), the ACS Division of Medicinal Chemistry, Pfizer, Inc., and the ARCS Foundation, Seattle Chapter.
Abbreviations
- TA
tienilic acid
- TAI
tienilic acid isomer
- MBI
mechanism-based inactivator
- kPi
potassium phosphate buffered saline
- NMR
nuclear magnetic resonance
- TLC
thin layer chromatography
- LC-MS
liquid chromatography mass spectrometry
- 5-OH-TA
5-hydroxy tienilic acid
- NADPH
nicotinamide adenine dinucleotide phosphate
- ACN
acetonitrile
- DFT
Density Functional Theory
- IRC
Intrinsic Reaction Coordinate
Footnotes
Supporting Information Available: LC ion chromatograms of the metabolites of TA and TAI, NMR spectrum of TAI-M1 and TAI-M2, synthetic procedures for the preparation of TA and TAI is available free of charge via the Internet at http://pubs.acs.org.
References
- 1.Treiber A, Dansette PM, El Amri H, Girault J-P, Ginderow D, Mornon J-P, Mansuy D. Chemical and biological oxidation of thiophene: preparation and complete characterization of thiophene S-oxide dimers and evidence for thiophene S-oxide as an intermediate in thiophene metabolism in vivo and in vitro. Journal of the American Chemical Society. 1997;119:1565–1571. [Google Scholar]
- 2.Pouzet P, Erdelmeier I, Ginderow D, Dansette PM, Mansuy D, Mornon J-P. Thiophene 1-oxides. V. Comparison of the crystal structures and thiophene ring aromaticity of 2,5-diphenylthiophene, its sulfoxide and sulfone. Journal of Heterocyclic Chemistry. 1997;34:1567–1574. [Google Scholar]
- 3.Treiber A, Dansette PM, Mansuy D. Mechanism of the aromatic hydroxylation of thiophene by acid-catalyzed peracid oxidation. J. Org. Chem . 2002;67:7261–7266. doi: 10.1021/jo0202177. [DOI] [PubMed] [Google Scholar]
- 4.Guroff G, Daly JW, Jerina DM, Renson J, Witkop B, Udenfriend S. Hydroxylation-induced migration: the NIH shift. Recent experiments reveal an unexpected and general result of enzymatic hydroxylation of aromatic compounds. Science. 1967;157:1524–1530. doi: 10.1126/science.157.3796.1524. [DOI] [PubMed] [Google Scholar]
- 5.Rittle J, Green MT. Cytochrome P450 compound I: capture, characterization, and C-H bond activation kinetics. Science. 2010;330:933–937. doi: 10.1126/science.1193478. [DOI] [PubMed] [Google Scholar]
- 6.Darbyshire JF, Iyer KR, Grogan J, Korzekwa KR, Trager WF. Substrate probe for the mechanism of aromatic hydroxylation catalyzed by cytochrome P450. Drug Metab. Dispos. 1996;24:1038–1045. [PubMed] [Google Scholar]
- 7.Bray HG, Carpanini FM, Waters BD. The metabolism of thiophen in the rabbit and the rat. Xenobiotica. 1971;1:157–168. doi: 10.3109/00498257109044387. [DOI] [PubMed] [Google Scholar]
- 8.Dansette PM, Thang DC, el Amri H, Mansuy D. Evidence for thiophene-S-oxide as a primary reactive metabolite of thiophene in vivo: formation of a dihydrothiophene sulfoxide mercapturic acid. Biochem. Biophys. Res. Commun. 1992;186:1624–1630. doi: 10.1016/s0006-291x(05)81594-3. [DOI] [PubMed] [Google Scholar]
- 9.Dansette PM, Amar C, Smith C, Pons C, Mansuy D. Oxidative activation of the thiophene ring by hepatic enzymes. Hydroxylation and formation of electrophilic metabolites during metabolism of tienilic acid and its isomer by rat liver microsomes. Biochem. Pharmacol. 1990;39:911–918. doi: 10.1016/0006-2952(90)90207-2. [DOI] [PubMed] [Google Scholar]
- 10.Lopez-Garcia MP, Dansette PM, Mansuy D. Thiophene derivatives as new mechanism-based inhibitors of cytochromes P-450: inactivation of yeast-expressed human liver cytochrome P-450 2C9 by tienilic acid. Biochemistry. 1994;33:166–175. doi: 10.1021/bi00167a022. [DOI] [PubMed] [Google Scholar]
- 11.Mansuy D, Dansette PM. New biological reactive intermediates: metabolic activation of thiophene derivatives. Adv. Exp. Med. Biol. 1996;387:1–6. [PubMed] [Google Scholar]
- 12.Valadon P, Dansette PM, Girault JP, Amar C, Mansuy D. Thiophene sulfoxides as reactive metabolites: formation upon microsomal oxidation of a 3-aroylthiophene and fate in the presence of nucleophiles in vitro and in vivo. Chem. Res. Toxicol. 1996;9:1403–1413. doi: 10.1021/tx9601622. [DOI] [PubMed] [Google Scholar]
- 13.Dansette PM, Bertho G, Mansuy D. First evidence that cytochrome P450 may catalyze both S-oxidation and epoxidation of thiophene derivatives. Biochem Biophys. Res. Commun. 2005;338:450–455. doi: 10.1016/j.bbrc.2005.08.091. [DOI] [PubMed] [Google Scholar]
- 14.Joshi EM, Heasley BH, Chordia MD, Macdonald TL. In vitro metabolism of 2-acetylbenzothiophene: relevance to zileuton hepatotoxicity. Chem. Res. Toxicol. 2004;17:137–143. doi: 10.1021/tx0341409. [DOI] [PubMed] [Google Scholar]
- 15.Medower C, Wen L, Johnson WW. Cytochrome P450 oxidation of the thiophene-containing anticancer drug 3-[(quinolin-4-ylmethyl)-amino]-thiophene-2-carboxylic acid (4-trifluoromethoxy-phenyl)-amide to an electrophilic intermediate. Chem. Res. Toxicol. 2008;21:1570–1577. doi: 10.1021/tx700430n. [DOI] [PubMed] [Google Scholar]
- 16.Shimizu S, Atsumi R, Nakazawa T, Fujimaki Y, Sudo K, Okazaki O. Metabolism of ticlopidine in rats: identification of the main biliary metabolite as a glutathione conjugate of ticlopidine S-oxide. Drug Metab, and Dispos. 2009;37:1904–1915. doi: 10.1124/dmd.109.027524. [DOI] [PubMed] [Google Scholar]
- 17.Talakad JC, Shah MB, Walker GS, Xiang C, Halpert JR, Dalvie D. Comparison of in vitro metabolism of ticlopidine by human cytochrome P450 2B6 and rabbit cytochrome P450 2B4. Drug Metab. Dispos. 39:539–550. doi: 10.1124/dmd.110.037101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fouda HG, Avery MJ, Dalvie D, Falkner FC, Melvin LS, Ronfeld RA. Disposition and metabolism of tenidap in the rat. Drug Metab. Dispos. 1997;25:140–148. [PubMed] [Google Scholar]
- 19.Savi P, Pereillo JM, Uzabiaga MF, Combalbert J, Picard C, Maffrand JP, Pascal M, Herbert JM. Identification and biological activity of the active metabolite of clopidogrel. Thromb. Haemost. 2000;84:891–896. [PubMed] [Google Scholar]
- 20.O'Donnell JP, Dalvie DK, Kalgutkar AS, Obach RS. Mechanism-based inactivation of human recombinant P450 2C9 by the nonsteroidal anti-inflammatory drug suprofen. Drug. Metab. Dispos. 2003;31:1369–1377. doi: 10.1124/dmd.31.11.1369. [DOI] [PubMed] [Google Scholar]
- 21.Lau K, Stote RM, Goldberg M, Agus ZS. Mechanisms of the uricosuric effect of the diuretic tienilic acid (Ticrynafen) in man. Clin. Sci. Mol. Med. 1977;53:379–386. doi: 10.1042/cs0530379. [DOI] [PubMed] [Google Scholar]
- 22.Beevers DG, Walker JM. Total ban on tienilic acid. The Lancet. 1980;315:1417. doi: 10.1016/s0140-6736(80)92680-x. [DOI] [PubMed] [Google Scholar]
- 23.Mansuy D, Dansette PM, Foures C, Jaouen M, Moinet G, Bayer N. Metabolic hydroxylation of the thiophene ring: isolation of 5-hydroxy-tienilic acid as the major urinary metabolite of tienilic acid in man and rat. Biochem. Pharmacol. 1984;33:1429–1435. doi: 10.1016/0006-2952(84)90409-x. [DOI] [PubMed] [Google Scholar]
- 24.Koenigs LL, Peter RM, Hunter AP, Haining RL, Rettie AE, Friedberg T, Pritchard MP, Shou M, Rushmore TH, Trager WF. Electrospray ionization mass spectrometric analysis of intact cytochrome P450: identification of tienilic acid adducts to P450 2C9. Biochemistry. 1999;38:2312–2319. doi: 10.1021/bi9823030. [DOI] [PubMed] [Google Scholar]
- 25.Lopez Garcia MP, Dansette PM, Valadon P, Amar C, Beaune PH, Guengerich FP, Mansuy D. Human-liver cytochromes P-450 expressed in yeast as tools for reactive-metabolite formation studies. Oxidative activation of tienilic acid by cytochromes P-450 2C9 and 2C10. Eur. J. Biochem. 1993;213:223–232. doi: 10.1111/j.1432-1033.1993.tb17752.x. [DOI] [PubMed] [Google Scholar]
- 26.Dansette PM, Amar C, Valadon P, Pons C, Beaune PH, Mansuy D. Hydroxylation and formation of electrophilic metabolites of tienilic acid and its isomer by human liver microsomes. Catalysis by a cytochrome P450 IIC different from that responsible for mephenytoin hydroxylation. Biochem. Pharmacol. 1991;41:553–560. doi: 10.1016/0006-2952(91)90627-h. [DOI] [PubMed] [Google Scholar]
- 27.Belghazi M, Jean P, Poli S, Schmitter JM, Mansuy D, Dansette PM. Use of isotopes and LC-MS-ESI-TOF for mechanistic studies of tienilic acid metabolic activation. Adv. Exp. Med. Biol. 2001;500:139–144. doi: 10.1007/978-1-4615-0667-6_17. [DOI] [PubMed] [Google Scholar]
- 28.Godfroid JJ, Thuillier JE. Phenoxyacetic acid derivatives as diuretic agents. US patent 3969529.
- 29.Thuillier G, Laforest J, Cariou B, Bessin P, Bonnet J, Thuillier J. Dérivés hétérocycliquesd’acides phénoxyacétiques. Synthèse et études préliminaires de leurs activités diurétiques et uricosuriques. Eur. J. Med. Chem. 1974;9:625. [Google Scholar]
- 30.Brauman JI. Least squares analysis and simplification of multi-isotope mass spectra. Analytical Chemistry (U.S.) Formerly Ind. Eng. Chem., Anal. Ed. 1966;38:607–610. [Google Scholar]
- 31.Wester MR, Yano JK, Schoch GA, Yang C, Griffin KJ, Stout CD, Johnson EF. The structure of human cytochrome P450 2C9 complexed with flurbiprofen at 2.0-Å. resolution. Journal of Biological Chemistry. 2004;279:35630–35637. doi: 10.1074/jbc.M405427200. [DOI] [PubMed] [Google Scholar]
- 32.Paulsen MD, Ornstein RL. A 175-psec molecular dynamics simulation of camphor-bound cytochrome P-450cam. Proteins. 1991;11:184–204. doi: 10.1002/prot.340110304. [DOI] [PubMed] [Google Scholar]
- 33.Paulsen MD, Ornstein RL. Predicting the product specificity and coupling of cytochrome P450cam. J. Comput Aided Mol. Des. 1992;6:449–460. doi: 10.1007/BF00130396. [DOI] [PubMed] [Google Scholar]
- 34.Poli-Scaife S, Attias R, Dansette PM, Mansuy D. The substrate binding site of human liver cytochrome P450 2C9: an NMR study. Biochemistry. 1997;36:12672–12682. doi: 10.1021/bi970527x. [DOI] [PubMed] [Google Scholar]
- 35.Huang Q, Szklarz GD. Significant increase in phenacetin oxidation on L382V substitution in human cytochrome P450 1A2. Drug Metab. Dispos. 2010;38:1039–1045. doi: 10.1124/dmd.109.030767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Neau E, Dansette PM, Andronik V, Mansuy D. Hydroxylation of the thiophene ring by hepatic monooxygenases. Evidence for 5-hydroxylation of 2-aroylthiophenes as a general metabolic pathway using a simple UV-visible assay. Biochem. Pharmacol. 1990;39:1101–1107. doi: 10.1016/0006-2952(90)90290-2. [DOI] [PubMed] [Google Scholar]
- 37.Farid NA, Smith RL, Gillespie TA, Rash TJ, Blair PE, Kurihara A, Goldberg MJ. The disposition of prasugrel, a novel thienopyridine, in humans. Drug Metab. Dispos. 2007;35:1096–1104. doi: 10.1124/dmd.106.014522. [DOI] [PubMed] [Google Scholar]
- 38.Heying MJ, Nag M, Jenks WS. Photochemistry of thiophene-S-oxide derivatives. Journal of Physical Organic Chemistry. 2008;21:915–924. [Google Scholar]
- 39.Dansette PM, Rosi J, Bertho G, Mansuy D. Cytochromes P450 Catalyze Both Steps of the Major Pathway of Clopidogrel Bioactivation, whereas Paraoxonase Catalyzes the Formation of a Minor Thiol Metabolite Isomer. Chem. Res. Toxicol. 2011 Dec. doi: 10.1021/tx2004085. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.