Abstract
The aim of this review is to discuss the contribution of cytochrome P450 (CYP) 1B1 in vascular smooth muscle cell growth, hypertension, and associated pathophysiology. CYP1B1 is expressed in cardiovascular and renal tissues, and mediates angiotensin II (Ang II)-induced activation of NADPH oxidase and generation of reactive oxygen species (ROS), and vascular smooth muscle cell migration, proliferation, and hypertrophy. Moreover, CYP1B1 contributes to the development and/or maintenance of hypertension produced by Ang II-, deoxycorticosterone Nω-nitro-(DOCA)-salt-, and L-arginine methyl ester-induced hypertension and in spontaneously hypertensive rats. The pathophysiological changes, including cardiovascular hypertrophy, increased vascular reactivity, endothelial and renal dysfunction, injury and inflammation associated with Ang II- and/or DOCA-salt induced hypertension in rats, and Ang II-induced hypertension in mice are minimized by inhibition of CYP1B1 activity with 2,4,3′,5′-tetramethoxystilbene or by Cyp1b1 gene disruption in mice. These pathophysiological changes appear to be mediated by increased production of ROS via CYP1B1-dependent NADPH oxidase activity and extracellular signal-regulated kinase 1/2, p38 mitogen-activated protein kinase, and c-Src.
Keywords: cytochrome P450 1B1, reactive oxygen species, angiotensin II-/DOCA-salt-hypertension, cardiovascular hypertrophy, renal dysfunction, inflammation
1. Introduction
Several neurohumoral factors including norepinephrine, angiotensin II (Ang II), and aldosterone contribute to the regulation of blood pressure and increased levels of these agents promote hypertension (1, 2). Both norepinephrine and Ang II activate one or more phospholipases, mainly cytosolic phospholipase A2 (cPLA2), which releases arachidonic acid (AA) (3–5). Metabolites of AA modulate the release of norepinephrine and mediate and/or modulate the actions of several vasoactive agents including norepinephrine and Ang II (6). Products of AA generated via cyclooxygenase, lipoxygenase, and cytochrome P450 (CYP) 4A have been implicated in the regulation of blood pressure (6–9). AA metabolism is also associated with generation of reactive oxygen species (ROS) that have been implicated in vascular smooth muscle cell (VSMC) growth and various models of experimental hypertension (10–15). In addition to CYP4A that expresses ω-hydroxylase activity, and CYP2B, 2C, and 2J, the main epoxygenases (16), CYP1 enzymes that are involved in the bioactivation of xenobiotics can also metabolize endobiotics including steroid hormones, retinoids, and fatty acids (17–20). Among the CYP1 enzymes, CYP1B1 that has been shown to metabolize AA into HETEs and EETs in vitro (20), may also contribute to the regulation of cardiovascular and renal function. Although the role of CYP1B1 in cancer and bioactivation of carcinogens have been well studied, there is very little information on the implications of CYP1B1 in the cardiovascular system. This brief review will focus on its contribution to hypertension and associated pathophysiology.
2. CYP1B1 and its expression in the cardiovascular system
CYP1B1 is a member of the cytochrome P450 enzyme family I, subfamily B, polypeptide 1. The CYP1B1 gene was cloned in 1994 from tetrachloro-dibenzo-1/2-dioxin-treated human keratinocyte cells and is currently the only known member of the CYP1B family (21, 22). CYP1B1 is present in several non-hepatic tissues including those within the cardiovascular system (23). Although CYP1B1 is expressed in normal tissues and is constitutively active, its expression is increased in various cancer cells, particularly steroid sensitive cells (23, 24). Several pro-carcinogenic polycyclic aromatic hydrocarbons that are metabolized by CYP1B1 also increase its expression in various tissues including the heart, kidney, and vascular cells (23, 25–27). In blood vessels, CYP1B1 is expressed mainly in VSMCs with very little expression in endothelial cells, and its expression is increased in endothelial cells by shear stress (28). CYP1B1 is also expressed in retinal endothelial cells and has been implicated in angiogenesis in response to low oxidative stress (29).
3. Contribution of CYP1B1 to VSMC migration and growth
Ang II-induced VSMC migration, proliferation and hypertrophy are mediated via AA release by cPLA2 (30). Moreover, the demonstration that inhibition of AA metabolism with 5,8,11,14-eicosatetraynoic acid, and CYP1B1 activity with 2,4,3′,5′-tetramethoxystilbene (TMS) or adenovirus CYP1B1 shRNA prevent Ang II- and AA-induced VSMC migration, proliferation, and hypertrophy, suggest that these effects of Ang II and AA are dependent on CYP1B1 activity (Fig. 1) (30). TMS or adenovirus CYP1B1 shRNA also inhibit Ang II- and AA-induced ROS generation and extracellular signal-regulated kinase 1/2 (ERK1/2) and p38 mitogen-activated protein kinase (p38 MAPK) activity without altering production of HETEs (30). Since Tempol, a ROS inactivator (31), that inhibited these effects as well as VSMC migration and growth produced by Ang II and AA without altering CYP1B1 activity, it appears that Ang II-induced VSMC migration and growth are mediated by ROS generated from AA by CYP1B1 and activation of ERK1/2 and p38 MAPK (30). Our preliminary experiments indicate that Ang II-induced neointimal growth is inhibited by TMS and adenovirus CYP1B1 shRNA in balloon-injured rat carotid artery, and in wire-injured mouse carotid artery by Cyp1b1 gene disruption (our unpublished data).
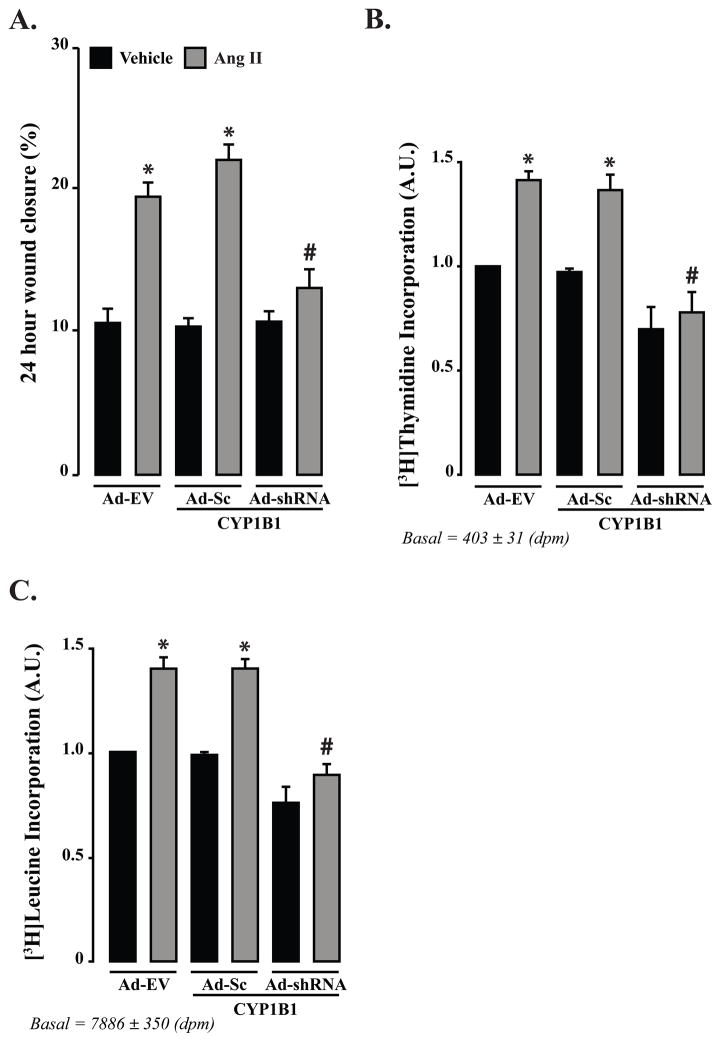
Figure 1. Ang II-induced migration and DNA and protein synthesis depend on CYP1B1 in VSMCs.
A–C. Quiescent VSMCs were transduced with Adenovirus CYP1B1 shRNA (Ad-shRNA), Ad-scrambled (Sc) CYP1B1 shRNA (Ad-Sc) or Ad empty vector (Ad-EV) (200 MOI each) for 24 hr and then treated with Ang II (200 nmol/L) or its vehicle in the presence of Ad for 24 hr for measurement of migration and proliferation or 48 hr for measurement of protein synthesis. Migration of VSMCs was determined by wound healing method, proliferation and protein synthesis by [3H]thymidine and [3H]leucine incorporation, respectively. *P<0.05 vehicle vs. corresponding value from Ang II-treated group; #P<0.05 Ad-shRNA vs. Ad-Sc (n = 3 for each group, and data are expressed as mean ± SEM) (Hypertension. 2010;55:1461–1467).
4. Contribution of CYP1B1 to hypertension and associated pathophysiology
4.1. Cardiac hypertrophy, fibrosis, and inflammation
The evidence that ROS is involved in various experimental animal models of hypertension (13–15), together with our finding that Ang II generates ROS and promotes VSMC growth via CYP1B1 (30), led us to investigate the role of CYP1B1 in Ang II-induced hypertension and associated cardiovascular pathology. Infusion of Ang II (150 ng/kg/min) by miniosmotic pump in rats increased mean arterial blood pressure (MAP). Administration of the CYP1B1 inhibitor, TMS (300 μg/kg i.p. every third day), did not alter basal MAP but abolished the Ang II-induced increase in MAP (32). In animals made hypertensive with Ang II infusion for 8 days, injections of TMS (300 μg/kg, i.p. every second day), brought blood pressure back to basal levels (32). Cardiac hypertrophy, as indicated by increased heart: body weight ratio and brain natriuretic peptide mRNA associated with Ang II-induced hypertension, were inhibited in animals treated with TMS. Moreover, cardiac fibrosis, as observed by increased accumulation of α-smooth muscle actin-positive cells, and inflammation, as indicated by an increase in macrophages in the myocardium, were inhibited (32). These observations, together with a decrease in CYP1B1 activity in hearts from TMS treated rats, suggested that CYP1B1 contributes to Ang II-induced hypertension and associated cardiac hypertrophy, fibrosis, and inflammation. Further evidence that CYP1B1 contributes to the development of hypertension and associated cardiac hypertrophy was the demonstration that in CYP1B1 knockout (Cyp1b1−/−) mice, the effect of Ang II to increase MAP was minimized (Figure 2) and heart: body weight ratio was reduced compared to that observed in wild type (Cyp1b1+/+) mice (32).
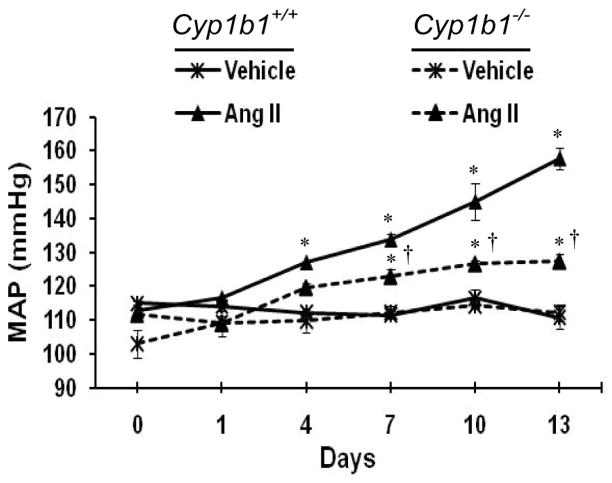
Figure 2. CYP1B1 contributes to the development of Ang II-induced hypertension in mice.
Cyp1b1+/+ and Cyp1b1−/− mice were infused with either Ang II (700 ng/kg/min) or vehicle with miniosmotic pumps for 13 days, and blood pressure was measured by tail cuff every 3rd day. *P < 0.05 vehicle vs. corresponding value from Ang II-treated animal; †P < 0.05 Cyp1b1+/+ Ang II vs. Cyp1b1−/− Ang II (n = 4 for all experiments, and data are expressed as mean ± SEM). (Hypertension. 2010;56:667–674).
CYP1B1 was also found to contribute to the development and/or maintenance of Ang II-independent and genetic models of hypertension. DOCA-salt treatment causes hypertension independent of Ang II by increasing salt and water retention and increasing levels of vasopressin, catecholamines, and endothelin-1 (ET-1), and by stimulating ROS production (33–36). These agents also activate cPLA2 and release AA (4, 37, 38) that could generate ROS through CYP1B1 and promote development and/or maintenance of hypertension and associated cardiac hypertrophy. Supporting this view were our findings that administration of TMS reversed the increase in blood pressure and associated cardiac hypertrophy produced by DOCA-salt treatment in rats (39). DOCA-salt- and Nω-nitro-L-arginine methyl ester hydrochloride (L-NAME)-induced hypertension are minimized in Cyp1b1−/− mice, and treatment with TMS reduces blood pressure in spontaneously hypertensive rats (our unpublished data).
4.2. Increased vascular reactivity, hypertrophy and endothelial dysfunction associated with hypertension
In experimental models of hypertension, the vascular response to vasoconstrictor agents is enhanced, and there is also endothelial dysfunction as a result of nitric oxide inactivation by increased oxidative stress (13–15, 40). Our studies indicate that increased vascular reactivity to the vasoconstrictor agents, phenylephrine and ET-1, associated with Ang II- and DOCA-salt-induced hypertension in the rat is abolished by inhibition of CYP1B1 activity with TMS (32, 39). Also the increase in vascular reactivity to these agents associated with Ang II-induced hypertension was diminished in Cyp1b1−/− mice (32). The increased vascular reactivity in Ang II-induced hypertension was also associated with hypertrophy of the aorta and mesenteric and femoral arteries in the rat, and of the aorta in mice, as indicated by an increase in media: lumen ratio that was inhibited by treatment with TMS or disruption of the Cyp1b1 gene in mice (32). Similarly, treatment with TMS prevented the increase in response of the mesenteric artery and aorta caused by DOCA-salt hypertension in the rat (39). Moreover, endothelial dysfunction, as indicated by a decrease in acetylcholine-induced relaxation of the aorta observed in Ang II-induced hypertension in rats and mice, or DOCA-salt induced hypertension in rats, was prevented by treatment with TMS, and by Cyp1b1 gene disruption in mice (32, 39). Since Ang II-induced hypertension in rats and mice and DOCA-salt-induced hypertension in rats was associated with increased NADPH oxidase activity and/or ROS production in the aorta that were inhibited by TMS (32, 39) or by Cyp1b1 gene disruption in mice (Figure 3) (32), it appears that ROS generated via CYP1B1 are most likely responsible for endothelial dysfunction in these models of experimental hypertension. Metabolite(s) of AA generated via CYP1B1 that activate NADPH oxidase, as observed in VSMCs, do not appear to be 5-, 12-, 15-, or 20-HETE (30). Whether it is one or more lipid peroxide(s) generated from AA that activates NADPH oxidase and produce ROS remains to be determined.
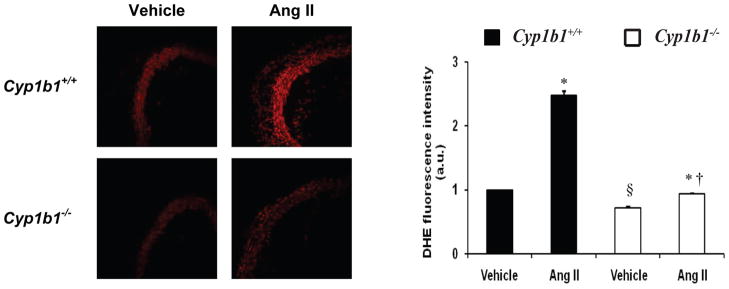
Figure 3. Cyp1b1 gene disruption reduces vascular oxidative stress associated with Ang II-induced hypertension in mice.
Cyp1b1+/+ and Cyp1b1−/− mice were infused with either Ang II or vehicle as described in Figure 2 legend. ROS production was determined in aortic sections by DHE fluorescence. §P < 0.05 Cyp1b1+/+ vehicle vs. Cyp1b1−/− vehicle; *P < 0.05 vehicle vs. corresponding value from Ang II-treated animal; †P < 0.05 Cyp1b1+/+ Ang II vs. Cyp1b1−/− Ang II (n = 4 for all experiments, and data are expressed as mean ± SEM). (Hypertension, 2010;56:667–674).
4.3. Renal dysfunction, injury and inflammation associated with hypertension
Hypertension is known to be associated with renal dysfunction and end organ damage, which has been attributed to increased renal perfusion pressure and ROS production (41–45). In a recent study, we found that Ang II infusion in rats at 150 ng/kg/min that caused hypertension, cardiovascular hypertrophy, and cardiac fibrosis and inflammation, did not result in renal dysfunction. However, infusion of Ang II at 300 ng/kg/min that produced a similar increase in systolic blood pressure to that of Ang II at a concentration of 150 ng/kg/min, also served to increase water consumption and urine output, increase urinary sodium and potassium excretion, cause proteinuria and albuminuria, and decrease urine osmolality in rat. Similar effects of Ang II infusion at 700 ng/kg/min were observed in Cyp1b1+/+ mice; these effects of Ang II were prevented or minimized by treatment with TMS in rats and by Cyp1b1 gene disruption in mice (46, 47). Moreover, Ang II infusion increased renal vascular resistance and vascular reactivity to vasoconstrictor agents and endothelial dysfunction in rats and in Cyp1b1+/+ mice that were inhibited by TMS in rats and in Cyp1b1−/− mice (46, 47). In addition, the renal dysfunction associated with DOCA-salt-induced hypertension is minimized by TMS in rats (39). These observations, together with our findings that these pathophysiological changes caused by Ang II- and DOCA-salt-induced hypertension are associated with increased renal NADPH oxidase activity and ROS production (39, 46, 47), suggest that CYP1B1 via ROS generation also contributes to renal dysfunction in these models of hypertension.
Ang II infusion at 300 ng/kg/min, but not 150 ng/kg/min in rats produced renal fibrosis as manifested by increased interstitial accumulation of α-smooth muscle actin, tubular damage, as indicated by tubular dilation, proteinaceous cast formation, and inflammation, as observed by increased T-lymphocyte (CD-3+ cells) infiltration in the glomerulus (46). Ang II infusion at 700 ng/kg/min for13 days that caused renal dysfunction in mice did not produce renal injury or inflammation (47). However, infusion of Ang II for 28 days produced a similar degree of renal damage in Cyp1b1+/+ mice, as that described above in the rat (46); these deleterious effects of Ang II in the kidney were minimized by treatment with TMS in the rat (46), and by Cyp1b1−/− gene disruption in the mice (47). It has been reported that renal damage produced by Ang II infusion at 2ng/kg/min i.v. with 4% salt in drinking water in rats is primarily due to increased renal perfusion pressure (42). Therefore, it appears that administration of salt with Ang II or a prolonged increase in pressure such as in mice without salt is obligatory for renal injury and inflammation. The renal injury and inflammation caused by Ang II infusion in rats and mice were also associated with increased renal NADPH oxidase activity and ROS production (46, 47). Since the increased renal perfusion pressure caused by hypertension can produce ROS independently that cause renal damage (21–25), and Ang II stimulates VSMC growth via CYP1B1-dependent ROS production in vitro (30), it is possible that ROS generated via CYP1B1 contributes to the renal injury and inflammation associated with Ang II-induced hypertension by a renal perfusion pressure- dependent as well as -independent mechanism. The renal damage caused by Ang II + L-NAME-induced hypertension has been reported to be independent of renal perfusion pressure, and is probably due to increased oxidative stress and inflammation resulting from ischemia as a result of increased renal vasoconstriction (48).
5. Mechanism of CYP1B1-mediated pathophysiological changes associated with hypertension
The increased ROS production associated with Ang II- and DOCA-salt-induced hypertension could result from AA metabolites generated via CYP1B1. CYP1B1 can metabolize AA in vitro into HETEs including 12- and 20-HETE (29) that can increase NADPH oxidase activity and/or ROS production (49–51). However, in rat VSMCs, the conversion of AA into HETEs was not affected by inhibition of CYP1B1 activity with TMS or CYP1B1 shRNA (30) and plasma levels of 12- and 20-HETE were also not altered in Ang II- and DOCA-salt-induced hypertension in rats (32, 39). Moreover, renal levels of these HETEs were not altered in rats infused with Ang II with or without TMS (46). Therefore, ROS produced by Ang II- or DOCA-salt-induced hypertension in rats appear to be independent of 12- or 20-HETE. However, Ang II-induced renal dysfunction was associated with increased levels of 12- and 20-HETE in Cyp1b1+/+ mice, but not in Cyp1b1−/− mice, suggesting that these HETEs may contribute to renal dysfunction by increasing ROS production and/or by their direct tubular and vascular actions (47). Since renal levels of 12/15 lipoxygenase, Cyp4a10/a12/a14, and Cyp4f13/f15 protein were not altered in the kidneys of Cyp1b1−/− mice; they appear unlikely to be involved in the increased production of 12- and 20-HETEs by Ang II in mice (47). These observations also suggest species-specific production of these HETEs via CYP1B1.
The mechanism by which ROS produced via CYP1B1 causes cardiovascular hypertrophy, increased vascular reactivity, endothelial dysfunction, and renal dysfunction, proteinuria, injury, and inflammation associated with Ang II-induced hypertension in rats and mice and DOCA-salt-induced hypertension in rats, is most likely due to activation of one or more signaling molecules including ERK1/2, p38 MAPK and c-Src. These kinases are known to be involved in various actions of Ang II including VSMC growth and various models of hypertension (52–55). Supporting this view is the demonstration that an increase in ERK1/2 and p38 MAPK activities in aorta, heart, and kidney associated with DOCA-salt induced hypertension was inhibited by treatment with TMS in rats (39). Moreover, Ang II-induced hypertension and renal dysfunction, injury and inflammation that are associated with increased activities of NADPH oxidase, ERK1/2, p38 MAPK and c-Src and ROS production in the kidneys of rats and Cyp1b1−/− mice are diminished by treatment with TMS, and in Cyp1b1−/− mice, respectively (46, 47).
6. Conclusion
The increased activity of neurohumoral factors including Ang II increase cPLA2 activity which releases AA. One or more metabolite(s) of AA generated via CYP1B1 stimulates NADPH oxidase that increases ROS production, which by activating various signaling molecules including ERK1/2, p38 MAPK, and c-Src stimulate VSMC growth, and contribute to the development of hypertension and associated pathophysiological changes including cardiovascular hypertrophy, increased vascular reactivity, endothelial and renal dysfunction, injury, and inflammation (Figure 4). Therefore, CYP1B1 could serve as a novel target for the development of therapeutic agents for the treatment of hypertension and associated cardiovascular and renal dysfunction, injury, and inflammation.
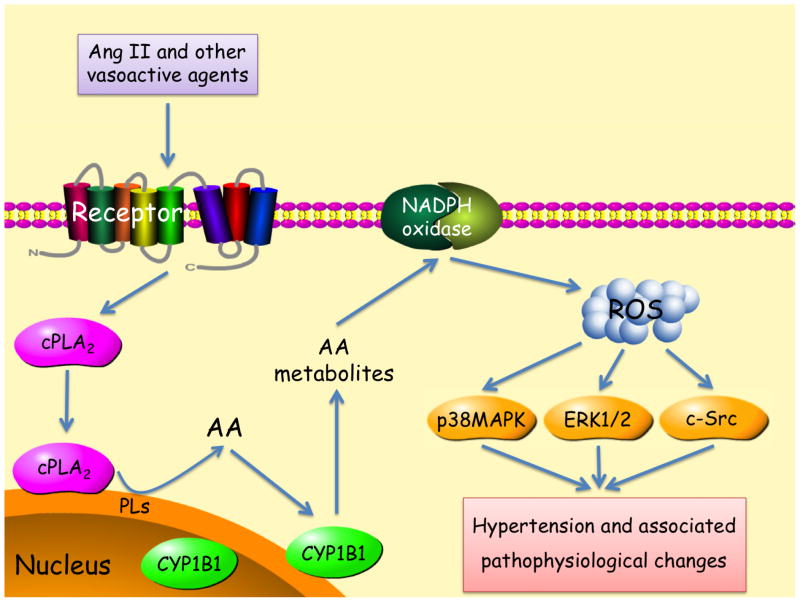
Figure 4.
Activation of cPLA2 by Ang II and other vasoactive agents releases AA from tissue phospholipids (PLs). One or more metabolite(s) of AA generated via CYP1B1 stimulates NADPH oxidase activity and generates ROS, that in turn by stimulating one or more signaling molecules (ERK 1/2, p38MAPK and c-Src) results in hypertension and associated pathophysiological changes.
Acknowledgments
This work was supported by the National Institutes of Health, National Heart, Lung, and Blood Institute, Grant R01-HL-19134-36 (K.U.M). B.L.J. was partly supported by a fellowship from the Neuroscience Institute, University of Tennessee Health Science Center. S.S.-F. was supported by a fellowship from the Scientific and Technical Research Council of Turkey (TUBITAK).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Esler M. The sympathetic system and hypertension. Am J Hypertens. 2000;13:S99–S105. doi: 10.1016/s0895-7061(00)00225-9. [DOI] [PubMed] [Google Scholar]
- 2.Carey RM. Overview of endocrine systems in primary hypertension. Endocrinol Metab Clin North Am. 2011;40:265–77. doi: 10.1016/j.ecl.2011.01.003. [DOI] [PubMed] [Google Scholar]
- 3.Rao GN, Lassègue B, Alexander RW, Griendling KK. Angiotensin II stimulates phosphorylation of high-molecular-mass cytosolic phospholipase A2 in vascular smooth-muscle cells. Biochem J. 1994;299:197–201. doi: 10.1042/bj2990197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Muthalif MM, Benter IF, Uddin MR, Malik KU. Calcium/calmodulin-dependent protein kinase IIα mediates activation of mitogen-activated protein kinase and cytosolic phospholipase A2 in norepinephrine-induced arachidonic acid release in rabbit aortic smooth muscle cells. J Biol Chem. 1996;271:30149–57. doi: 10.1074/jbc.271.47.30149. [DOI] [PubMed] [Google Scholar]
- 5.Muthalif MM, Benter IF, Uddin MR, Harper JL, Malik KU. Signal transduction mechanisms involved in angiotensin-(1–7)-stimulated arachidonic acid release and prostanoid synthesis in rabbit aortic smooth muscle cells. J Pharmacol Exp Ther. 1998;284:388–98. [PubMed] [Google Scholar]
- 6.Nasjletti A, Malik KU. Interrelations between prostaglandins and vasoconstrictor hormones: contribution to blood pressure regulation. Fed Proc. 1982;41:2394–9. [PubMed] [Google Scholar]
- 7.McGiff JC. Prostaglandins, prostacyclin, and thromboxanes. Annu Rev Pharmacol Toxicol. 1981;21:479–509. doi: 10.1146/annurev.pa.21.040181.002403. [DOI] [PubMed] [Google Scholar]
- 8.Nasjletti A. Arthur C. Corcoran Memorial Lecture: The role of eicosanoids in angiotensin-dependent hypertension. Hypertension. 1998;31:194–200. doi: 10.1161/01.hyp.31.1.194. [DOI] [PubMed] [Google Scholar]
- 9.McGiff JC, Quilley J. 20-Hydroxyeicosatetraenoic acid and epoxyeicosatrienoic acids and blood pressure. Curr Opin Nephrol Hypertens. 2001;10:231–7. doi: 10.1097/00041552-200103000-00012. [DOI] [PubMed] [Google Scholar]
- 10.Nozawa K, Tuck ML, Golub M, Eggena P, Nadler JL, Stern N. Inhibition of lipoxygenase pathway reduces blood pressure in renovascular hypertensive rats. Am J Physiol Heart Circ Physiol. 1990;259:H1774–80. doi: 10.1152/ajpheart.1990.259.6.H1774. [DOI] [PubMed] [Google Scholar]
- 11.Smith RL, Weidemann MJ. Reactive oxygen production associated with arachidonic acid metabolism by peritoneal macrophages. Biochem Biophys Res Commun. 1980;97:973–80. doi: 10.1016/0006-291x(80)91472-2. [DOI] [PubMed] [Google Scholar]
- 12.Lee MY, Griendling KK. Redox signaling, vascular function, and hypertension. Antioxid Redox Signal. 2008;10:1045–59. doi: 10.1089/ars.2007.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hong HJ, Hsiao G, Cheng TH, Yen MH. Supplemention with tetrahydrobiopterin suppresses the development of hypertension in spontaneously hypertensive rats. Hypertension. 2001;38:1044–8. doi: 10.1161/hy1101.095331. [DOI] [PubMed] [Google Scholar]
- 14.Rajagopalan S, Kurz S, Münzel T, Tarpey M, Freeman BA, Griendling KK, Harrison DG. Angiotensin II–mediated hypertension in the rat increases vascular superoxide production via membrane NADH/NADPH oxidase activation. Contribution to alterations of vasomotor tone. J Clin Invest. 1996;97:1916–23. doi: 10.1172/JCI118623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Landmesser U, Dikalov S, Price SR, McCann L, Fukai T, Holland SM, Mitch WE, Harrison DG. Oxidation of tetrahydrobiopterin leads to uncoupling of endothelial cell nitric oxide synthase in hypertension. J Clin Invest. 2003;111:1201–9. doi: 10.1172/JCI14172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Roman RJ. P-450 metabolites of arachidonic acid in the control of cardiovascular function. Physiol Rev. 2002;82:131–85. doi: 10.1152/physrev.00021.2001. [DOI] [PubMed] [Google Scholar]
- 17.Shimada T, Hayes CL, Yamazaki H, Amin S, Hecht SS, Guengerich FP, Sutter TR. Activation of chemically diverse procarcinogens by human cytochrome P-450 1B1. Cancer Res. 1996;56:2979–84. [PubMed] [Google Scholar]
- 18.Shimada T, Fujii-Kuriyama Y. Metabolic activation of polycyclic aromatic hydrocarbons to carcinogens by cytochromes P450 1A1 and 1B1. Cancer Sci. 2004;95:1–6. doi: 10.1111/j.1349-7006.2004.tb03162.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hayes CL, Spink DC, Spink BC, Cao JQ, Walker NJ, Sutter TR. 17β-Estradiol hydroxylation catalyzed by human cytochrome P450 1B1. Proc Natl Acad Sci USA. 1996;93:9776–81. doi: 10.1073/pnas.93.18.9776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Choudhary D, Jansson I, Stoilov I, Sarfarazi M, Schenkman JB. Metabolism of retinoids and arachidonic acid by human and mouse cytochrome P450 1B1. Drug Metab Dispos. 2004;32:840–7. doi: 10.1124/dmd.32.8.840. [DOI] [PubMed] [Google Scholar]
- 21.Sutter TR, Tang YM, Hayes CL, Wo YYP, Jabs EW, Lee X, Yin H, Cody CW, Greenlee WF. Complete cDNA sequence of a human dioxin-inducible mRNA identifies a new gene subfamily of cytochrome P450 that maps to chromosome 2. J Biol Chem. 1994;269:13092–9. [PubMed] [Google Scholar]
- 22.Tang YM, Wo Y-Y, Stewart J, Hawkins AL, Griffins CA, Sutter TR, Greenlee WF. Isolation and characterization of the human CYP1B1 gene. J Biol Chem. 1996;271:28324–30. doi: 10.1074/jbc.271.45.28324. [DOI] [PubMed] [Google Scholar]
- 23.Korashy HM, El-Kadi AOS. The role of aryl hydrocarbon receptor in the pathogenesis of cardiovascular diseases. Drug Metab Rev. 2006;38:411–50. doi: 10.1080/03602530600632063. [DOI] [PubMed] [Google Scholar]
- 24.McFadyen MC, Melvin WT, Murray GI. Cytochrome P450 enzymes: novel options for cancer therapeutics. Mol Cancer Ther. 2004;3:363–71. [PubMed] [Google Scholar]
- 25.Ryu DY, Hodgson E. Constitutive expression and induction of CYP1B1 mRNA in the mouse. J Biochem Mol Toxicol. 1999;13:249–51. doi: 10.1002/(sici)1099-0461(1999)13:5<249::aid-jbt4>3.0.co;2-l. [DOI] [PubMed] [Google Scholar]
- 26.Zhao W, Parrish AR, Ramos KS. Constitutive and inducible expression of cytochrome P450IA1 and P450IB1 in human vascular endothelial and smooth muscle cells. In Vitro Cell Dev Biol Anim. 1998;34:671–3. doi: 10.1007/s11626-998-0060-7. [DOI] [PubMed] [Google Scholar]
- 27.Kerzee JK, Ramos KS. Constitutive and inducible expression of Cyp1a1 and Cyp1b1 in vascular smooth muscle cells. Role of the Ahr bHLH/PAS transcription factor. Circ Res. 2001;89:573–82. doi: 10.1161/hh1901.097083. [DOI] [PubMed] [Google Scholar]
- 28.Conway DE, Sakurai Y, Weiss D, Vega JD, Taylor WR, Jo H, Eskin SG, Marcus CB, McIntire LV. Expression of CYP1A1 and CYP1B1 in human endothelial cells: regulation by fluid shear stress. Cardiovasc Res. 2009;81:669–77. doi: 10.1093/cvr/cvn360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tang Y, Scheef EA, Wang S, Sorenson CM, Marcus CB, Jefcoate CR, Sheibani N. CYP1B1 expression promotes the proangiogenic phenotype of endothelium through decreased intracellular oxidative stress and thrombospondin-2 expression. Blood. 2009;113:744–54. doi: 10.1182/blood-2008-03-145219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yaghini FA, Song CY, Lavrentyev EN, Ghafoor HUB, Fang XR, Estes AM, Campbell WB, Malik KU. Angiotensin II-induced vascular smooth muscle cell migration and growth are mediated by cytochrome P450 1B1-dependent superoxide generation. Hypertension. 2010;55:1461–7. doi: 10.1161/HYPERTENSIONAHA.110.150029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wilcox CS, Pearlman A. Chemistry and antihypertensive effects of tempol and other nitroxides. Pharmacol Rev. 2008;60:418–69. doi: 10.1124/pr.108.000240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Jennings BL, Sahan-Firat S, Estes AM, Das K, Farjana N, Fang XR, Gonzalez FJ, Malik KU. Cytochrome P450 1B1 contributes to angiotensin II–induced hypertension and associated pathophysiology. Hypertension. 2010;56:667–74. doi: 10.1161/HYPERTENSIONAHA.110.154518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Crofton JT, Share L, Shade RE, Lee-Kwon WJ, Manning M, Sawyer WH. The importance of vasopressin in the development and maintenance of DOC-salt hypertension in the rat. Hypertension. 1979;1:31–8. doi: 10.1161/01.hyp.1.1.31. [DOI] [PubMed] [Google Scholar]
- 34.de Champlain J, Bouvier M, Drolet G. Abnormal regulation of the sympathoadrenal system in deoxycorticosterone acetate-salt hypertensive rats. Can J Physiol Pharmacol. 1987;65:1605–14. doi: 10.1139/y87-252. [DOI] [PubMed] [Google Scholar]
- 35.Drolet G, Bouvier M, de Champlain J. Enhanced sympathoadrenal reactivity to haemorrhagic stress in DOCA-salt hypertensive rats. J Hypertens. 1989;7:237–42. doi: 10.1097/00004872-198903000-00011. [DOI] [PubMed] [Google Scholar]
- 36.Schiffrin EL, Sventek P, Li JS, Turgeon A, Reudelhuber T. Antihypertensive effect of an endothelin receptor antagonist in DOCA-salt spontaneously hypertensive rats. Br J Pharmacol. 1995;115:1377–81. doi: 10.1111/j.1476-5381.1995.tb16626.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bonventre JV, Swidler M. Calcium dependency of prostaglandin E2 production in rat glomerular mesangial cells. Evidence that protein kinase C modulates the Ca2+ dependent activation of phospholipase A2. J Clin Invest. 1988;82:168–76. doi: 10.1172/JCI113566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Trevisi L, Bova S, Cargnelli G, Ceolotto G, Luciani S. Endothelin-1 induced arachidonic acid release by cytosolic phospholipase A2 activation in rat vascular smooth muscle via extracellular signal-regulated kinases pathway. Biochem Pharmacol. 2000;264:425–31. doi: 10.1016/s0006-2952(02)01066-3. [DOI] [PubMed] [Google Scholar]
- 39.Sahan-Firat S, Jennings BL, Yaghini FA, Song CY, Estes AM, Fang XR, Farjana N, Khan AI, Malik KU. 2,3′,4,5′-Tetramethoxystilbene prevents deoxycorticosterone-salt-induced hypertension: contribution of cytochrome P-450 1B1. Am J Physiol Heart Circ Physiol. 2010;299:H1891–901. doi: 10.1152/ajpheart.00655.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sarr M, Chataigneau M, Martins S, Schott C, El Bedoui J, Oak M-H, Muller B, Chataigneau T, Schini-Kerth VB. Red wine polyphenols prevent angiotensin II-induced hypertension and endothelial dysfunction in rats: role of NADPH oxidase. Cardiovasc Res. 2006;71:794–802. doi: 10.1016/j.cardiores.2006.05.022. [DOI] [PubMed] [Google Scholar]
- 41.Mori T, Cowley AW., Jr Role of pressure in angiotensin II-induced renal injury. Chronic servo-control of renal perfusion pressure in rats. Hypertension. 2004;43:752–9. doi: 10.1161/01.HYP.0000120971.49659.6a. [DOI] [PubMed] [Google Scholar]
- 42.Jin C, Hu C, Polichnowski A, Mori T, Skelton M, Ito S, Cowley AW., Jr Effects of renal perfusion pressure on renal medullary hydrogen peroxide and nitric oxide production. Hypertension. 2009;53:1048–53. doi: 10.1161/HYPERTENSIONAHA.109.128827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Agarwal R. Proinflammatory effects of oxidative stress in chronic kidney disease: role of additional angiotensin II blockade. Am J Physiol Renal Physiol. 2003;284:F863–9. doi: 10.1152/ajprenal.00385.2002. [DOI] [PubMed] [Google Scholar]
- 44.Reckelhoff JF, Romero JC. Role of oxidative stress in angiotensin-induced hypertension. Am J Physiol Regul Integr Comp Physiol. 2003;284:R893–912. doi: 10.1152/ajpregu.00491.2002. [DOI] [PubMed] [Google Scholar]
- 45.Welch WJ, Blau J, Xie H, Chabrashvili T, Wilcox CS. Angiotensin-induced defects in renal oxygenation: role of oxidative stress. Am J Physiol Heart Circ Physiol. 2005;288:H22–8. doi: 10.1152/ajpheart.00626.2004. [DOI] [PubMed] [Google Scholar]
- 46.Jennings BL, Anderson LJ, Estes AM, Fang XR, Song CY, Campbell WB, Malik KU. Involvement of cytochrome P450 1B1 to renal dysfunction, injury and inflammation associated with angiotensin II-induced hypertension in rats. Am J Physiol Renal Physiol. 2011 Nov 16; doi: 10.1152/ajprenal.00542.2011. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Jennings BL, Anderson LJ, Estes AM, Yaghini FA, Fang XR, Porter J, Gonzalez FJ, Campbell WB, Malik KU. Cytochrome P450 1B1 contributes to renal dysfunction and damage caused by angiotensin II in mice. Hypertension. doi: 10.1161/HYPERTENSIONAHA.111.183301. (accepted for publication) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Polichnowski AJ, Lu L, Cowley AW., Jr Renal injury in angiotensin II + L-NAME-induced hypertensive rats is independent of elevated blood pressure. Am J Physiol Renal Physiol. 2011;300:F1008–16. doi: 10.1152/ajprenal.00354.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Medhora M, Chen Y, Gruenloh S, Harland D, Bodiga S, Zielonka J, Gebremedhin D, Gao Y, Falck JR, Anjaiah S, Jacobs ER. 20-HETE increases superoxide production and activates NADPH oxidase in pulmonary artery endothelial cells. Am J Physiol Lung Cell Mol Physiol. 2008;294:L902–11. doi: 10.1152/ajplung.00278.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wen Y, Gu J, Vandenhoff GE, Liu X, Nadler JL. Role of 12/15-lipoxygenase in the expression of MCP-1 in mouse macrophages. Am J Physiol Heart Circ Physiol. 2008:H1933–8. doi: 10.1152/ajpheart.00260.2007. [DOI] [PubMed] [Google Scholar]
- 51.Eid AA, Gorin Y, Fagg BM, Maalouf R, Barnes JL, Block K, Abboud HE. Mechanisms of podocyte injury in diabetes. Role of cytochrome P450 and NADPH oxidases. Diabetes. 2009;58:1201–11. doi: 10.2337/db08-1536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Cat AND, Touyz RM. Cell signaling of angiotensin II on vascular tone: novel mechanisms. Curr Hypertens Rep. 2011;13:122–8. doi: 10.1007/s11906-011-0187-x. [DOI] [PubMed] [Google Scholar]
- 53.Higuchi S, Ohtsu H, Suzuki H, Shirai H, Frank GD, Eguchi S. Angiotensin II signal transduction through the AT1 receptor: novel insights into mechanisms and pathophysiology. Clin Sci. 2007;112:417–28. doi: 10.1042/CS20060342. [DOI] [PubMed] [Google Scholar]
- 54.Callera GE, Montezano ACI, Yogi A, Tostes RC, He Y, Schiffrin EL, Touyz RM. Responses to c-Src–dependent nongenomic signaling responses to aldosterone are increased in vascular myocytes from spontaneously hypertensive rats. Hypertension. 2005;46:1032–8. doi: 10.1161/01.HYP.0000176588.51027.35. [DOI] [PubMed] [Google Scholar]
- 55.Mehta PK, Griendling KK. Angiotensin II cell signaling: physiological and pathological effects in the cardiovascular system. Am J Physiol Cell Physiol. 2007;292:C82–97. doi: 10.1152/ajpcell.00287.2006. [DOI] [PubMed] [Google Scholar]