Abstract
The regenerative capacity of the mammary gland following post-lactational involution depends on the presence of multipotent stem or progenitor cells. Mammary progenitor cells exist as a quiescent and self-renewing population capable of differentiating into luminal epithelial and myoepithelial cells and generating ductal and alveolar structures. The fate choices of these cells are regulated by several soluble signals as well as their surrounding extracellular matrix. Whereas matrix stiffness has been implicated in organ-specific differentiation of embryonic and mesenchymal stem cells, the effects of substratum compliance on the more limited fate switches typical of tissue-specific progenitor cells is unknown. Here we examined how the mechanical properties of the microenvironment affect the differentiation of mammary progenitor cells. Immortalized human mammary progenitor cells were cultured on synthetic hydrogels of varying stiffness and their self-renewal and fate decisions were quantified. We found that cells cultured on soft substrata differentiated preferentially into luminal epithelial cells, whereas those cultured on stiff substrata differentiated preferentially into myoepithelial cells. Furthermore, pharmacological manipulations of cytoskeletal tension in conjunction with analysis of gene expression revealed that mechanical properties of the microenvironment signal through the small GTPase RhoA and cytoskeletal contractility to modulate the differentiation of mammary progenitor cells. These data suggest that subtle variations in the mechanical compliance of a tissue can direct the fate decisions of its resident progenitor cells.
Keywords: mammary stem cells, differentiation, matrix compliance, RhoA, mechanical stress, branching morphogenesis
1 Introduction
Multipotent stem cells, or progenitor cells, maintain homeostasis of adult tissues and are unique in their ability to self-renew and differentiate into specialized cell types. In the adult human mammary gland, progenitor cells reside in nests within the terminal ducts (Villadsen et al. 2007) and are responsible for the formation of terminal ductal lobular units (TDLUs), the basic components of mammary epithelial architecture (Tiede and Kang 2011; Visvader and Smith 2011). Mammary progenitor cells differentiate into the luminal epithelial and myoepithelial cells that make up the bilayered structure of the duct (Visvader and Smith 2011; Woodward et al. 2005). The differentiation of such progenitors is governed by a multitude of signals from other cells and the extracellular matrix (ECM) (LaBarge et al. 2009; Lim et al. 2010; Taddei et al. 2008; Anderson et al. 2011; Liu et al. 2006; Dontu et al. 2004; Korkaya et al. 2009).
The morphogenesis and functional differentiation of the mammary epithelium depends also on its mechanical environment (Gjorevski and Nelson 2011). The formation and branching morphogenesis of tubular structures in culture is tuned by the compliance of the surrounding microenvironment (Wozniak et al. 2003; Gjorevski and Nelson 2010), and abnormally stiff settings can promote tissue dysplasia (Paszek et al. 2005). In the mouse mammary gland in vivo, genetic alterations that enhance tissue stiffness lead to distortions in epithelial structure and promote tumor formation and progression (Levental et al. 2009; Sternlicht et al. 1999). Furthermore, luminal epithelial cells respond to basement membrane and lactogenic hormones by synthesizing and secreting milk proteins when in soft, but not stiff, environments (Alcaraz et al. 2008; Emerman et al. 1979). Based on these data, it is intriguing to speculate that the mechanical environment might also play a role in the maintenance and differentiation of the progenitor cells that occupy the gland.
The mechanical environment of a tissue is defined by the inherent contractility of its resident cells and the mechanical properties, or compliance, of their surrounding matrix. Cell-ECM adhesions contain mechanically sensitive proteins such as vinculin, which both respond to force and allow the force generated from actomyosin contraction to be transmitted to the substratum (Alenghat and Ingber 2002; Beningo et al. 2001; Bershadsky et al. 2003; Tamada et al. 2004). In response to contraction, the counterbalancing resistance from the substratum results in the generation of tensile stresses in the cytoskeleton (Ingber 2004; Engler et al. 2006), allowing cells to pull on and sense their physical environments. Cellular contractility is regulated in part by the small GTPase RhoA, which modulates cytoskeletal tension through its effectors, including Rho-associated kinase (ROCK) (Ishizaki et al. 1997; Kimura et al. 1996). Activated ROCK and myosin light chain kinase (MLCK) increase the level of phosphorylated myosin light chain, which promotes myosin activation (Amano et al. 1996; Ishizaki et al. 1997; Kimura et al. 1996), a necessary component for cells to contract and pull against a matrix to generate cellular tension.
Substratum stiffness and actomyosin contractility play a critical role in regulating the differentiation of pluripotent cells including human mesenchymal stem cells (MSCs) and embryonic stem (ES) cells. In contrast to tissue-specific progenitor cells such as those in the mammary gland, MSCs and ES cells can generate a diverse array of cell types from several different organs (Pittenger et al. 1999; Thomson et al. 1998), each with widely different mechanical properties. For example, MSCs can differentiate into osteogenic cells that occupy rigid bone or neurons typical of those found in the brain; bone is >100-fold stiffer than brain. Soft substrata that mimic the elasticity of brain tissue promote neuronal differentiation, intermediate substrata that mimic the elasticity of muscle tissue are myogenic, and stiff substrata that mimic bone are osteogenic (Engler et al. 2006). Information about the elasticity of the substratum is likely transmitted to changes in gene expression that drive these switches in differentiation through cellular contractility. Inhibiting RhoA by ectopic expression of a dominant negative mutant causes MSCs to differentiate into adipocytes, whereas activating RhoA induces differentiation into bone (McBeath et al. 2004). Similarly, ES cells express mesendodermal genes and undergo osteogenic differentiation on stiff but not soft substrata (Evans et al. 2009). Substratum compliance and cellular contractility thus play significant roles in the differentiation of pluripotent MSCs and ES cells.
Whereas these previous studies have demonstrated that substratum compliance is capable of modulating the fate of pluripotent stem cells by directing them down different lineages, the effects of substratum compliance on the more limited differentiation programs of organ-specific progenitor cells is unknown. Here we examined how the mechanical properties of the microenvironment affect the differentiation of mammary progenitors. We cultured immortalized human mammary progenitor cells on synthetic substrata of varying elastic modulus and monitored the resulting changes in cell fate. We found that substratum compliance profoundly affected the self-renewal of the mammary progenitor cells and their differentiation into luminal epithelial or myoepithelial cells. Using this system in conjunction with pharmacological manipulations of cytoskeletal tension and analysis of gene expression, we implicate the RhoA pathway as a possible mechanism through which mechanical signals from the microenvironment may modulate the differentiation of mammary progenitor cells.
2 Materials and methods
2.1 Cell culture and reagents
Immortalized human mammary progenitor D920 cells (Villadsen et al. 2007) were maintained in H14 medium (1:1 DMEM/F12, 250 ng/ml insulin (Sigma), 10 µg/ml human transferrin (Sigma), 2.6 ng/ml sodium selenite (BD Bioscience), 0.1 nM estradiol (Sigma), 1.4 µM hydrocortisone (Sigma), 5 µg/ml prolactin (Sigma), and 0.1 µM gentamicin (Sigma)) and used between passages 8 and 18. Progenitor cells were cultured on collagen-functionalized substrata with a range of compliances. For pharmacological manipulation, samples were seeded and cultured for 24 hours, at which point the H14 medium was replaced with media containing one of the following reagents, including blebbistatin (12.5 µM, Sigma), Y27632 (10 µM, Tocris), or calyculin A (2 nM, Calbiochem). Samples were then maintained in the presence of these agents for the remainder of the experiments.
2.2 Synthesis and functionalization of synthetic substrata
Polyacrylamide gels with a fixed acrylamide monomer concentration of 5% and bis-acrylamide crosslinker concentrations ranging from 0.01% to 0.35% were prepared using a protocol adapted from Pelham and Wang (Pelham and Wang 1997). Briefly, glass coverslips were etched in 0.1 N NaOH for 5 minutes followed by rinsing in Millipore water and drying. Coverslips were then soaked in a 2% (v/v) solution of aminopropyltrimethoxysilane (Sigma) in acetone for 10 minutes, rinsed extensively with acetone, and air dried. Next, the coverslips were incubated in a solution of 0.5% (v/v) glutaraldehyde (Sigma) in phosphate-buffered saline (PBS) for 30 minutes followed by extensive rinsing with Millipore water. Acrylamide was mixed with bis-acrylamide, ammonium persulfate (1/200), and N,N,N',N'-tetramethylethylenediamine (1/2000, TEMED; BioRad) and was placed on the surface of the activated coverslip. The acrylamide solution was then covered with a dichlorodimethylsilane-treated (Sigma) coverslip and allowed to polymerize for 30 minutes. The top coverslip was carefully removed and the gel was rinsed with PBS.
The bulk viscoelastic properties of ~1 mm-thick polyacrylamide gels prepared as described above were characterized using an Anton Paar MCR 501 rheometer (Ashland, VA). Measurements were performed in the linear viscoelastic regime for gels hydrated with culture media and maintained at a temperature of 37°C. Young’s moduli (E) of the polyacrylamide gels were computed from measured shear moduli, G, using the following equation: E = 2G(1+v), where v is the Poisson ratio (v = 0.48 for polyacrylamide (Boudou et al. 2006)).
Type I collagen was crosslinked to the surfaces of the gel through the use of sulfosuccinimidyl-6-(4'-azido-2'-nitrophenyl-amino)-hexanoate (sulpho-SANPAH; Pierce) chemistry. Polyacrylamide gels were rinsed in a solution of 50 mM HEPES, pH 8.5. The gel surfaces were then treated with a 2 mM solution of sulfo-SANPAH and exposed to a germicidal UV lamp for 10 minutes. Gels were rinsed once with 50 mM HEPES, pH 8.5 and then the sulfo-SANPAH treatment was repeated. Gels were rinsed twice with HEPES and then treated with 0.2 mg/mL of bovine collagen (Koken, Japan) overnight at 4°C. Before plating cells, gels were rinsed extensively with PBS followed by incubation in culture media at 37°C for 1 hour.
2.3 Real-time PCR
Total RNA was isolated using Trizol reagent (Invitrogen) followed by cDNA synthesis using a Super Script First-Strand Synthesis kit (Invitrogen). Transcript levels were measured by quantitative real-time PCR (qRT-PCR) using SYBR Green chemistry and a Bio-Rad Mini-Opticon instrument. Amplification was followed by melting curve analysis to verify the presence of a single PCR product. Primers for keratin-8, keratin-14, keratin-19, p63, E-cadherin, P-cadherin, alphasmooth muscle actin (αSMA), vimentin and 18S were designed using Beacon Designer software (BioRad) and determined to be specific by BLAST and dissociation curve analysis (Table 1).
Table 1.
Primers used for quantitative real-time PCR
| Gene | Sequences |
|---|---|
| 18S rRNA | Fwd: CGCCGACGACCCATTCGAAC Rev : GAATCGAACCCTGATTCCCCGTC |
| Keratin-8 | Fwd: AGTTACGGTCAACCAGAG Rev : GTCTCCAGCATCTTGTTC |
| Keratin-14 | Fwd: AACCACGAGGAGGAGATG Rev : GTTCAGAATGCGGCTCAG |
| Keratin-19 | Fwd: GCGACTACAGCCACTACTAC Rev : GTCTCAAACTTGGTTCGGAAG |
| E-cadherin | Fwd: TGAAGGTGACAGAGCCTCTGGAT Rev : TGGGTGAATTCGGGCTTGTT |
| P-cadherin | Fwd: GCTGAACATCACGGACAAG Rev : CCTTCCTCGTTGACCTCTG |
| p63 | Fwd: TTGTTGGAAAGTAACTGTGAGAAC Rev : CAAGGGAACTCTTCGTTTAAGTG |
| αSMA | Fwd: GAGTTACGAGTTGCCTGATG Rev : GGTCCTTCCTGATGTCAATATC |
2.4 Immunofluorescence analysis
Samples were rinsed once with PBS prior to fixation in 4% paraformaldehyde solution for 15 minutes. The samples were then rinsed twice with PBS, and permeabilized with 0.3% Triton-X-100 in PBS for 10 minutes. The samples were blocked with 0.1% Triton-X-100 in 10% goat serum in PBS for 6 hours at room temperature or overnight at 4°C. Incubation with rabbit anti-keratin-14 (1:1000, Covance) and mouse anti-keratin-8 (1:100, AbCam) primary antibodies overnight was followed by three consecutive 15 minute rinses with PBS and a two hour incubation with the following secondary antibodies: Alexa 594 goat-anti-rabbit (1:1000) and Alexa 488 goat-anti-mouse (1:1000). The samples were then washed for 15 minutes three times with PBS and incubated for 10 minutes with Hoechst 33342 (1:1000, Invitrogen) to counterstain the nuclei. Samples were then washed for 15 minutes three times with PBS prior to imaging.
2.5 Microscopy and analysis
Samples were imaged using a 20× air objective on a Nikon Eclipse Ti-U inverted fluorescence microscope equipped with a Hamamatsu ORCA CCD camera. Each sample was examined at five random locations, and image analysis was performed using ImageJ. Original images were merged and the numbers of cells expressing keratin-8, keratin-14, both or neither of the markers was recorded. Analysis was performed on at least 300 cells over at least three independent experiments. Confocal images were taken with a Hamamatsu ER camera coupled to a spinning disk confocal microscope (BioRad). Statistical analysis was conducted with Tukey’s multiple comparison test and the Bonferroni post-tests. Graphs were created in GraphPad PRISM 5 and figures were assembled with Macromedia FreeHand MXa.
3 Results
The fate decisions of stem and progenitor cells are typically assessed by tracking the expression of lineage-specific markers at the mRNA and protein levels. Human mammary progenitor cells express, among other markers, keratin-14 (K14) and keratin-19 (K19), two epithelial-specific intermediate filament proteins (LaBarge et al. 2009). In two-dimensional culture on type I collagen-coated surfaces, human mammary progenitors stochastically differentiate into a mixture of luminal epithelial and myoepithelial cells (LaBarge et al. 2009). In three-dimensional culture, these cells can give rise to structures reminiscent of TDLUs, with an inner layer of luminal epithelial cells and an outer layer of myoepithelial cells (LaBarge et al. 2009; Villadsen et al. 2007). Luminal epithelial cells retain expression of K19 and also express keratin-8 (K8) and E-cadherin (LaBarge et al. 2009; Villadsen et al. 2007). In contrast, myoepithelial cells retain expression of K14 while also expressing P-cadherin, α-smooth muscle actin (αSMA), and p63 (LaBarge et al. 2009; Villadsen et al. 2007; Batistatou et al. 2003). The three cell types can thus be distinguished from one another by virtue of their expression patterns: progenitors are K8−/K14+/K19+, luminal epithelial cells are K8+/K14−/K19+ and express E-cadherin, whereas myoepithelial cells are K8−/K14+/K19− and express P-cadherin, αSMA, and p63.
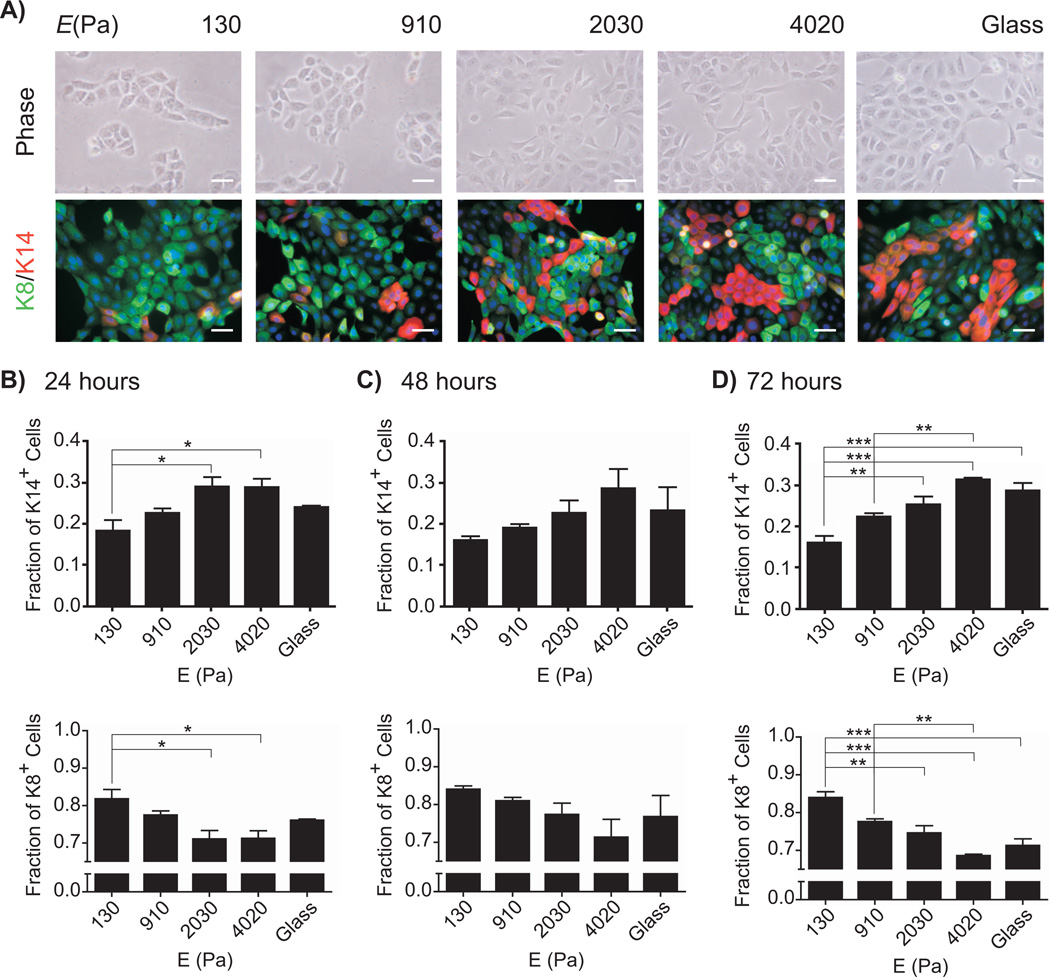
The human mammary gland presents a heterogeneous mechanical environment, with elastic moduli ranging from 0.5 – 7 kPa, depending on measurement technique, location in the breast, and stage in the menstrual cycle (Samani et al. 2007; Lorenzen et al. 2002; Srivastava et al. 2011; Lorenzen et al. 2003). To investigate whether the mechanical properties of the microenvironment affected the fate of mammary progenitors, we cultured immortalized mammary progenitor cells on gels of polyacrylamide (PA) in which the elastic modulus was tuned by controlling the density of crosslinker (Pelham and Wang 1997). Progenitor cells were seeded on collagen-coated PA gels or glass coverslips and assessed for marker expression via immunofluorescence analysis as a function of time (Figure 1A). On soft gels (those with Young’s modulus, E < 1 kPa), the cells exhibited a cobblestone morphology typical of a simple epithelium. On stiff gels (those with E > 2 kPa), a subset of individual cells displayed a more elongated morphology. Within 24 hours of culture, we also observed statistically significant differences in the expression of keratin markers as a function of the elastic modulus of the PA gels (Figure 1B). A larger population of cells expressed the progenitor/myoepithelial marker K14 when cultured on stiff gels than on soft gels. Conversely, cells cultured on soft gels expressed preferentially the luminal epithelial marker K8. These differences persisted over three days of culture (Figure 1C-D). These data suggest that soft substrata favor differentiation into the luminal epithelial phenotype. Because K14 is expressed by both myoepithelial cells and progenitor cells, these data also suggest that stiff substrata either promote differentiation into myoepithelium or maintenance of the progenitor state.
Fig. 1.
Matrix compliance affects mammary progenitor cell differentiation. (A) Phase contrast and fluorescence images of mammary progenitor cells cultured on polyacrylamide gels of varying compliances and collagen-coated glass, stained for keratin-14 (red) and keratin-8 (green). Scale bar, 50 µm. Fraction of keratin-14- and keratin-8-expressing cells on substrata of varying compliance after 24 hours (B), 48 hours (C), and 72 hours (D) after plating, as quantified from immunofluorescence images. (*, P<0.05; **, P<0.01; ***, P<0.001)
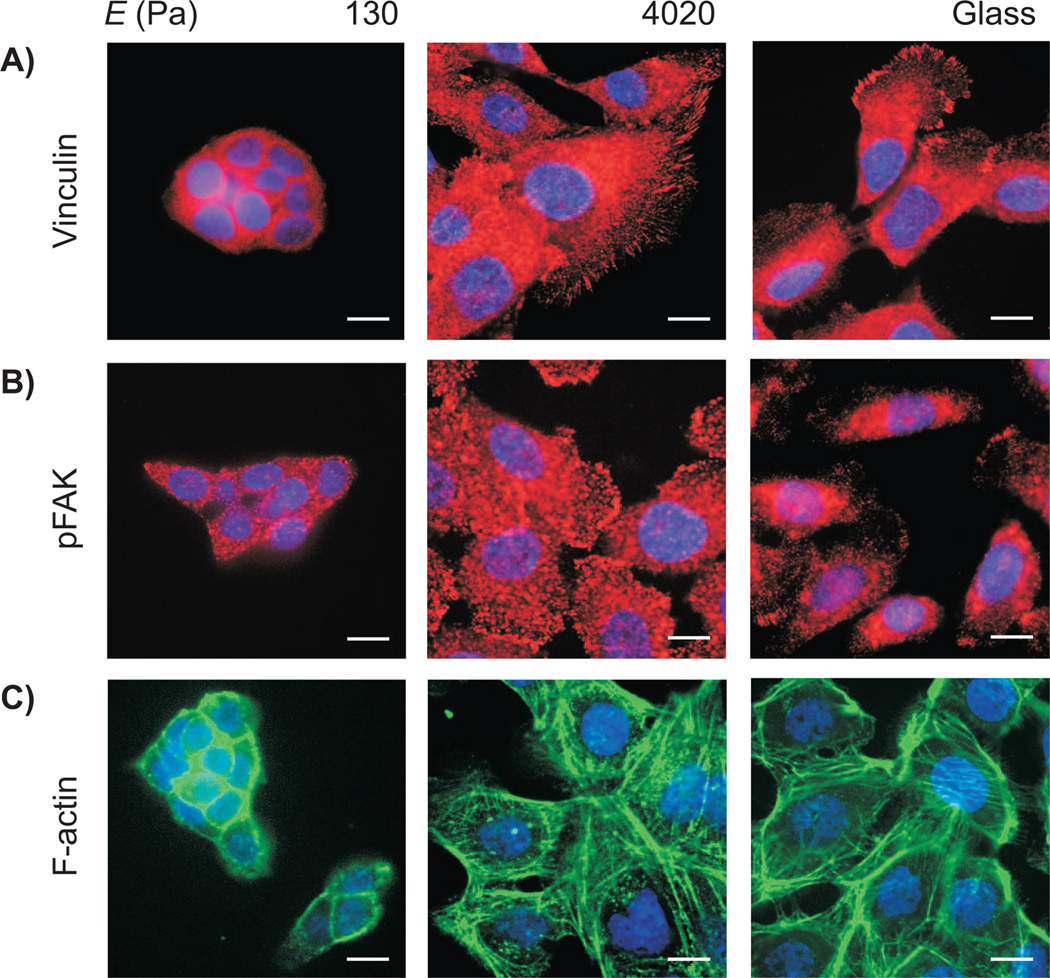
In general, substratum compliance influences cellular phenotype by altering the assembly of focal adhesions and subsequent downstream signaling through the small GTPase RhoA (Eyckmans et al. 2011; Schwartz 2010). Indeed, mammary progenitor cells formed prominent focal adhesions that contained both vinculin and active phosphorylated focal adhesion kinase (FAK) when cultured on stiff substrata (Figure 2A, B). Cells cultured on stiff substrata also contained abundant stress fibers, as demonstrated by labeling filamentous actin with phalloidin (Figure 2C). In contrast, progenitors cultured on soft substrata formed very few adhesions containing vinculin or phosphorylated FAK, and contained predominantly cortical actin rather than stress fibers. Culture on stiff substrata thus appeared to induce cytoskeletal contraction and focal adhesion formation in mammary progenitor cells, whereas soft substrata appeared to relax the cells.
Fig. 2.
Fluorescence images of mammary progenitor cells cultured on 130 and 4020 Pa polyacrylamide gels and collagen-coated glass, stained for vinculin (A), pFAK (B), and F-actin. Scale bar, 10 µm.
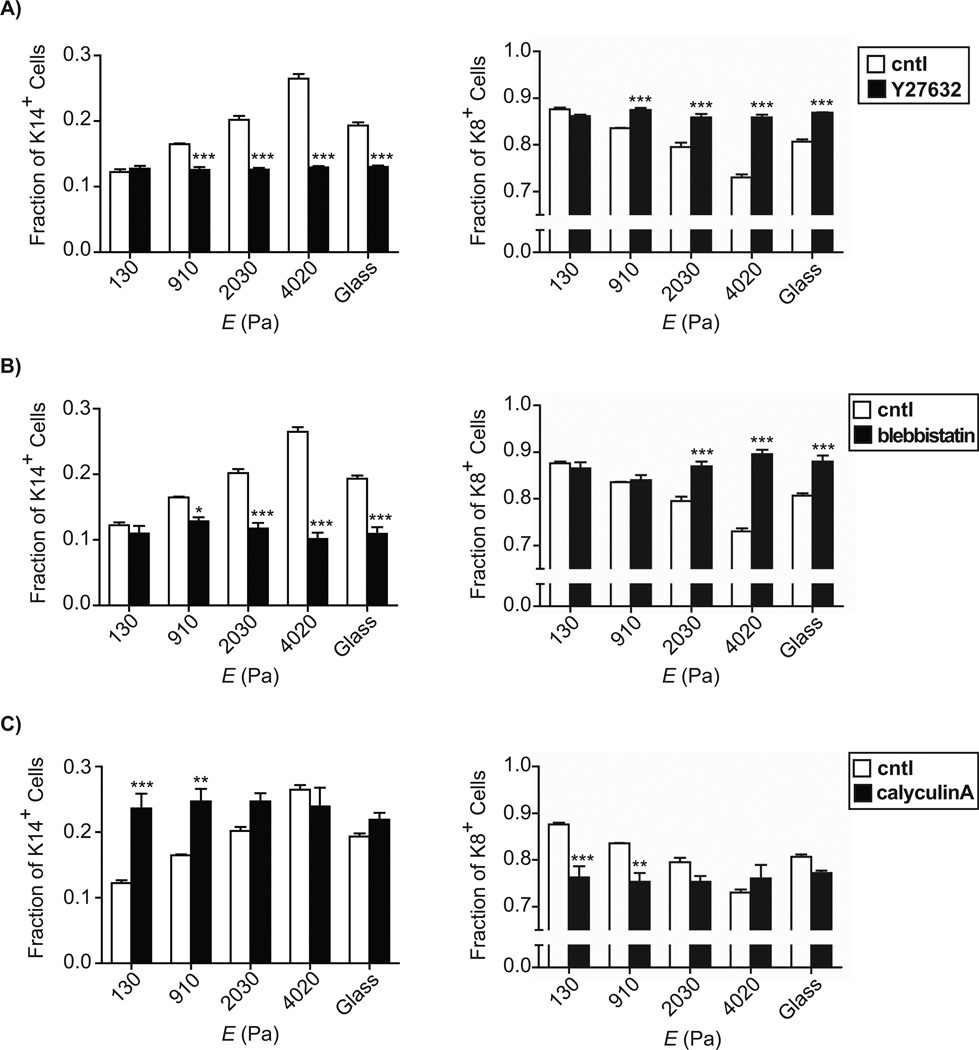
To determine directly whether substratum stiffness modulates the differentiation of mammary progenitor cells through the RhoA pathway, we disrupted downstream signaling in progenitors cultured on PA gels. Blocking signaling through ROCK by using the pharmacological inhibitor Y27632 (Davies et al. 2000) abrogated the stiffness-dependent increase in K14-expressing cells (Figure 3A); cells preferentially expressed K8 on all substrata. Similarly, blocking actomyosin contraction by using blebbistatin, a selective inhibitor of non-muscle myosin II ATPase activity (Straight et al. 2003), reduced the fraction of K14-expressing cells on all substrata, again in favor of the expression of K8 (Figure 3B). Conversely, increasing contractility by using the myosin light chain phosphatase inhibitor calyculin A (Ishihara et al. 1989) resulted in statistically significant increases in the fraction of K14-expressing cells on soft substrata (Figure 3C). These data suggest that soft substrata promote progenitor differentiation into luminal epithelial cells in part by decreasing signaling through RhoA and reducing cytoskeletal contractility.
Fig. 3.
Fractions of keratin-14- and keratin-8-expressing cells on substrata of varying compliances for untreated controls (white) and pharmacologically manipulated samples (black) for Y27632 treatment (A), blebbistatin treatment (B), and calyculin A treatment (C), as quantified from fluorescence images. (*, P<0.05; **, P<0.01; ***, P<0.001)
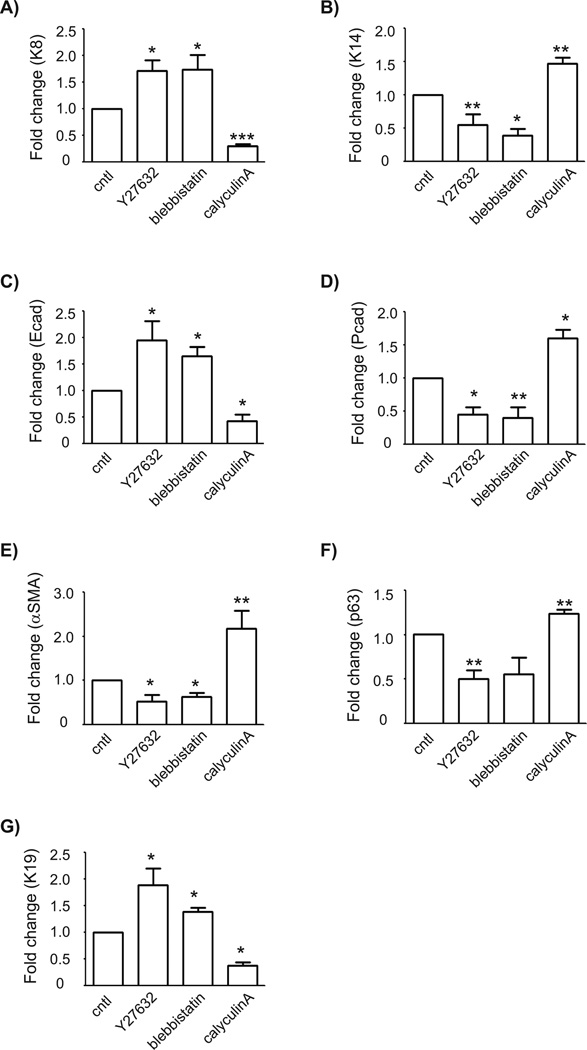
To complement our immunofluorescence assays of markers at the protein level, we used qRT-PCR analysis to monitor changes in marker expression at the mRNA level (Figure 4). Consistently, the expression levels of K8 and K14 showed opposing responses to treatment with Y27632, blebbistatin, and calyculin A. In particular, relaxing the cells increased the levels of K8 and decreased those of K14, whereas enhancing contractility had the inverse effect (Figure 4A, B). These data are consistent with the hypothesis that soft substrata induce differentiation to the luminal epithelial phenotype by reducing actomyosin contractility. Our confidence in this conclusion was further bolstered by examining the effects of these manipulations on the levels of E-cadherin, which is expressed by luminal epithelial but not myoepithelial or progenitor cells. Treatment with Y27632 and blebbistatin increased the levels of E-cadherin, whereas treatment with calyculin A decreased the levels of E-cadherin (Figure 4C).
Fig. 4.
Modulating RhoA signaling pathway regulates the expression levels of myoepithelial and luminal epithelial markers. Mammary progenitor cells cultured on collagen-coated glass were treated with Y27632, blebbistatin, or calyculinA and analyzed for the expression levels of keratin-8 (A), keratin-14 (B), E-cadherin (C), P-cadherin (D), αSMA (E), p63 (F), and keratin-19 (G). The mRNA levels were normalized to the levels of 18S rRNA in each sample and each value was expressed relative to the levels in vehicle (cntl); shown are mean ± SEM (n = 3). (*, P<0.05; **, P<0.01; ***, P<0.001) vs cntl (1-way ANOVA with Bonferroni comparison).
However, the data regarding expression levels of K14 still left us with two possibilities for the effects of stiff substrata: either maintenance of the progenitor state or differentiation toward a myoepithelial phenotype. To distinguish between these possibilities, we examined the expression of several additional markers of myoepithelial cell fate. In particular, we focused on P-cadherin, αSMA, and p63, all of which are expressed predominantly in the myoepithelium. We found that enhancing cytoskeletal contractility using calyculin A increased the expression levels of all three markers (Figures 4D-F); relaxing the cells had the opposite effect. Furthermore, the levels of K19, which is expressed by progenitor cells and lost when they differentiate into myoepithelial cells, were decreased in response to induction of cytoskeletal contractility (Figure 4G). These data suggest that enhancing contractility induces mammary epithelial progenitor cells to differentiate preferentially into myoepithelium, and are consistent with the hypothesis that stiff substrata promote differentiation toward a myoepithelial phenotype by enhancing cytoskeletal contractility.
Discussion
Here we report that substratum compliance plays an integral role in modulating the differentiation of mammary progenitor cells into either myoepithelial or luminal epithelial cells. Immunofluorescence analysis of keratin expression combined with quantitative RT-PCR demonstrated that soft substrata promote differentiation to the luminal epithelial lineage, whereas stiff substrata promote differentiation to the myoepithelial lineage. Additionally, pharmacological manipulations of intracellular tension implicate the RhoA pathway as the possible route through which mechanical signals affect the differentiation of mammary progenitor cells.
Signaling through FAK can activate RhoA, which leads to the local assembly of actin into stress fibers, reorganization of the cytoskeleton, and increased intracellular tension (Mitra et al. 2005). Consistent with previous evidence, our immunofluorescence images of cells cultured on stiff substrata revealed an increased prevalence of focal adhesions and the formation of prominent stress fibers, all of which indicate increased intracellular tension as compared to cells cultured on soft substrata. Furthermore, immunofluorescence analysis indicated a correlation between substratum compliance and the fraction of cells expressing luminal and myoepithelial markers, suggesting RhoA as a possible pathway through which mechanical signals affect differentiation.
Signaling through RhoA was previously shown to direct the differentiation of MSCs into bone or fat (McBeath et al. 2004). Our findings from the pharmacological treatments of cells cultured on substrata of varying compliances mimicking that of normal human breast tissue indicate that the RhoA pathway is also instrumental in regulating the differentiation of mammary progenitors downstream of substratum compliance. Disrupting signaling downstream of RhoA decreased the fraction of K14-expressing cells on all substrata. Conversely, treatment with calyculin A to induce focal adhesion assembly, promote actin phosphorylation and inhibit myosin light chain phosphatase led to increased intracellular tension and significantly increased fractions of K14-expressing cells on the softest substrata. Furthermore, qRT-PCR analysis of pharmacologically manipulated cells cultured on glass demonstrated that inhibiting the RhoA pathway resulted in downregulation of myoepithelial markers and upregulation of luminal epithelial markers, whereas activating cytoskeletal contractility downstream of RhoA resulted in downregulation of luminal epithelial markers and upregulation of myoepithelial markers.
Previous analysis of the biochemical regulation of human mammary progenitor cells identified the ECM protein laminin-111 as important for inducing a quiescent state, and the Notch-family ligand Jagged-1 as important for maintaining the progenitor phenotype (LaBarge et al. 2009). The microenvironment of the mammary gland in vivo presents cells with both these biochemical moieties and the biophysical signals we describe in this study. It will be interesting to determine how the identity of the ECM, interactions with neighboring cells, and the compliance of the surrounding microenvironment integrate in the fate decisions of these tissue-specific progenitor cells.
Taken together, the results from this study demonstrate that signals from the mechanical microenvironment, specifically substratum compliance, may regulate the differentiation of mammary progenitor cells through the RhoA pathway, with stiff substrata promoting differentiation to the myoepithelial lineage, and soft substrata promoting differentiation to the luminal epithelial lineage. The range of substratum compliance examined here was representative of that of the normal human mammary gland, and suggests regional and temporal variations in the mechanical tone of the gland may play an important role in progenitor fate. Just as cytoskeletal contractility and matrix compliance affect the branching morphogenesis process that builds the epithelial tree during puberty (Gjorevski and Nelson 2010), these same mechanical parameters may modulate remodeling of the gland during the pregnancy-lactation-involution cycles.
Acknowledgments
This work was supported in part by grants from the NIH (CA128660, HL110335, and GM083997), Susan G. Komen for the Cure (FAS07038550), the David & Lucile Packard Foundation, and the Alfred P. Sloan Foundation. C.M.N. holds a Career Award at the Scientific Interface from the Burroughs Wellcome Fund. C.L. was supported by the Lidow Senior Thesis Fund.
Abbreviations
- ECM
extracellular matrix
- ES
embryonic stem
- FAK
focal adhesion kinase
- MLCK
myosin light chain kinase
- MSCs
mesenchymal stem cells
- PA
polyacrylamide
- ROCK
Rho-associated kinase
- TDLU
terminal ductal lobular unit
References
- Alcaraz J, Xu R, Mori H, Nelson CM, Mroue R, Spencer VA, Brownfield D, Radisky DC, Bustamante C, Bissell MJ. Laminin and biomimetic extracellular elasticity enhance functional differentiation in mammary epithelia. EMBO J. 2008;27(21):2829–2838. doi: 10.1038/emboj.2008.206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alenghat FJ, Ingber DE. Mechanotransduction: all signals point to cytoskeleton, matrix, and integrins. Science"s STKE [electronic resource] : signal transduction knowledge environment 2002. 2002;119 doi: 10.1126/stke.2002.119.pe6. [DOI] [PubMed] [Google Scholar]
- Amano M, Ito M, Kimura K, Fukata Y, Chihara K, Nakano T, Matsuura Y, Kaibuchi K. Phosphorylation and Activation of Myosin by Rho-associated Kinase (Rho-kinase) Journal of Biological Chemistry. 1996;271(34):20246–20249. doi: 10.1074/jbc.271.34.20246. [DOI] [PubMed] [Google Scholar]
- Anderson LH, Boulanger CA, Smith GH, Carmeliet P, Watson CJ. Stem cell marker prominin-1 regulates branching morphogenesis, but not regenerative capacity, in the mammary gland. Dev Dyn. 2011;240(3):674–681. doi: 10.1002/dvdy.22539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Batistatou A, Stefanou D, Arkoumani E, Agnantis NJ. The usefulness of p63 as a marker of breast myoepithelial cells. In Vivo. 2003;17(6):573–576. [PubMed] [Google Scholar]
- Beningo KA, Dembo M, Kaverina I, Small JV, Wang Y-l. Nascent focal adhesions are responsible for the generation of strong propulsive forces in migrating fibroblasts. J Cell Biol. 2001;153(4):881–888. doi: 10.1083/jcb.153.4.881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bershadsky AD, Balaban NQ, Geiger B. Adhesion-dependent cell mechanosensitivity. Annu Rev Cell Dev Biol. 2003;19(1):677–695. doi: 10.1146/annurev.cellbio.19.111301.153011. [DOI] [PubMed] [Google Scholar]
- Boudou T, Ohayon J, Picart C, Tracqui P. An extended relationship for the characterization of Young's modulus and Poisson's ratio of tunable polyacrylamide gels. Biorheology. 2006;43(6):721–728. [PubMed] [Google Scholar]
- Davies SP, Reddy H, Caivano M, Cohen P. Specificity and mechanism of action of some commonly used protein kinase inhibitors. Biochem J. 2000;351(Pt 1):95–105. doi: 10.1042/0264-6021:3510095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dontu G, Jackson KW, McNicholas E, Kawamura MJ, Abdallah WM, Wicha MS. Role of Notch signaling in cell-fate determination of human mammary stem/progenitor cells. Breast Cancer Res. 2004;6(6):R605–R615. doi: 10.1186/bcr920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emerman JT, Burwen SJ, Pitelka DR. Substrate properties influencing ultrastructural differentiation of mammary epithelial cells in culture. Tissue and Cell. 1979;11(1):109–119. doi: 10.1016/0040-8166(79)90011-9. [DOI] [PubMed] [Google Scholar]
- Engler AJ, Sen S, Sweeney HL, Discher DE. Matrix Elasticity Directs Stem Cell Lineage Specification. Cell. 2006;126(4):677–689. doi: 10.1016/j.cell.2006.06.044. [DOI] [PubMed] [Google Scholar]
- Evans ND, Minelli C, Gentleman E, LaPointe V, Patankar SN, Kallivretaki M, Chen X, Roberts CJ, Stevens MM. Substrate stiffness affects early differentiation events in embryonic stem cells. Eur Cell Mater. 2009;18:1–13. doi: 10.22203/ecm.v018a01. discussion 13-14. [DOI] [PubMed] [Google Scholar]
- Eyckmans J, Boudou T, Yu X, Chen CS. A hitchhiker's guide to mechanobiology. Dev Cell. 2011;21(1):35–47. doi: 10.1016/j.devcel.2011.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gjorevski N, Nelson CM. Endogenous patterns of mechanical stress are required for branching morphogenesis. Integr Biol (Camb) 2010;2(9):424–434. doi: 10.1039/c0ib00040j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gjorevski N, Nelson CM. Integrated morphodynamic signalling of the mammary gland. Nature reviews. 2011;12:581–593. doi: 10.1038/nrm3168. [DOI] [PubMed] [Google Scholar]
- Ingber DE. The mechanochemical basis of cell and tissue regulation. Mechanics & chemistry of biosystems : MCB. 2004;1(1):53–68. [PubMed] [Google Scholar]
- Ishihara H, Martin BL, Brautigan DL, Karaki H, Ozaki H, Kato Y, Fusetani N, Watabe S, Hashimoto K, Uemura D, et al. Calyculin A and okadaic acid: inhibitors of protein phosphatase activity. Biochem Biophys Res Commun. 1989;159(3):871–877. doi: 10.1016/0006-291x(89)92189-x. [DOI] [PubMed] [Google Scholar]
- Ishizaki T, Naito M, Fujisawa K, Maekawa M, Watanabe N, Saito Y, Narumiya S. p160ROCK, a Rho-associated coiled-coil forming protein kinase, works downstream of Rho and induces focal adhesions. FEBS Letters. 1997;404(2–3):118–124. doi: 10.1016/s0014-5793(97)00107-5. [DOI] [PubMed] [Google Scholar]
- Kimura K, Ito M, Amano M, Chihara K, Fukata Y, Nakafuku M, Yamamori B, Feng J, Nakano T, Okawa K, Iwamatsu A, Kaibuchi K. Regulation of Myosin Phosphatase by Rho and Rho-Associated Kinase (Rho-Kinase) Science. 1996;273(5272):245–248. doi: 10.1126/science.273.5272.245. [DOI] [PubMed] [Google Scholar]
- Korkaya H, Paulson A, Charafe-Jauffret E, Ginestier C, Brown M, Dutcher J, Clouthier SG, Wicha MS. Regulation of mammary stem/progenitor cells by PTEN/Akt/beta-catenin signaling. PLoS Biol. 2009;7(6):e1000121. doi: 10.1371/journal.pbio.1000121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LaBarge MA, Nelson CM, Villadsen R, Fridriksdottir A, Ruth JR, Stampfer MR, Petersen OW, Bissell MJ. Human mammary progenitor cell fate decisions are products of interactions with combinatorial microenvironments. Integr Biol. 2009;1:70–79. doi: 10.1039/b816472j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levental KR, Yu H, Kass L, Lakins JN, Egeblad M, Erler JT, Fong SF, Csiszar K, Giaccia A, Weninger W, Yamauchi M, Gasser DL, Weaver VM. Matrix crosslinking forces tumor progression by enhancing integrin signaling. Cell. 2009;139:891–906. doi: 10.1016/j.cell.2009.10.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim E, Wu D, Pal B, Bouras T, Asselin-Labat ML, Vaillant F, Yagita H, Lindeman GJ, Smyth GK, Visvader JE. Transcriptome analyses of mouse and human mammary cell subpopulations reveal multiple conserved genes and pathways. Breast Cancer Res. 2010;12(2):R21. doi: 10.1186/bcr2560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu S, Dontu G, Mantle ID, Patel S, Ahn NS, Jackson KW, Suri P, Wicha MS. Hedgehog signaling and Bmi-1 regulate self-renewal of normal and malignant human mammary stem cells. Cancer Res. 2006;66(12):6063–6071. doi: 10.1158/0008-5472.CAN-06-0054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lorenzen J, Sinkus R, Biesterfeldt M, Adam G. Menstrual-cycle dependence of breast parenchyma elasticity: estimation with magnetic resonance elastography of breast tissue during the menstrual cycle. Invest Radiol. 2003;38(4):236–240. doi: 10.1097/01.RLI.0000059544.18910.BD. [DOI] [PubMed] [Google Scholar]
- Lorenzen J, Sinkus R, Lorenzen M, Dargatz M, Leussler C, Roschmann P, Adam G. MR elastography of the breast:preliminary clinical results. Rofo. 2002;174(7):830–834. doi: 10.1055/s-2002-32690. [DOI] [PubMed] [Google Scholar]
- McBeath R, Pirone DM, Nelson CM, Bhadriraju K, Chen CS. Cell Shape, Cytoskeletal Tension, and RhoA Regulate Stem Cell Lineage Commitment. Developmental Cell. 2004;6(4):483–495. doi: 10.1016/s1534-5807(04)00075-9. [DOI] [PubMed] [Google Scholar]
- Mitra SK, Hanson DA, Schlaepfer DD. Focal adhesion kinase: in command and control of cell motility. Nat Rev Mol Cell Biol. 2005;6(1):56–68. doi: 10.1038/nrm1549. [DOI] [PubMed] [Google Scholar]
- Paszek MJ, Zahir N, Johnson KR, Lakins JN, Rozenberg GI, Gefen A, Reinhart-King CA, Margulies SS, Dembo M, Boettiger D, Hammer DA, Weaver VM. Tensional homeostasis and the malignant phenotype. Cancer Cell. 2005;8(3):241–254. doi: 10.1016/j.ccr.2005.08.010. [DOI] [PubMed] [Google Scholar]
- Pelham RJ, Wang Y-l. Cell locomotion and focal adhesions are regulated by substrate flexibility. Proc Natl Acad Sci USA. 1997;94(25):13661–13665. doi: 10.1073/pnas.94.25.13661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pittenger MF, Mackay AM, Beck SC, Jaiswal RK, Douglas R, Mosca JD, Moorman MA, Simonetti DW, Craig S, Marshak DR. Multilineage potential of adult human mesenchymal stem cells. Science. 1999;284(5411):143–147. doi: 10.1126/science.284.5411.143. [DOI] [PubMed] [Google Scholar]
- Samani A, Zubovits J, Plewes D. Elastic moduli of normal and pathological human breast tissues: an inversion-technique-based investigation of 169 samples. Physics in medicine and biology. 2007;52(6):1565–1576. doi: 10.1088/0031-9155/52/6/002. [DOI] [PubMed] [Google Scholar]
- Schwartz MA. Integrins and extracellular matrix in mechanotransduction. Cold Spring Harb Perspect Biol. 2010;2(12):a005066. doi: 10.1101/cshperspect.a005066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srivastava A, Verma Y, Rao KD, Gupta PK. Determination of elastic properties of resected human breast tissue samples using optical coherence tomographic elastography. Strain. 2011;47:75–87. [Google Scholar]
- Sternlicht MD, Lochter A, Sympson CJ, Huey B, Rougier JP, Gray JW, Pinkel D, Bissell MJ, Werb Z. The stromal proteinase MMP3/stromelysin-1 promotes mammary carcinogenesis. Cell. 1999;98(2):137–146. doi: 10.1016/s0092-8674(00)81009-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Straight AF, Cheung A, Limouze J, Chen I, Westwood NJ, Sellers JR, Mitchison TJ. Dissecting temporal and spatial control of cytokinesis with a myosin II Inhibitor. Science. 2003;299(5613):1743–1747. doi: 10.1126/science.1081412. [DOI] [PubMed] [Google Scholar]
- Taddei I, Deugnier MA, Faraldo MM, Petit V, Bouvard D, Medina D, Fassler R, Thiery JP, Glukhova MA. Beta1 integrin deletion from the basal compartment of the mammary epithelium affects stem cells. Nat Cell Biol. 2008;10(6):716–722. doi: 10.1038/ncb1734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamada M, Sheetz MP, Sawada Y. Activation of a Signaling Cascade by Cytoskeleton Stretch. Dev Cell. 2004;7(5):709–718. doi: 10.1016/j.devcel.2004.08.021. [DOI] [PubMed] [Google Scholar]
- Thomson JA, Itskovitz-Eldor J, Shapiro SS, Waknitz MA, Swiergiel JJ, Marshall VS, Jones JM. Embryonic stem cell lines derived from human blastocysts. Science. 1998;282(5391):1145–1147. doi: 10.1126/science.282.5391.1145. [DOI] [PubMed] [Google Scholar]
- Tiede B, Kang Y. From milk to malignancy: the role of mammary stem cells in development, pregnancy and breast cancer. Cell Res. 2011;21(2):245–257. doi: 10.1038/cr.2011.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villadsen R, Fridriksdottir AJ, Ronnov-Jessen L, Gudjonsson T, Rank F, LaBarge MA, Bissell MJ, Petersen OW. Evidence for a stem cell hierarchy in the adult human breast. J Cell Biol. 2007;177(1):87–101. doi: 10.1083/jcb.200611114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Visvader JE, Smith GH. Murine Mammary Epithelial Stem Cells: Discovery, Function, and Current Status. Cold Spring Harb Perspect Biol. 2011 doi: 10.1101/cshperspect.a004879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodward WA, Chen MS, Behbod F, Rosen JM. On mammary stem cells. J Cell Sci. 2005;118(Pt 16):3585–3594. doi: 10.1242/jcs.02532. [DOI] [PubMed] [Google Scholar]
- Wozniak MA, Desai R, Solski PA, Der CJ, Keely PJ. ROCK-generated contractility regulates breast epithelial cell differentiation in response to the physical properties of a three-dimensional collagen matrix. J Cell Biol. 2003;163(3):583–595. doi: 10.1083/jcb.200305010. [DOI] [PMC free article] [PubMed] [Google Scholar]