Abstract
Papillary thyroid carcinoma (PTC) is the most common malignant thyroid tumor, and 36-69% of PTC cases are caused by mutations in the BRAF gene. The substitution of a valine for a glutamic acid (V600E) comprises up to 95-100% of BRAF mutations; therefore, most diagnostic methods, including allele-specific PCR and real-time PCR, are designed to detect this mutation. Nevertheless, other mutations can also comprise the genetic background of PTC. Recently, a novel and sensitive technique called mutant enrichment with 3'-modified oligonucleotides (MEMO) PCR has been introduced. When we applied allelespecific PCR and MEMO-PCR for the detection of the BRAF V600E mutation, we found an unusual 3' bp deletion mutation (c.1799_1801delTGA) only when using MEMO-PCR. This deletion results in the introduction of a glutamic acid into the B-Raf activation segment (p.V600_K601delinsE), leading to an elevated basal kinase activity of BRAF. This is the first report of a rare 3 bp BRAF deletion in a PTC patient that could not be detected by allele-specific PCR.
Keywords: Papillary thyroid carcinoma, BRAF, Deletion, Mutation, Mutant enrichment with 3'-modified oligonucleotides (MEMO) PCR, Korean
INTRODUCTION
Papillary thyroid carcinoma (PTC) is the most common malignant thyroid tumor. In the past, RET/PTC oncogene activation was considered the genetic hallmark of PTC, despite the relatively low prevalence (5-30%) of RET/PTC oncogene activation in adult PTC patients [1]. However, after Davies et al. [2] reported that the BRAF gene is frequently mutated in a variety of human tumors, many studies have reported a particularly high prevalence of BRAF mutations in PTC (36-69%) [1, 3, 4].
The BRAF gene encodes a cytoplasmic serine/threonine kinase that is a member of the Raf family of kinases located downstream of Ras in the Ras/Raf/extracellular signal-regulated kinase (ERK) cell signaling pathway. Constitutive activation of the Ras-Raf kinase-signaling pathway promotes uncontrolled cell division, resulting in carcinogenesis [4]. A single substitution, valine to glutamic acid at codon 600 (V600E), accounts for 95%-100% of all BRAF mutations in thyroid carcinoma [1, 4, 5]. Only a few other BRAF mutations have been documented, such as the substitution of a lysine with a glutamate at codon 601 (K601E) in patients with follicular variant of PTC [5]. Therefore, most diagnostic methods, including allele-specific PCR (AS-PCR) and real-time PCR, are designed to detect the BRAF V600E mutation.
Recently, a novel and sensitive technique called mutant enrichment with 3'-modified oligonucleotides (MEMO) PCR has been introduced [6]. The method is based on the use of a 3'-modified oligonucleotide primer that blocks extension of the normal allele but enables extension of the mutated alleles. In this study, we describe an unusual 3 bp deletion mutation (c.1799_1801delTGA) in a thyroid nodule that was identified only by using the MEMO-PCR and sequencing method.
CASE REPORT
A 47-yr-old woman visited our hospital for further evaluation of multiple thyroid masses. These masses were detected based on abnormal results of thyroid ultrasonography at a local hospital. The largest mass was isoechoic, measured approximately 2×2.8 cm, and had the appearance of a benign nodule. However, a smaller 0.5-cm-sized nodule located in the lower pole of left thyroid was hypoechoic and had a spiculated appearance, and was suspected to be malignant. The patient underwent thyroid function tests, including triiodothyronine (T3), thyroxine (T4), and thyroid-stimulating hormone (TSH) tests. Fine-needle aspiration biopsy and BRAF mutational analysis were performed on the suspicious lesion.
A thyroid aspiration sample was collected from the patient after obtaining informed consent. Genomic DNA was isolated from the sample using the QIAamp DNA Mini Kit (Qiagen, Chatsworth, CA, USA) according to the manufacturer's instructions. AS-PCR was performed to detect V600E substitutions in the BRAF gene using the Seeplex V600E ACE detection kit (Seegene, Seoul, Korea). Although AS-PCR of a positive control for the BRAF V600E mutation produced PCR products at 538 and 335 bp as expected, only the 538 bp band, characteristic of the wild-type sequence, was amplified from our patient (Fig. 1). MEMO-PCR was then performed. The ability of this method to detect trace mutant alleles is superior to that of conventional methods [6]. In MEMO-PCR, 2 generic primers and a blocking primer were used to amplify BRAF exon 15. The 3'-end of the blocking primer was modified by the addition of a C3 spacer, a phosphate, or a C6 amine; these 3' modifications inhibit the amplification of normal alleles. Five microliters of the amplification product was treated with 2 U shrimp alkaline phosphatase and 10 U exonuclease I (USB Corp., Cleveland, OH, USA). Direct sequencing was performed using the BigDye Terminator Cycle Sequencing Ready Reaction Kit on an ABI Prism 3130 Genetic Analyzer (Applied Biosystems, Foster City, CA, USA). The obtained sequences were analyzed using the Sequencher program (Gene Codes Corp., Ann Arbor, MI, USA) and were compared to a reference sequence (GenBank accession number NM_004333.4).
Fig. 1.
Allele-specific PCR results. At left, two PCR products at 538 and 335 bp were amplified for the positive control of BRAF V600E; at right, the patient with the BRAF deletion mutation only showed a wild-type band at 538 bp.
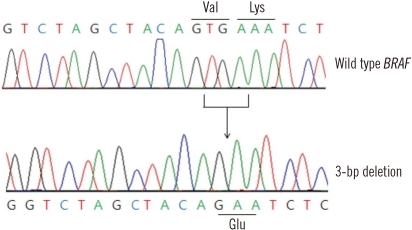
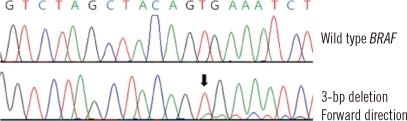
Thyroid function tests of the patient indicated that the levels of the following test items were all within normal limits: T3, 95.6 ng/dL (reference interval (RI), 76-190 ng/dL); T4, 1.02 ng/dL (RI, 0.64-1.72 ng/dL); and TSH, 1.25 µIU/mL (RI 0.3-6.5 µIU/mL). According to the MEMO-PCR analysis, the BRAF mutation in our case was a 3 bp deletion in coding nucleotides 1799 to 1801 (c.1799_1801delTGA), which fall within the BRAF mutational hotspot region in which the V600E and K601E mutations are found (Fig. 2). The result of the deletion mutation is the conversion of codons 600 (GTG) and 601 (AAA) into a single codon (GAA), resulting in the introduction of glutamic acid into the BRAF activation segment (p.V600_K601delinsE), mimicking the BRAF V600E and K601E mutations. To confirm this result, we performed conventional PCR and sequencing of the BRAF gene; heterozygous peaks were observed from a starting position at nucleotide 1799 (Fig. 3).
Fig. 2.
Direct sequencing of exon 15 of the BRAF gene after MEMO-PCR. Top, wild-type BRAF; bottom, a 3 bp deletion of coding nucleotides 1799 to 1801 (c.1799_1801delTGA; arrow).
Abbreviation: MEMO-PCR, mutant enrichment with 3'-modified oligonucleotides-PCR.
Fig. 3.
Confirmation of the deletion mutation using conventional PCR and sequencing. Top, wild-type BRAF; bottom, heterozygous peaks were observed from a starting position at nucleotide 1799 (arrow).
Later, the patient underwent left lobectomy for a lesion that was suspected of being a thyroid carcinoma. The histological study of the nodule revealed classical papillary microcarcinoma. The cut surface of the confirmed lesion showed a whitish, round, and firm nodule measuring 0.5×0.5 cm. Metastatic deposits were detected in 1 out of 7 resected lymph nodes.
DISCUSSION
In the present study, we identified of a rare 3 bp BRAF gene deletion in a thyroid nodule by using a MEMO-PCR and sequencing method. This unusual deletion mutation could not be detected by AS-PCR. Until now, the 3 bp deletion (c.1799_1801delTGA) that was detected in this study has previously been reported in only 3 cases: a melanoma, a serrated adenoma, and a solid variant of papillary carcinoma [7-9]. The rarity of BRAF deletion mutations suggests that this mutation may have less tumorigenic potential than the BRAF V600E and K601E mutations [9]. Another hypothesis is that a deletion mutation has less chance of occurring than a single-nucleotide substitution. Finally, the possibility of underestimating the incidence of these deletion mutations because of "mutation-specific" screenings, such as mutant-allele-specific PCR, should not be overlooked. Currently, many laboratories perform AS-PCR and real-time PCR for detection of BRAF V600E, which is the most prevalent mutation in PTC. However, initial restriction of screening to the hotspot region of BRAF mutations in thyroid tumors, specifically a thymidine to adenine substitution at nucleotide position 1799, may obscure detection of a deletion or other mutations in exon 15 of the BRAF gene, as described in our current case.
Recently, the ability to identify minority mutant DNAs has become essential for characterizing the early and post-treatment tumor status in cancer patients. Mutant-enrichment processes that increase the amount of mutant allele relative to the wild-type allele have therefore attracted great interest [10]. There are many moderate- to high-selectivity PCR methods for mutant enrichment, such as amplification refractory mutation system PCR, thermostable restriction endonuclease-mediated selective PCR, and PCR clamping mediated by peptide nucleic acid (PNA) or locked nucleic acid (LNA) [10]. These techniques are attractive, but the time and cost required for optimization may hamper their widespread use. MEMO-PCR is a novel mutant enrichment technique that is simpler and more practical method than prior enrichment PCR methods. This technique is based on the use of a 3'-modified oligonucleotide primer for blocking extension of the normal allele, which is much less expensive and is easy to design compared with PNA or LNA. In MEMO-PCR, 2 generic primers (which amplify both mutant and wild-type alleles) and a blocking primer constitute the PCR reaction mixture. The 3'-modified blocking primer is designed to be modified with an extension-inhibiting compound, to encompass the target BRAF V600E site, and is complementary to the wild-type sequence. If there is a BRAF V600E mutation present in the patient, the affinity of the primer for the mutant allele is less than that for the wild-type allele, due to nucleotide mismatches. As a result, mutant alleles will be selectively amplified. It has been documented that the MEMO-PCR and sequencing method can enrich for minority alleles that are present in wild-type DNA at concentrations as low as 10-2 to 10-6 depending on the concentrations and thermodynamics of the primers [6]. The enrichment efficiency of MEMO-PCR is comparable to that of PNA-mediated PCR clamping when reaction conditions are optimized [6]. This accurate and cost-effective technique may be practically applicable in clinical and diagnostic settings. In the current study, MEMO-PCR with sequencing was capable of detecting a rare BRAF deletion mutation successfully. The 3 bp deletion sequence was easily distinguished from that of the wild-type sequence (Fig. 2).
In 2005, V. Trovisco et al. reported a new BRAF triplet deletion for the first time in a case of PTC [9]. A 47-yr-old woman underwent a total thyroidectomy after the clinical diagnosis of a multinodular goiter. The tumor had the characteristics of PTC, displaying a predominantly solid growth pattern [9]. A BRAF mutation was identified using PCR with single-strand conformation polymorphism analysis, and a 3 bp deletion was detected in the tumor tissue (c.1799_1801delTGA). Our case provides another example of a BRAF deletion mutation in PTC. The histological type of the current case is the classical type of PTC, not the solid variant. Given the rarity of BRAF deletions, it is not possible to determine whether the mutation is associated with a specific histological type of PTC. Further studies using a large series of cases are needed to clarify this point.
In conclusion, we identified a rare 3 bp deletion of the coding nucleotides 1799 to 1801 of BRAF in a PTC patient using MEMO-PCR and sequencing. The MEMO-PCR and sequencing method may be a useful tool for successful identification of a rare deletion mutation of the BRAF gene as well as the detection for clinically significant low-level mutant alleles.
Acknowledgement
This study was supported by a grant of the Korea Healthcare Technology R&D Project, Ministry for Health & Welfare Affairs, Republic of Korea (A092255), and the Research Grant Number CB-2011-03-02 of the Korean Foundation for Cancer Research.
Footnotes
No potential conflict of interest relevant to this article was reported.
References
- 1.Kimura ET, Nikiforova MN, Zhu Z, Knauf JA, Nikiforov YE, Fagin JA. High prevalence of BRAF mutations in thyroid cancer: genetic evidence for constitutive activation of the RET/PTC-RAS-BRAF signaling pathway in papillary thyroid carcinoma. Cancer Res. 2003;63:1454–1457. [PubMed] [Google Scholar]
- 2.Davies H, Bignell GR, Cox C, Stephens P, Edkins S, Clegg S, et al. Mutations of the BRAF gene in human cancer. Nature. 2002;417:949–954. doi: 10.1038/nature00766. [DOI] [PubMed] [Google Scholar]
- 3.Cohen Y, Xing M, Mambo E, Guo Z, Wu G, Trink B, et al. BRAF mutation in papillary thyroid carcinoma. J Natl Cancer Inst. 2003;95:625–627. doi: 10.1093/jnci/95.8.625. [DOI] [PubMed] [Google Scholar]
- 4.Fukushima T, Suzuki S, Mashiko M, Ohtake T, Endo Y, Takebayashi Y, et al. BRAF mutations in papillary carcinomas of the thyroid. Oncogene. 2003;22:6455–6457. doi: 10.1038/sj.onc.1206739. [DOI] [PubMed] [Google Scholar]
- 5.Trovisco V, Vieira de Castro I, Soares P, Maximo V, Silva P, Magalhaes J, et al. BRAF mutations are associated with some histological types of papillary thyroid carcinoma. J Pathol. 2004;202:247–251. doi: 10.1002/path.1511. [DOI] [PubMed] [Google Scholar]
- 6.Lee ST, Kim JY, Kown MJ, Kim SW, Chung JH, Ahn MJ, et al. Mutant enrichment with 3'-modified oligonucleotides a practical PCR method for detecting trace mutant DNAs. J Mol Diagn. 2011;13:657–668. doi: 10.1016/j.jmoldx.2011.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lee EJ, Park CK, Kim JW, Chang DK, Kim KM. Deletion mutation of BRAF in a serrated adenoma from a patient with familial adenomatous polyposis. APMIS. 2007;115:982–986. doi: 10.1111/j.1600-0463.2007.apm_670.x. [DOI] [PubMed] [Google Scholar]
- 8.Reifenberger J, Knobbe CB, Sterzinger AA, Blaschke B, Schulte KW, Ruzicka T, et al. Frequent alterations of Ras signaling pathway genes in sporadic malignant melanomas. Int J Cancer. 2004;109:377–384. doi: 10.1002/ijc.11722. [DOI] [PubMed] [Google Scholar]
- 9.Trovisco V, Soares P, Soares R, Magalhães J, Sá-Couto P, Sobrinho-Simões M. A new BRAF gene mutation detected in a case of a solid variant of papillary thyroid carcinoma. Hum Pathol. 2005;36:694–697. doi: 10.1016/j.humpath.2005.04.011. [DOI] [PubMed] [Google Scholar]
- 10.Milbury CA, Li J, Makrigiorgos GM. PCR-based methods for the enrichment of minority alleles and mutations. Clin Chem. 2009;55:632–640. doi: 10.1373/clinchem.2008.113035. [DOI] [PMC free article] [PubMed] [Google Scholar]