Abstract
In the present study, the antinociceptive profiles of Agrimonia pilosa Ledeb extract were examined in ICR mice. Agrimonia pilosa Ledeb extract administered orally (200 mg/kg) showed an antinociceptive effect as measured by the tail-flick and hot-plate tests. In addition, Agrimonia pilosa Ledeb extract attenuated the writhing numbers in the acetic acid-induced writhing test. Furthermore, the cumulative nociceptive response time for intrathecal (i.t.) injection of substance P (0.7 µg) was diminished by Agrimonia pilosa Ledeb extract. Intraperitoneal (i.p.) pretreatment with yohimbine (α2-adrenergic receptor antagonist) attenuated antinociceptive effect induced by Agrimonia pilosa Ledeb extract in the writhing test. However, naloxone (opioid receptor antagonist) or methysergide (5-HT serotonergic receptor antagonist) did not affect antinociception induced by Agrimonia pilosa Ledeb extract in the writhing test. Our results suggest that Agrimonia pilosa Ledeb extract shows an antinociceptive property in various pain models. Furthermore, this antinociceptive effect of Agrimonia pilosa Ledeb extract may be mediated by α2-adrenergic receptor, but not opioidergic and serotonergic receptors.
Keywords: Agrimonia pilosa Ledeb, Anti-nociception, Inflammatory pain, α2 adrenoceptor
INTRODUCTION
Recently, natural products from plant origin possessing immense ethnopharmacological importance have been given the utmost priority as treatments for inflammation and allergic reactions [1,2]. Agrimonia pilosa Ledeb has been used traditionally for treatment of abdominal pain, sore throat, headaches, bloody discharge, parasitic infections and eczema in Korea and other Asian countries since centuries [3]. Pharmacologically, Agrimonia pilosa Ledeb has been reported to possess anti-tumor [4,5], anti-viral [6,7], anti-oxidant [8], anti-microbial [9] and anti-hyperglycemic activity [10]. Reports also indicated that Agrimonia pilosa Ledeb extract inhibited the inflammatory process by suppression of iNOS, ROS and inflammatory cytokine production in microglial cells [11,12].
However the detailed mechanisms of Agrimonia pilosa Ledeb in delivering anti-nociceptive profiles were not fully elucidated. Further, to date no report exists on the anti-nociceptive effect of Agrimonia pilosa Ledeb. Therefore, in this study, we attempted to characterize antinociceptive profiles and mechanisms of Agrimonia pilosa Ledeb extract in various pain models.
METHODS
These experiments were approved by the University of Hallym Animal Care and Use Committee (Registration Number: Hallym 2009-05-01). All procedures were conducted in accordance with the 'Guide for Care and Use of Laboratory Animals' published by the National Institutes of Health and the ethical guidelines of the International Association for the Study of Pain.
Experimental animals
Male ICR mice (MJ Co., Seoul, Korea) weighing 20~25 g were used for all the experiments. Animals were housed 5 per cage in a room maintained at 22±0.5℃ with an alternating 12 hr light-dark cycle. Food and water were available ad libitum. The animals were allowed to adapt to the laboratory for at least 2 hr before testing and were only used once. Experiments were performed during the light phase of the cycle (10:00~17:00).
Oral administration, and intraperitoneal (i.p.) and intrathecal (i.t.) injections
Oral administration was performed with gage in a volume of 500 µl/25 g body weight. I.p. injection was conducted to unanesthesized mice with volume of 250 µl. The i.t. administration was performed following the method of Hylden and Wilcox [13,14] using a 30-gauge needle connected to a 25 µl Hamilton syringe with polyethylene tubing. The i.t. injection volume was 5 µl and the injection site was verified by injecting a similar volume of 1% methylene blue solution and determining the distribution of the injected dye in the spinal cord. The dye injected i.t. was distributed both rostrally and caudally but with short distance (about 0.5 cm from the injection site) and no dye was found visually in the brain. The success rate for the injections was consistently found to be over 95%, before the experiments were done.
Assessment of antinociception and experimental protocols
All assessments for measuring antinociceptive properties of Agrimonia pilosa Ledeb extract were carried out by blinded observers.
Tail-flick and hot-plate tests
Antinociception was determined by the tail-flick [15] and the hot-plate paw-licking tests [16]. For the measurement of the tail-flick latency, mice were gently held with one hand with the tail positioned in the apparatus (EMDIE Instrument Co., Maidens, VA, USA, Model TF6) and the tail-flick response was elicited by applying radiant heat to the dorsal surface of the tail. The intensity of radiant heat was adjusted so that the animal flicked its tail within 3 to 5 sec. For the hot-plate test, mice were individually placed on the 55℃ hot-plate apparatus (Itic Life Science, Woodland Hills, CA, USA, Model 39 Hot Plate) and then, the reaction time starting from the placement of the mouse on the hotplate to the time of licking the front paw was measured. Basal latency for the hot-plate test was approximately 9 sec. Animals were pretreated orally once with vehicle (control) or Agrimonia pilosa Ledeb extract at 200 mg/kg doses 30 min prior to performing the tail-flick or hot-plate tests.
Acetic acid-induced writhing test
For the writhing test [17], 1% acetic acid was injection i.p. and then, the animals were immediately placed in an acrylic observation chamber (20 cm high, 20 cm diameter). The number of writhes was counted during 30 min after the injection of acetic acid. A writhe was defined as a contraction of the abdominal muscles accompanied by an extension of the forelimbs and elongation of the body. Animals were pretreated orally once with vehicle (control) or Agrimonia pilosa Ledeb extract at 200 mg/kg doses 30 min prior to performing the acetic acid-induced writhing and formalin tests.
Substance P-induced nociceptive behavioral test
Vehicle (control) or 200 mg/kg of Agrimonia pilosa Ledeb extract was pretreated orally 30 min prior to performing i.t. injection of substance P (0.7 µg/5 µl). Immediately after i.t. injection with substance P the mice were placed in an observation chamber (20 cm high, 20 cm diameter) and their nociceptive behavioral responses were recorded during 30 min. The cumulative response time of licking, scratching and biting episodes directed toward the lumbar and caudal region of spinal cord were measured with a stop-watch timer [14].
Pretreatment of antagonists
At first, mice were pretreated i.p. with either saline, naloxone (5 mg/kg), methysergide (5 mg/kg), or yohimbine (5 mg/kg), 10 min before oral administration of vehicle as a control or a fixed dose of Agrimonia pilosa Ledeb extract (200 mg/kg). And then, the writhing response was tested 30 min after the treatment with either vehicle or Agrimonia pilosa Ledeb extract [18-22].
Drugs
Acetic acid, substance P, naloxone, methysergide and yohimbine were purchased from Sigma Chemical Co. (St. Louis, MO, USA). Agrimonia pilosa Ledeb (300 g) was dissolved in 80% ethanol (1,500 ml) and extracted as refluxing for 3 hours, and then the extract was filtered for obtaining A. This process was repeated again once to obtain B from residue. A and B were mixed. This mixture was decompressed and dried for using as Agrimonia pilosa Ledeb extract. Agrimonia pilosa Ledeb extract, naloxone, methysergide and yohimbine were dissolved in saline. All drugs were prepared just before use.
Statistical analysis
Data were presented as the mean±SEM. The statistical significance of differences between groups was assessed with one-way ANOVA with Bonferroni's post-hoc test using GraphPad Prism version 4.0 for Windows Vista (GraphPad Software, San Diego, CA, USA); p<0.05 was considered significant.
RESULTS
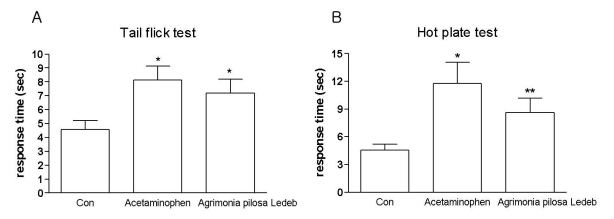
Effect of Agrimonia pilosa Ledeb extract on the tail-flick and hot-plate paw-licking responses
As revealed in Fig. 1, oral treatment of Agrimonia pilosa Ledeb extract at the dose of 200 mg/kg increased latencies of the tail-flick and hot-plate paw-licking responses compare to the control group of mice. The sedative effect was manifested, when the mice were treated with Agrimonia pilosa Ledeb extract orally at the dose of 200 mg/kg. However, there were no paralysis and motor changes.
Fig. 1.
The antinociceptive effect of Agrimonia pilosa Ledeb extract administered orally in the tail-flick and hot-plate tests. Mice were administered orally with either vehicle or 200 mg/kg of Agrimonia pilosa Ledeb extract and the tail-flick (A) or hot-plate (B) response was measured at 30 min after treatment. Acetaminophen is positive control. The vertical bars denote the standard error of the mean. The number of animal used for each group was 8~10 (*p<0.05, **p<0.01 compared to the vehicle-treated control group of mice).
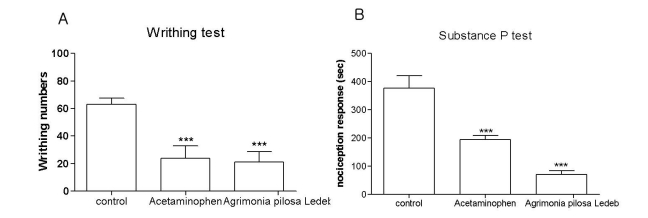
Effect of Agrimonia pilosa Ledeb extract on the nociceptive behavior induced by acetic acid and substance P
Agrimonia pilosa Ledeb extract attenuated the acetic acid-induced writhing numbers (Fig. 2A). Treatment with Agrimonia pilosa Ledeb extract at the dose of 200 mg/kg led to 68% decrease in the acetic acid-induced writhing response compare to the control group of mice. In vehicle-treated control mice, i.t. injection of substance P (0.7 µg) caused acute, immediate behavioral responses, i.e., licking, scratching and biting the lumbar or caudal region, which lasted about 30 min. As shown in Fig. 2B, cumulative nociceptive response times for i.t. administration of substance P was significantly diminished by 85%.
Fig. 2.
Effect of Agrimonia pilosa Ledeb extract on the nociceptive response induced by various pain models. Agrimonia pilosa Ledeb extract (200 mg/kg) was administered orally and then, 0.25 ml of 1% acetic acid solution was injected intraperitoneally 30 min after treatment. The number of writhing was counted for 30 min following acetic acid injection (A). Agrimonia pilosa Ledeb extract (200 mg/kg) was administered orally for 30 min prior to the substance P (0.7 µg per 5 µl) injection intrathecally (B). The cumulative response time of licking, scratching and biting episodes was measured for 30 min. Acetaminophen is positive control. The vertical bars indicate the standard error of the mean. The number of animal used for each group was 8~10 (***p<0.001, compared with control group).
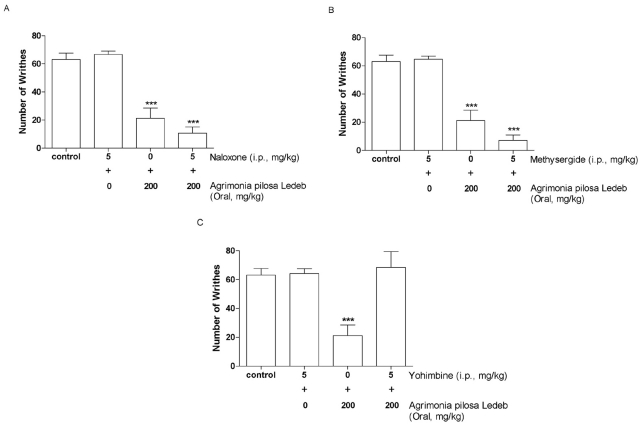
Effect of opioidergic, serotonergic and adrenergic system on the inhibition of writhing response induced by Agrimonia pilosa Ledeb extract
We examined the possible involvement of opioidergic, serotonergic and adrenergic system in the Agrimonia pilosa Ledeb extract-induced antinociception. The pretreatment with naloxone (opioid receptor antagonist, Fig. 3A) or methysergide (serotonergic receptor antagonist, Fig. 3B) did not affect Agrimonia pilosa Ledeb extract-induced antinociception. However, the blockade of α2-adrenergic receptor with systemic pre-administration of yohimbine abolished the Agrimonia pilosa Ledeb extract-induced inhibition of the writhing response (Fig. 3C). The treatment of naloxone, methysergide or yohimbine itself did not affect the writhing response (Fig. 3).
Fig. 3.
Effect of naloxone (A), methysergide (B) and yohimbine (C) injected intraperitoneally (i.p.) on inhibition of the writhing response induced by Agrimonia pilosa Ledeb extract administered orally. Naloxone, methysergide, or yohimbine (5 mg/kg) was pretreated intraperitoneally for 10 min, before oral administration of vehicle or Agrimonia pilosa Ledeb extract (200 mg/kg). Agrimonia pilosa Ledeb extract or vehicle was administered orally and then, 0.25 ml of 1% acetic acid solution was injected i.p. 30 min after treatment. The number of writhing was counted for 30 min following acetic acid injection. The vertical bars denote the standard error of the mean. The number of animal used for each group was 8~10 (***p<0.001, compared with control group).
DISCUSSION
In the present study, we found that Agrimonia pilosa Ledeb extract administered orally produces antinociception in various pain models. The tail-flick response is believed to be a spinally mediated reflex and the paw-licking hotplate response is a more complex supraspinally organized behavior (for review, see [23]). Moreover, Grumbach [24] has shown that the effectiveness of analgesic agents in the tail-flick pain model is highly correlated with relief of human pain. Our results demonstrate that Agrimonia pilosa Ledeb extract causes to prolong the tailflick and hot-plate response latencies, indicating the increase of nociceptive threshold.
We also examined the effect of Agrimonia pilosa Ledeb extract on the acetic acid-induced writhing test. I.p. injection of acetic acid can produce the peritoneal inflammation (acute peritonitis), which cause a response characterized by contraction of the abdominal muscles accompanying an extension of the forelimbs and elongation of the body. This writhing response is considered as a visceral inflammatory pain model [17 for review, see 25]. In the present study, we clearly showed the antinociceptive effect of Agrimonia pilosa Ledeb extract in an acetic acid-induced writhing test. Furthermore, it has been reported that i.t. injection of substance P in mice can also elicit nociceptive responses, consisting of biting, scratching and licking the caudal parts of the body [14,26]. We found in the present study that Agrimonia pilosa Ledeb extract was also effective in attenuating substance P-induced nociceptive responses. These results suggest furthermore that Agrimonia pilosa Ledeb extract may exert their antinociceptive effect via the central sites, possibly spinally mediated mechanisms.
The roles of opioid, serotonergic and adrenergic receptors in the regulation of modulation of nociceptive processing have been demonstrated in many previous studies. For example, it is well known that opioid receptors are involved in the antinociception [27-29]. Also, it has been reported that blockade of the spinal serotonergic or noradrenergic receptors by spinal injection of methysergide or yohimbine antagonize the antinociception induced by morphine administered supraspinally [28,30,31]. We observed in the present study that α2-adrenergic receptor, but not opioidergic and serotonergic receptors, appear to be involved in orally administered Agrimonia pilosa Ledeb extract-induced antinociception.
In conclusion, our results suggest that Agrimonia pilosa Ledeb extract shows an antinociceptive property in various pain models. Furthermore, this antinociceptive effect of Agrimonia pilosa Ledeb extract may be mediated by α2-adrenergic receptor, but not opioidergic and serotonergic receptors.
ACKNOWLEDGEMENTS
This research was supported by Priority Research Centers (2011-0030750) and Basic Science Research (2012-0000313 & 2011-0011156) Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education, Science and Technology.
ABBREVIATIONS
- i.t.
intrathecal
- i.p.
intraperitoneal
References
- 1.Talhouk RS, Karam C, Fostok S, El-Jouni W, Barbour EK. Anti-inflammatory bioactivities in plant extracts. J Med Food. 2007;10:1–10. doi: 10.1089/jmf.2005.055. [DOI] [PubMed] [Google Scholar]
- 2.Lee J, Bielory L. Complementary and alternative interventions in atopic dermatitis. Immunol Allergy Clin North Am. 2010;30:411–424. doi: 10.1016/j.iac.2010.06.006. [DOI] [PubMed] [Google Scholar]
- 3.Kato H, Li W, Koike M, Wang Y, Koike K. Phenolic glycosides from Agrimonia pilosa. Phytochemistry. 2010;71:1925–1929. doi: 10.1016/j.phytochem.2010.08.007. [DOI] [PubMed] [Google Scholar]
- 4.Koshiura R, Miyamoto K, Ikeya Y, Taguchi H. Antitumor activity of methanol extract from roots of Agrimonia pilosa Ledeb. Jpn J Pharmacol. 1985;38:9–16. doi: 10.1254/jjp.38.9. [DOI] [PubMed] [Google Scholar]
- 5.Miyamoto K, Kishi N, Koshiura R. Antitumor effect of agrimoniin, a tannin of Agrimonia pilosa Ledeb., on transplantable rodent tumors. Jpn J Pharmacol. 1987;43:187–195. doi: 10.1254/jjp.43.187. [DOI] [PubMed] [Google Scholar]
- 6.Li Y, Ooi LS, Wang H, But PP, Ooi VE. Antiviral activities of medicinal herbs traditionally used in southern mainland China. Phytother Res. 2004;18:718–722. doi: 10.1002/ptr.1518. [DOI] [PubMed] [Google Scholar]
- 7.Shin WJ, Lee KH, Park MH, Seong BL. Broad-spectrum antiviral effect of Agrimonia pilosa extract on influenza viruses. Microbiol Immunol. 2010;54:11–19. doi: 10.1111/j.1348-0421.2009.00173.x. [DOI] [PubMed] [Google Scholar]
- 8.Zhu L, Tan J, Wang B, He R, Liu Y, Zheng C. Antioxidant activities of aqueous extract from Agrimonia pilosa Ledeb and its fractions. Chem Biodivers. 2009;6:1716–1726. doi: 10.1002/cbdv.200800248. [DOI] [PubMed] [Google Scholar]
- 9.Yamaki M, Kashihara M, Ishiguro K, Takagi S. Antimicrobial Principles of Xian he cao (Agrimonia pilosa) Planta Med. 1989;55:169–170. doi: 10.1055/s-2006-961915. [DOI] [PubMed] [Google Scholar]
- 10.Jung CH, Zhou S, Ding GX, Kim JH, Hong MH, Shin YC, Kim GJ, Ko SG. Antihyperglycemic activity of herb extracts on streptozotocin-induced diabetic rats. Biosci Biotechnol Biochem. 2006;70:2556–2559. doi: 10.1271/bbb.60238. [DOI] [PubMed] [Google Scholar]
- 11.Jung CH, Kim JH, Park S, Kweon DH, Kim SH, Ko SG. Inhibitory effect of Agrimonia pilosa Ledeb. on inflammation by suppression of iNOS and ROS production. Immunol Invest. 2010;39:159–170. doi: 10.3109/08820130903501790. [DOI] [PubMed] [Google Scholar]
- 12.Bae H, Kim HJ, Shin M, Lee H, Yin CS, Ra J, Kim J. Inhibitory effect of Agrimoniae Herba on lipopolysaccharide-induced nitric oxide and proinflammatory cytokine production in BV2 microglial cells. Neurol Res. 2010;32(Suppl 1):53–57. doi: 10.1179/016164109X12537002794002. [DOI] [PubMed] [Google Scholar]
- 13.Hylden JL, Wilcox GL. Intrathecal morphine in mice: a new technique. Eur J Pharmacol. 1980;67:313–316. doi: 10.1016/0014-2999(80)90515-4. [DOI] [PubMed] [Google Scholar]
- 14.Hylden JL, Wilcox GL. Intrathecal substance P elicits a caudally-directed biting and scratching behavior in mice. Brain Res. 1981;217:212–215. doi: 10.1016/0006-8993(81)90203-1. [DOI] [PubMed] [Google Scholar]
- 15.D'Amour FE, Smith DL. A method for determining loss of pain sensation. J Pharmacol Exp Ther. 1941;72:74–79. [Google Scholar]
- 16.Eddy NB, Leimbach D. Synthetic analgesics. II. Dithienylbutenyl- and dithienylbutylamines. J Pharmacol Exp Ther. 1953;107:385–393. [PubMed] [Google Scholar]
- 17.Koster R, Anderson M, Beer EJ. Acetic acid for analgesic screening. Federal Proceeding. 1959;18:412. [Google Scholar]
- 18.Choi SS, Han KJ, Lee JK, Lee HK, Han EJ, Kim DH, Suh HW. Antinociceptive mechanisms of orally administered decursinol in the mouse. Life Sci. 2003;73:471–485. doi: 10.1016/s0024-3205(03)00311-4. [DOI] [PubMed] [Google Scholar]
- 19.Park SH, Sim YB, Choi SM, Seo YJ, Kwon MS, Lee JK, Suh HW. Antinociceptive profiles and mechanisms of orally administered vanillin in the mice. Arch Pharm Res. 2009;32:1643–1649. doi: 10.1007/s12272-009-2119-8. [DOI] [PubMed] [Google Scholar]
- 20.Suh HW, Song DK, Son KH, Wie MB, Lee KH, Jung KY, Do JC, Kim YH. Antinociceptive mechanisms of dipsacus saponin C administered intracerebroventricularly in the mouse. Gen Pharmacol. 1996;27:1167–1172. doi: 10.1016/s0306-3623(96)00052-3. [DOI] [PubMed] [Google Scholar]
- 21.Suh HW, Song DK, Kim YH. Differential effects of adenosine receptor antagonists injected intrathecally on antinociception induced by morphine and beta-endorphin administered intracerebroventricularly in the mouse. Neuropeptides. 1997;31:339–344. doi: 10.1016/s0143-4179(97)90069-x. [DOI] [PubMed] [Google Scholar]
- 22.Suh HW, Chung KM, Kim YH, Huh SO, Song DK. Effects of histamine receptor antagonists injected intrathecally on antinociception induced by opioids administered intracerebroventricularly in the mouse. Neuropeptides. 1999;33:121–129. doi: 10.1054/npep.1999.0006. [DOI] [PubMed] [Google Scholar]
- 23.Chapman CR, Casey KL, Dubner R, Foley KM, Gracely RH, Reading AE. Pain measurement: an overview. Pain. 1985;22:1–31. doi: 10.1016/0304-3959(85)90145-9. [DOI] [PubMed] [Google Scholar]
- 24.Grumbach L. The prediction of analgesic activity in man by animal testing. In: Knighton RS, Dumke PR, editors. Pain. Boston: Little Brown and Co.; 1966. pp. 163–182. [Google Scholar]
- 25.Vyklicky L. The techniques for the study of pain in animals. In: Bonica JJ, Liebeskind JC, Albe-Fessard DG, editors. Advances in Pain Research and Theraphy. Vol. 3. New York: Raven Press; 1979. pp. 727–745. [Google Scholar]
- 26.Cumberbatch MJ, Herrero JF, Headley PM. Exposure of rat spinal neurones to NMDA, AMPA and kainate produces only short-term enhancements of responses to noxious and non-noxious stimuli. Neurosci Lett. 1994;181:98–102. doi: 10.1016/0304-3940(94)90569-x. [DOI] [PubMed] [Google Scholar]
- 27.Schmauss C, Yaksh TL. In vivo studies on spinal opiate receptor systems mediating antinociception. II. Pharmacological profiles suggesting a differential association of mu, delta and kappa receptors with visceral chemical and cutaneous thermal stimuli in the rat. J Pharmacol Exp Ther. 1984;228:1–12. [PubMed] [Google Scholar]
- 28.Yaksh TL. Direct evidence that spinal serotonin and noradrenaline terminals mediate the spinal antinociceptive effects of morphine in the periaqueductal gray. Brain Res. 1979;160:180–185. doi: 10.1016/0006-8993(79)90616-4. [DOI] [PubMed] [Google Scholar]
- 29.Yaksh TL. Multiple opioid receptor systems in brain and spinal cord: Part I. Eur J Anaesthesiol. 1984;1:171–199. [PubMed] [Google Scholar]
- 30.Jensen TS, Yaksh TL. Spinal monoamine and opiate systems partly mediate the antinociceptive effects produced by glutamate at brainstem sites. Brain Res. 1984;321:287–297. doi: 10.1016/0006-8993(84)90181-1. [DOI] [PubMed] [Google Scholar]
- 31.Wigdor S, Wilcox GL. Central and systemic morphine-induced antinociception in mice: contribution of descending serotonergic and noradrenergic pathways. J Pharmacol Exp Ther. 1987;242:90–95. [PubMed] [Google Scholar]