Abstract
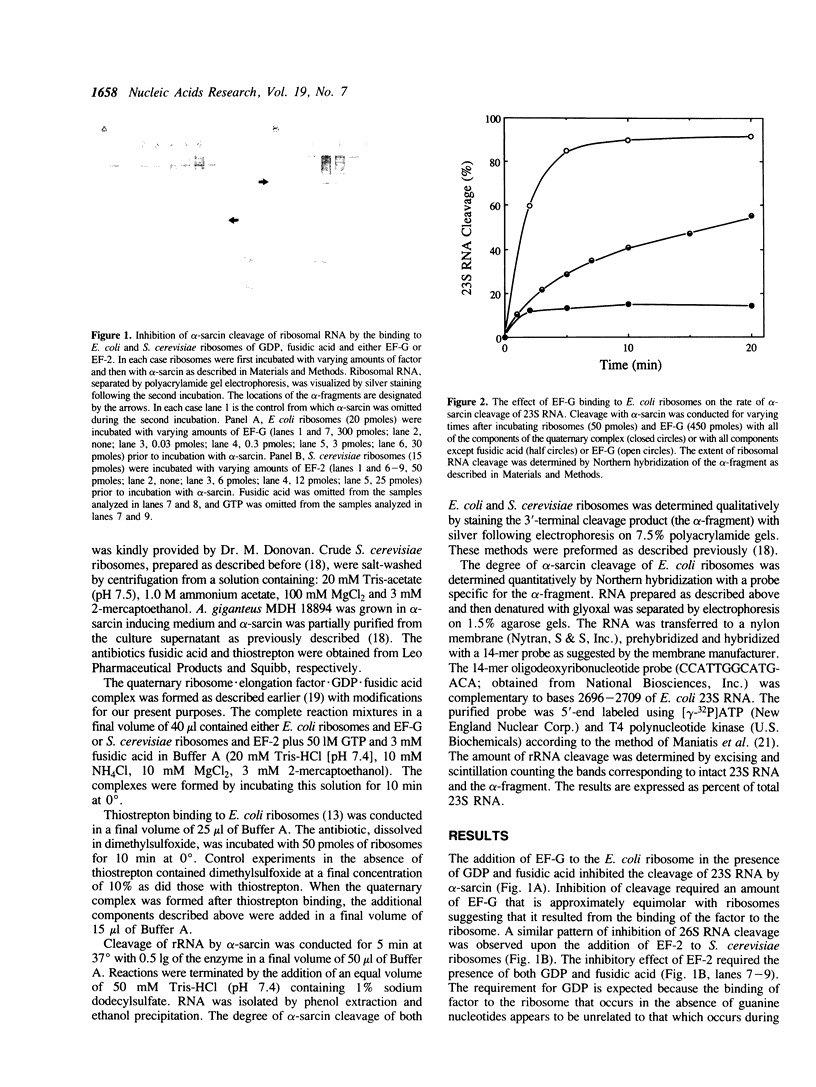
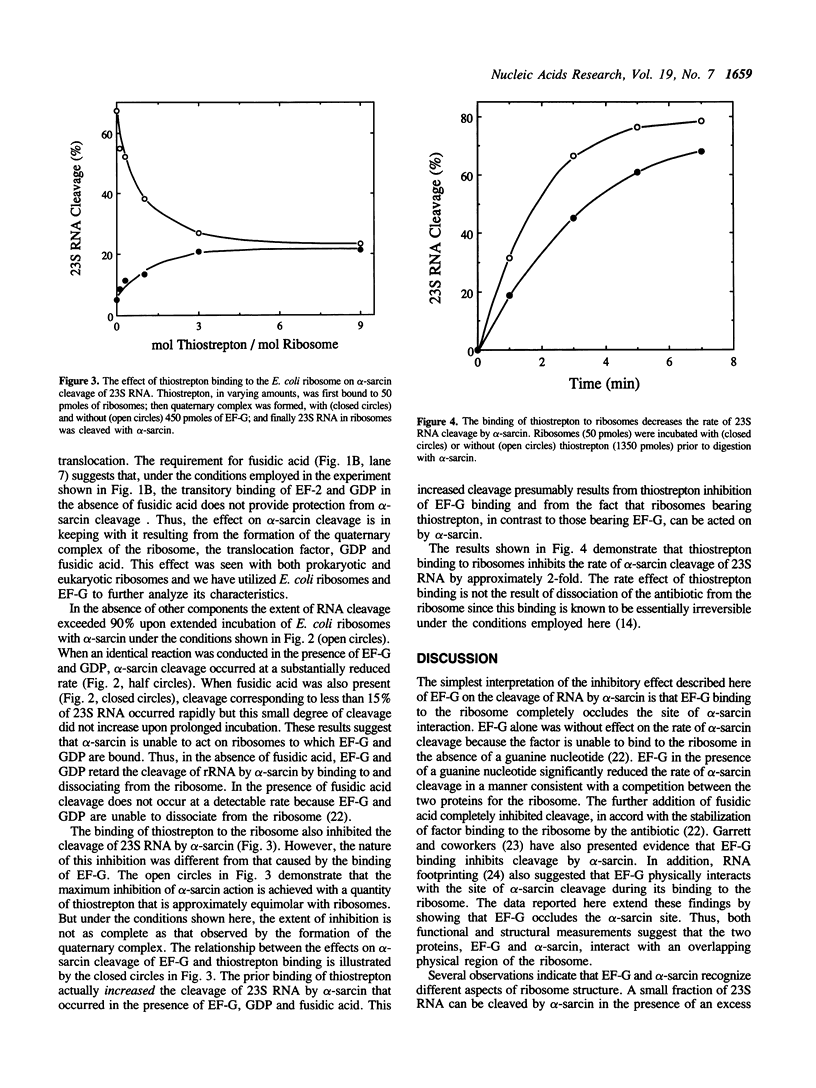
The translocation reaction catalyzed by elongation factor G (EF-G) is inhibited either by alpha-sarcin cleavage of 23S rRNA or by the binding of thiostrepton to the E. coli ribosome. Here we show that the transitory binding of EF-G and GDP to the ribosome inhibited the rate of alpha-sarcin cleavage and that stabilization of this binding with fusidic acid completely prevented alpha-sarcin cleavage. A similar pattern of inhibition was seen upon the binding of elongation factor 2 to the S. cerevisiae ribosome. The irreversible binding of the antibiotic thiostrepton to the E. coli ribosome, on the other hand, decreased the rate of cleavage by alpha-sarcin approximately 2-fold. These results suggest that the alpha-sarcin site is located within the ribosomal domain for EF-G binding and that the conformation of this site is affected by the binding of thiostrepton.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baca O. G., Rohrbach M. S., Bodley J. W. Equilibrium measurements of the interactions of guanine nucleotides with Escherichia coli elongation factor G and the ribosome. Biochemistry. 1976 Oct 19;15(21):4570–4574. doi: 10.1021/bi00666a004. [DOI] [PubMed] [Google Scholar]
- Bodley J. W., Lin L., Highland J. H. Studies on translocation. VI. Thiostrepton prevents the formation of a ribosome-G factor-guanine nucleotide complex. Biochem Biophys Res Commun. 1970 Dec 24;41(6):1406–1411. doi: 10.1016/0006-291x(70)90543-7. [DOI] [PubMed] [Google Scholar]
- Bodley J. W., Lin L. Studies on the nature of the G-factor binding site on the 50S ribosomal subunit. Biochemistry. 1972 Feb 29;11(5):782–786. doi: 10.1021/bi00755a016. [DOI] [PubMed] [Google Scholar]
- Bodley J. W., Weissbach H., Brot N. The binding of Escherichia coli elongation factor G to the ribosome. Methods Enzymol. 1974;30:235–238. doi: 10.1016/0076-6879(74)30026-2. [DOI] [PubMed] [Google Scholar]
- Brigotti M., Rambelli F., Zamboni M., Montanaro L., Sperti S. Effect of alpha-sarcin and ribosome-inactivating proteins on the interaction of elongation factors with ribosomes. Biochem J. 1989 Feb 1;257(3):723–727. doi: 10.1042/bj2570723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dahlberg A. E. The functional role of ribosomal RNA in protein synthesis. Cell. 1989 May 19;57(4):525–529. doi: 10.1016/0092-8674(89)90122-0. [DOI] [PubMed] [Google Scholar]
- Endo Y., Glück A., Chan Y. L., Tsurugi K., Wool I. G. RNA-protein interaction. An analysis with RNA oligonucleotides of the recognition by alpha-sarcin of a ribosomal domain critical for function. J Biol Chem. 1990 Feb 5;265(4):2216–2222. [PubMed] [Google Scholar]
- Endo Y., Mitsui K., Motizuki M., Tsurugi K. The mechanism of action of ricin and related toxic lectins on eukaryotic ribosomes. The site and the characteristics of the modification in 28 S ribosomal RNA caused by the toxins. J Biol Chem. 1987 Apr 25;262(12):5908–5912. [PubMed] [Google Scholar]
- Endo Y., Tsurugi K. RNA N-glycosidase activity of ricin A-chain. Mechanism of action of the toxic lectin ricin on eukaryotic ribosomes. J Biol Chem. 1987 Jun 15;262(17):8128–8130. [PubMed] [Google Scholar]
- Hausner T. P., Atmadja J., Nierhaus K. H. Evidence that the G2661 region of 23S rRNA is located at the ribosomal binding sites of both elongation factors. Biochimie. 1987 Sep;69(9):911–923. doi: 10.1016/0300-9084(87)90225-2. [DOI] [PubMed] [Google Scholar]
- Highland J. H., Lin L., Bodley J. W. Protection of ribosomes from thiostrepton inactivation by the binding of G factor and guanosine diphosphate. Biochemistry. 1971 Nov 23;10(24):4404–4409. doi: 10.1021/bi00800a009. [DOI] [PubMed] [Google Scholar]
- Leffers H., Egebjerg J., Andersen A., Christensen T., Garrett R. A. Domain VI of Escherichia coli 23 S ribosomal RNA. Structure, assembly and function. J Mol Biol. 1988 Dec 5;204(3):507–522. doi: 10.1016/0022-2836(88)90351-8. [DOI] [PubMed] [Google Scholar]
- Miller S. P., Bodley J. W. Alpha-sarcin cleaves ribosomal RNA at the alpha-sarcin site in the absence of ribosomal proteins. Biochem Biophys Res Commun. 1988 Jul 15;154(1):404–410. doi: 10.1016/0006-291x(88)90700-0. [DOI] [PubMed] [Google Scholar]
- Moazed D., Robertson J. M., Noller H. F. Interaction of elongation factors EF-G and EF-Tu with a conserved loop in 23S RNA. Nature. 1988 Jul 28;334(6180):362–364. doi: 10.1038/334362a0. [DOI] [PubMed] [Google Scholar]
- Paleologue A., Reboud J. P., Reboud A. M. Modifications of 60 S ribosomal subunits induced by the ricin A chain. FEBS Lett. 1986 Nov 24;208(2):373–377. doi: 10.1016/0014-5793(86)81052-3. [DOI] [PubMed] [Google Scholar]
- Rohrbach M. S., Dempsey M. E., Bodley J. W. Preparation of homogeneous elongation factor G and examination of the mechanism of guanosine triphosphate hydrolysis. J Biol Chem. 1974 Aug 25;249(16):5094–5101. [PubMed] [Google Scholar]
- Skogerson L. Separation and characterization of yeast elongation factors. Methods Enzymol. 1979;60:676–685. doi: 10.1016/s0076-6879(79)60063-0. [DOI] [PubMed] [Google Scholar]
- Stirpe F., Bailey S., Miller S. P., Bodley J. W. Modification of ribosomal RNA by ribosome-inactivating proteins from plants. Nucleic Acids Res. 1988 Feb 25;16(4):1349–1357. doi: 10.1093/nar/16.4.1349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terao K., Uchiumi T., Endo Y., Ogata K. Ricin and alpha-sarcin alter the conformation of 60S ribosomal subunits at neighboring but different sites. Eur J Biochem. 1988 Jun 15;174(3):459–463. doi: 10.1111/j.1432-1033.1988.tb14120.x. [DOI] [PubMed] [Google Scholar]
- Thompson J., Schmidt F., Cundliffe E. Site of action of a ribosomal RNA methylase conferring resistance to thiostrepton. J Biol Chem. 1982 Jul 25;257(14):7915–7917. [PubMed] [Google Scholar]
- Walker T. A., Endo Y., Wheat W. H., Wool I. G., Pace N. R. Location of 5.8 S rRNA contact sites in 28 S rRNA and the effect of alpha-sarcin on the association of 5.8 S rRNA with 28 S rRNA. J Biol Chem. 1983 Jan 10;258(1):333–338. [PubMed] [Google Scholar]




