Summary
How mutant prion protein (PrP) leads to neurological dysfunction in genetic prion diseases is unknown. Tg(PG14) mice synthesize a misfolded mutant PrP which is partially retained in the neuronal endoplasmic reticulum (ER). As these mice age, they develop ataxia and massive degeneration of cerebellar granule neurons (CGNs). Here, we report that motor behavioral deficits in Tg(PG14) mice emerge before neurodegeneration and are associated with defective glutamate exocytosis from granule neurons due to impaired calcium dynamics. We found that mutant PrP interacts with the voltage-gated calcium channel α2δ-1 subunit, which promotes the anterograde trafficking of the channel. Owing to ER retention of mutant PrP, α2δ-1 accumulates intracellularly, impairing delivery of the channel complex to the cell surface. Thus, mutant PrP disrupts cerebellar glutamatergic neurotransmission by reducing the number of functional channels in CGNs. These results link intracellular PrP retention to synaptic dysfunction, indicating new modalities of neurotoxicity and potential therapeutic strategies.
Highlights
► Mutant PrP dysrupts glutamatergic transmission in cerebellar granule neurons (CGNs) ► Mutant PrP impairs depolarization-evoked calcium dynamics in CGNs ► Mutant PrP binds to the α2δ-1 subunit of voltage-gated calcium channels (VGCCs) ► Mutant PrP misfolding and intracellular retention impairs membrane delivery of VGCCs
How mutant prion protein causes neurological dysfunction in genetic prion diseases is not fully known. Senatore et al. report intracellular accumulation of misfolded prion impairs voltage-gated calcium channel transport to synapses, altering glutamatergic neurotransmission and cerebellum-dependent motor function.
Introduction
Evidence is emerging that neurological symptoms in prion diseases precede neuronal loss and are due to an adverse effect of misfolded prion protein (PrP) on synaptic function. Therapeutic intervention, therefore, requires identification of the mechanisms by which abnormal PrP disrupts normal neuronal activity. Here, we describe the mechanism underlying the neurotransmission defect associated with early motor impairment in transgenic (Tg) mouse models of genetic prion disease. This has brought to light an unexpected effect of misfolded PrP on the intracellular trafficking of voltage-gated calcium channels (VGCCs).
Prion diseases, including Creutzfeldt-Jakob disease (CJD), Gerstmann-Sträussler-Scheinker syndrome, and fatal insomnia, are rare neurodegenerative disorders characterized pathologically by neuronal loss, astrocytosis, and deposition of insoluble PrP aggregates throughout the brain (Prusiner, 1998). They usually involve loss of motor coordination and other motor abnormalities, dementia and neurophysiological deficits, and are invariably fatal (Knight and Will, 2004). Approximately 15% of human prion diseases are inherited in an autosomal-dominant fashion and are linked to point mutations or insertions in the gene encoding PrP on chromosome 20 (Mastrianni, 2010). The neurotoxic pathways activated by mutant PrP are not clear, but misfolding and oligomerization of the mutant protein are thought to trigger the pathogenic process (Chiesa and Harris, 2001).
Tg mice expressing a mouse PrP homolog of a 72 amino acid insertion (PG14), which in humans is associated with progressive dementia and ataxia, synthesize a misfolded form of mutant PrP in their brains that is aggregated into small oligomers (Chiesa et al., 1998, 2003). As these mice age, they develop a fatal neurological disorder characterized clinically by ataxia, and neuropathologically by cerebellar atrophy due to loss of synaptic endings in the molecular layer and massive apoptosis of granule neurons (Chiesa et al., 2000). Deletion of the proapoptotic gene Bax in Tg(PG14) mice rescues cerebellar granule cells but does not prevent synaptic loss in the molecular layer and development of clinical symptoms (Chiesa et al., 2005); thus, mutant PrP causes neurological disease by disrupting the normal neuronal connectivity or function in the cerebellum. PG14 PrP molecules misfold soon after synthesis in the endoplasmic reticulum (ER) (Daude et al., 1997), and their exit from the ER is impaired (Drisaldi et al., 2003). However, ER stress-related pathways are not activated (Quaglio et al., 2011), suggesting that intracellular retention of PG14 PrP may trigger some other pathogenic mechanisms.
Here, we report that motor behavioral deficits in Tg(PG14) mice emerge before neurodegeneration and are associated with defective depolarization-induced glutamate exocytosis from cerebellar granule neurons (CGNs). Altered calcium influx due to inefficient membrane delivery of VGCCs accounts for the exocytosis defect and is causally linked to intracellular retention of mutant PrP. Confirming this, alterations in VGCC transport and glutamate exocytosis are also found in cells and in Tg mice expressing a mouse PrP homolog of the D178N mutation linked to inherited CJD. These results provide new insights into the mechanism of neuronal dysfunction in genetic prion diseases.
Results
Tg(PG14) Mice Develop Early Impairment in Motor and Balance Coordination Associated with Low Glutamate Release in the Cerebellum
The Tg(PG14) mice used in this study express mutant PrP at a level similar to endogenous PrP in wild-type mice; they develop ataxia, kyphosis, and foot clasp reflex at ∼240 days of age and die prematurely at ∼450 days (Chiesa et al., 1998, 2000).
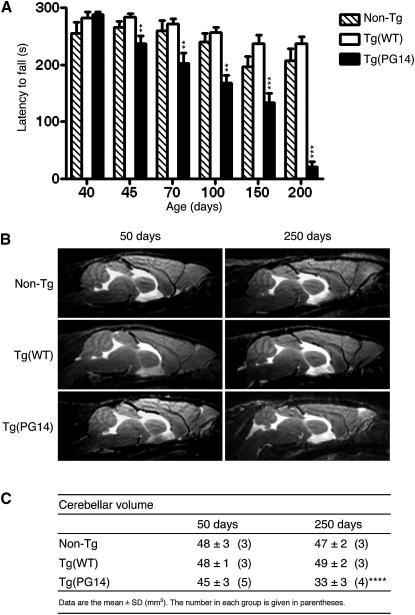
To find out the earliest appearance of motor dysfunction, Tg(PG14) mice were tested on the accelerating Rotarod. This motor behavioral task requires the mice to walk on an accelerating rotating rod with latency to fall as readout, and is a sensitive indicator of cerebellar abnormalities (Hilber and Caston, 2001; Yamamoto et al., 2003). Tg(PG14) mice performed well between 19 and 25 days of age (see Figure S1A available online), confirming normal postnatal development of the cerebellum (which is completed in the third week of life) and motor learning (Chiesa et al., 2000). From 45 days on, however, the mice showed a significantly shorter latency to fall than non-Tg littermates and Tg(WT) mice expressing wild-type PrP (Chiesa et al., 1998); their performance worsened with aging until they became virtually unable to perform the task (Figure 1A).
Figure 1.
Tg(PG14) Mice Develop Deficits in Motor and Balance Coordination before Cerebellar Degeneration
(A) Groups of 6–15 Tg(WT), 7–12 Tg(PG14) mice, and 6–17 non-Tg littermates were tested on a Rotarod at the ages indicated. Each mouse was tested three times. Bars indicate the mean ± SEM latency to fall (s); ∗∗p < 0.01, ∗∗∗p < 0.001 versus Tg(WT) by one-way analysis of variance (ANOVA), Bonferroni's post hoc test.
(B) Brain anatomy of non-Tg, Tg(WT), and Tg(PG14) mice at the ages indicated. Representative T2-weighted images (TE/TR = 50/2,500 ms).
(C) Cerebellar volumes (mm3) of non-Tg, Tg(WT), and Tg(PG14) mice at the ages indicated. Mean ± SD. The number of animals is given in parentheses. ∗∗∗∗p < 0.0001 versus non-Tg and Tg(WT) by two-way ANOVA, Bonferroni's post hoc test.
We used MRI to see whether the emergence of the motor deficit was associated with cerebellar degeneration. Tg(PG14) mice were examined at ∼50 and ∼250 days of age, with age-matched non-Tg and Tg(WT) controls. At 50 days the cerebellar volume of Tg(PG14) mice was comparable to controls (Figures 1B and 1C). In older Tg(PG14) mice there was a significant atrophy of the cerebellum (Figures 1B and 1C), consistent with previous histological analysis showing age-dependent cerebellar degeneration (Chiesa et al., 2000).
To investigate changes in cerebellar synaptic structures, we immunostained the brains of young and old animals with an antibody against the vesicular glutamate transporter VGLUT1, which specifically labels glutamatergic projections of CGNs in the molecular layer of the cerebellum (Fremeau et al., 2001). There was no difference in VGLUT1 immunostaining between young non-Tg and Tg(PG14) mice, whereas a significant decrease was seen in older Tg(PG14) mice (Figures S1B–S1D). There were also no differences between ∼50-day-old non-Tg and Tg(PG14) mice in the levels of the synaptic vesicle-associated proteins synaptophysin and synapsin I, the SNARE protein SNAP-25, the synaptic vesicle fusion protein synaptotagmin I, and the secretory vesicle chaperone CSPα (data not shown), confirming that there was no synaptic degeneration at this stage.
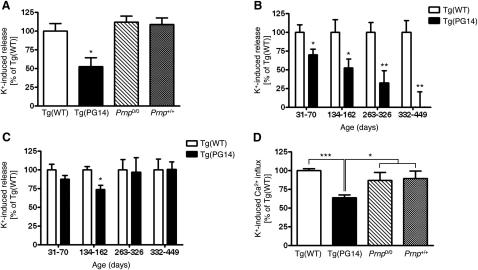
To identify abnormalities in cerebellar neurotransmission, we did functional studies in purified synaptosomal fractions. Synaptosomes were isolated from the cerebellum and cerebral cortex of Tg(WT) and Tg(PG14) mice, and characterized biochemically (Figures S2A–S2C). Synaptosomal PG14 PrP was detergent insoluble (seen in the pellet fraction after ultracentrifugation, Figures S2D and S2E), and was immunoprecipitated by monoclonal antibody 15B3 (Figure S2F), which selectively recognizes aggregated forms of misfolded PrP (Biasini et al., 2009). We analyzed synaptosomal uptake and release of glutamate and GABA, which are the main excitatory and inhibitory neurotransmitters in the cerebellum. There were no differences in [3H]glutamate and [3H]GABA uptake or spontaneous or depolarization-induced [3H]GABA release between Tg(WT) and Tg(PG14) mice up to 300 days old (data not shown). To assess release from glutamatergic terminals, we used [3H]D-aspartate, a nonmetabolizable analog of glutamate (Stigliani et al., 2006). We found a significant reduction in depolarization-induced release in the cerebellar synaptosomes from Tg(PG14) mice compared to Tg(WT), PrP knockout (Prnp0/0), and C57BL/6 (Prnp+/+) mice (Figure 2A). Release was already significantly reduced in cerebellar synaptosomes from 30- to 70-day-old animals, correlating with the onset of the motor deficit, and was almost completely impaired by the time mice had advanced clinical disease (Figure 2B). In the cerebral cortex a significant decrease in [3H]D-aspartate release was found only in mice between 134 and 162 days old (Figure 2C).
Figure 2.
Cerebellar Tg(PG14) Synaptosomes Show Impaired Depolarization-Evoked Glutamate Release and Calcium Influx
(A) Cerebellar synaptosomes from 230- to 250-day-old mice were preloaded with [3H]D-aspartate and stratified on filters in a 20 chamber superfusion apparatus. After equilibration, synaptosomes were exposed to 15 mM KCl for 1.5 min. The K+-induced [3H]D-aspartate overflow was measured in the collected fractions. Each error bar indicates the mean ± SEM of 5–20 replicate chambers from 2 to 4 independent experiments. Data are expressed as percentages of the values of Tg(WT) mice. ∗p < 0.05 versus corresponding value in Tg(WT) by Student's t test.
(B) K+-induced release was determined in cerebellar synaptosomes from Tg(WT) and Tg(PG14) mice of the ages indicated. Each bar indicates the mean ± SEM of 5–14 replicates from 1 to 3 independent experiments. ∗p < 0.05 and ∗∗p < 0.01 by Student's t test.
(C) Depolarization-induced release was determined in synaptosomes from the cerebral cortex of the same Tg(WT) and Tg(PG14) mice used in (B). Each bar indicates the mean ± SEM. ∗p < 0.05 versus corresponding value of Tg(WT) by Student's t test.
(D) Cerebellar synaptosomes were preloaded with fura-2 AM and depolarized with 50 mM KCl. The K+-induced [Ca2+]i rise is expressed as percent ΔF (difference of the fluorescence ratio F340/380 before and after the stimulus) of K+-induced calcium responses in synaptosomes of Tg(WT) mice. Each bar indicates the mean ± SEM of 9–21 replicates from 2 to 4 independent synaptosomal preparations pooling 13 Tg(WT), 12 Tg(PG14), 4 Prnp0/0, and 4 Prnp+/+ mice of 230–250 days. ∗p < 0.05 and ∗∗∗p < 0.001 versus corresponding value for Tg(WT) by Student's t test.
Defective Glutamate Release Is Due to Impaired VGCC Function in CGNs
Depolarization induces neurotransmitter release from synaptic terminals by triggering calcium influx through the VGCC, followed by exocytosis of synaptic vesicles (Sudhof, 2004). To determine whether the release defect in the cerebellum of Tg(PG14) mice was due to defective exocytosis, we used ionomycin, a calcium ionophore that allows calcium influx independently of VGCCs. Ionomycin evoked efficient calcium-dependent [3H]D-aspartate release from PG14 cerebellar synaptosomes unresponsive to depolarization (Figures S3A–S3C), indicating that the glutamate exocytotic machinery functioned normally in the mutant mice, and pointing to a VGCC defect.
Next, we measured depolarization- and ionomycin-induced calcium rise in synaptosomes preloaded with the calcium-sensitive dye fura-2 AM. Depolarization-induced calcium influx was significantly lower in PG14 cerebellar synaptosomes than in controls (Figures 2D and S3D), whereas there was no difference after stimulus with ionomycin (Figures S3E and S3F). No difference in depolarization-induced calcium rise was seen in synaptosomes from the cerebral cortex (data not shown).
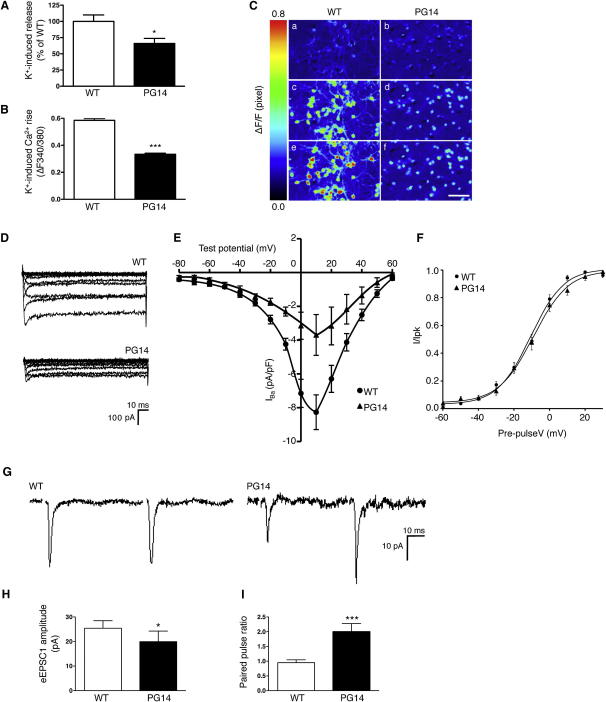
Because CGNs are the main glutamatergic cell population in the cerebellum, and specifically targeted in Tg(PG14) mice, we reasoned that the defect could pertain to this cell type. We, therefore, measured depolarization-evoked [3H]D-aspartate release in primary CGN cultures from the Tg(PG14) mice. Release was significantly lower in PG14 than in wild-type cells (Figure 3A). Single-cell calcium imaging found impaired calcium influx in response to depolarization (Figures 3B and 3C), and whole-cell patch-clamp recordings showed reduced calcium current densities in PG14 CGNs (Figures 3D and 3E). There were no apparent differences between wild-type and PG14 neurons in VGCC activation and inactivation kinetics (Figure 3D), and in the voltage dependence of activation (Figure 3F), suggesting a reduction in the number of functional channels rather than changes to their biophysical properties.
Figure 3.
Depolarization-Evoked [3H]D-Aspartate Release, Calcium Currents, and Glutamatergic Neurotransmission Are Impaired in Primary CGNs from Tg(PG14) Mice
(A) K+-induced [3H]D-aspartate release from primary cultures of CGNs from Tg(WT) and Tg(PG14) mice. Each value represents the mean ± SEM of 13–20 replicates from 3 independent experiments. ∗p < 0.05 by Student's t test.
(B) CGNs preloaded with 10 μM fura-2 AM were depolarized with 30 mM KCl. K+-induced [Ca2+]i rise was measured in single cells and expressed as ΔF. Values are the mean ± SEM of 129 cells from Tg(WT) and 286 cells from Tg(PG14) mice. ∗∗∗p < 0.001 by Student's t test.
(C) Representative pseudocolor images of fura-2 AM-loaded CGNs stimulated with 30 mM KCl. The images were taken before the stimulus (a and b), at an intermediate (c and d), and at the peak response (e and f). The color scale is shown on the left. Scale bar, 50 μm.
(D) Representative whole-cell VGCC inward Ba2+ currents in response to 10 mV increment step depolarizations from −80 to 60mV recorded in CGNs from Tg(WT) and Tg(PG14).
(E) Mean IV relationship of peak IBa recorded in CGNs from Tg(WT) and Tg(PG14), in response to 250 ms voltage pulses in 10mV increments from −80 to 60mV. IBa current densities (pA/pF) are shown as mean ± SEM; n = 17 for Tg(WT) and n = 10 for Tg(PG14).
(F) Voltage-dependent activation curves of VGCC currents generated in Tg(WT) (n = 8) and Tg(PG14) (n = 6) CGNs by measuring tail currents stimulated by a repolarization to −40mV, normalizing to the largest tail current in the series and then plotting against the prepulse voltage.
(G) Cultured CGNs from wild-type (C57BL/6J) and Tg(PG14) mice were voltage clamped at a holding potential of −70mV, and EPSCs were evoked by pairs of 1 ms depolarizing pulse to 30mV at 50 ms interpulse interval. Representative sample traces are shown.
(H) Amplitude of the first evoked EPSC in wild-type and PG14 neurons.
(I) The PPR value was calculated as the ratio of the amplitudes of the second to the first EPSC.
Bars in (H) and (I) indicate the mean ± SEM of 10 wild-type and 11 PG14 cells. ∗p < 0.05, ∗∗∗p < 0.001 by Mann-Whitney U test.
Evoked excitatory postsynaptic currents (EPSCs) recorded in cultured PG14 CGN by dual whole-cell patch clamp were significantly smaller than in wild-type cells, supporting the view that reduced calcium influx in the mutant neurons impaired glutamate release (Figures 3G and 3H). The decrease in EPSC amplitude in PG14 neurons was not due to reduced postsynaptic sensitivity to glutamate, as suggested by the increased amplitude (wild-type = 12.07 ± 0.89 pA; PG14 = 16.51 ± 0.88 pA; mean ± SEM, n = 14 for wild-type and n = 13 for PG14; p < 0.01 by Mann-Whitney U test), and not frequency of miniature events (wild-type = 0.34 ± 0.05 Hz; PG14 = 0.28 ± 0.03 Hz, mean ± SEM; not significant by Mann-Whitney U test). The decrease in EPSC amplitude was rather due to reduced presynaptic calcium currents, as revealed by the increase in facilitation in a protocol of short-term plasticity (Figure 3I), which is sensitive to the amount of calcium entry (Zucker and Regehr, 2002). These results, which are in line with previous reports for mutations of calcium channels affecting excitatory synaptic transmission (Liu and Friel, 2008; Ly et al., 2008; Qian and Noebels, 2000), indicated abnormal VGCC function and impaired glutamatergic neurotransmission in CGN of Tg(PG14) mice.
Defective Calcium Influx in CGNs Is Associated with PG14 PrP Accumulation in Transport Organelles
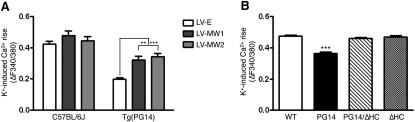
We used two complementary approaches to demonstrate that the VGCC defect was due to mutant PrP expression. First, we tested whether silencing PG14 PrP expression by lentivector-mediated RNAi restored the depolarization-induced calcium rise in mutant CGNs. CGNs from Tg(PG14) mice were transduced with a control lentivirus carrying enhanced green fluorescent protein (EGFP) cDNA (LV-E), or two different lentiviruses encoding EGFP and anti-PrP shRNAs (LV-MW1 and LV-MW2) that efficiently knock down PrP expression (Figure S4) (White et al., 2008), and the intracellular calcium rise in response to depolarization was measured in transduced neurons identified by EGFP fluorescence. LV-MW1 and LV-MW2 significantly enhanced the calcium rise in PG14 PrP-expressing but not control cells (Figure 4A), indicating that knockdown of mutant PrP rescued the cells from the calcium influx defect.
Figure 4.
Defective Calcium Influx in CGNs Is Due to PG14 PrP Expression and Accumulation in Secretory Organelles
(A) Primary CGNs from C57BL/6J and Tg(PG14) mice were transduced with 1–5 × 106 TU/ml of lentiviruses encoding anti-PrP shRNA (LV-MW1 or LV-MW2), or with a lentivirus lacking shRNA (LV-E). The depolarization-induced [Ca2+]i rise was measured in lentivirus-exposed CGNs and expressed as ΔF. Each bar indicates the mean ± SEM of cells transduced by the LV-E (40 C57BL/6J and 20 PG14 cells), LV-MW1 (19 C57BL/6J and 13 PG14 cells), and LV-MW2 (9 C57BL/6J and 27 PG14 cells). ∗∗p < 0.01 and ∗∗∗p < 0.001 by two-way ANOVA, Bonferroni's post hoc test.
(B) Primary CGNs from C57BL/6J mice were transfected with pBUD-GFP plasmid carrying the wild-type, PG14, PG14/ΔHC, or ΔHC PrP cDNA. The K+-induced [Ca2+]i rise was measured in GFP-positive cells and expressed as ΔF. Each bar is the mean ± SEM of 330 wild-type, 235 PG14, 299 PG14/ΔHC, and 192 ΔHC PrP-expressing cells. ∗∗∗p < 0.001 versus the corresponding value of Tg(WT) by one-way ANOVA, Bonferroni's post hoc test.
Second, we investigated whether PG14 PrP induced an abnormal calcium response in wild-type neurons. CGNs from C57BL/6J mice were transfected with a bigenic plasmid that drives efficient PrP and EGFP expression in CGNs (Drisaldi et al., 2004), and the depolarization-induced calcium rise was measured in EGFP-positive cells. PG14 PrP-transfected cells had a significantly smaller rise in calcium than untransfected or wild-type PrP-transfected neurons (Figure 4B), indicating that acute PG14 PrP expression was sufficient to impair VGCC function.
The biosynthetic maturation of misfolded PG14 PrP molecules in the ER is delayed, and they accumulate in the neuronal secretory pathway (Drisaldi et al., 2003; Fioriti et al., 2005). To assess whether intracellular PG14 PrP retention plays a role in the VGCC defect, we analyzed the depolarization-induced calcium rise in CGNs transfected with a version of PG14 PrP with a deletion of amino acids 114–121 in the hydrophobic core (PG14/ΔHC). This molecule is less prone to misfolding and delivered to the cell surface more efficiently than its full-length counterpart (Biasini et al., 2010). CGNs from C57BL/6 mice were transfected with PG14/ΔHC PrP, or with a version of PrP carrying the hydrophobic core deletion but not the PG14 mutation (ΔHC). The calcium responses of PG14/ΔHC PrP-expressing cells were comparable to those of the wild-type and ΔHC controls (Figure 4B), suggesting that misfolding and intracellular retention of mutant PrP were necessary to induce the defect in calcium influx.
PG14 PrP Impairs VGCC α2δ-1 Subunit Trafficking to the Plasma Membrane
Reduced intracellular calcium influx and current amplitude in PG14 CGNs might be due to changes in VGCC expression, biophysical properties, or membrane targeting. VGCCs are heteromeric proteins consisting of the pore-forming CaVα1 subunit, which governs the biophysical and pharmacological properties of the channel, and the auxiliary α2δ and CaVβ subunits, which regulate the cellular trafficking and activity of CaVα1 (Dolphin, 2009). Glutamate release from CGNs is mainly governed by P/Q-type channels made of the CaVα1A, α2δ-1, and CaVβ4 subunit isoforms (Mintz et al., 1995). To test whether expression of these channels was altered in Tg(PG14) mice, we measured CaVα1A and α2δ-1 levels in cerebellar postnuclear supernatants and cultured CGNs. There were no differences in these proteins between Tg(PG14) and Tg(WT) mice (data not shown; Figure S7B), indicating that the calcium defect in the mutant mice was not due to altered VGCC expression.
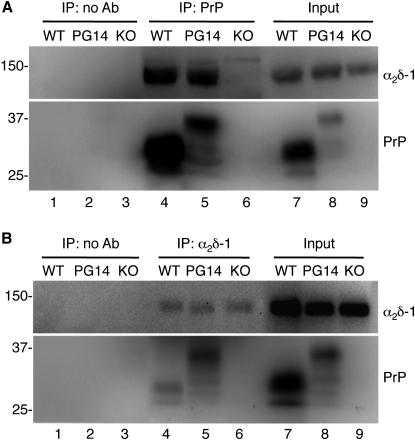
Because our data pointed to a role of intracellular PG14 PrP retention, we hypothesized that mutant PrP interacted with VGCCs in transport organelles, interfering with their trafficking toward the plasma membrane. Of the different channel subunits, α2δ-1 was the best candidate for an interaction with PrP because PrP is a glycosylphosphatidylinositol (GPI)-anchored sialoglycoprotein (Stahl et al., 1987) exposed on the outer leaflet of the plasma membrane in cholesterol-rich microdomains (also known as lipid rafts), and there is evidence that α2δ-1 is also GPI anchored (Davies et al., 2010). To test whether PrP and α2δ-1 interacted physically, we immunoprecipitated PrP from cerebellar extracts of Tg(WT) and Tg(PG14) mice, and immunoblotted the precipitated fractions with an antibody raised against the α2 polypeptide of α2δ-1. As shown in Figure 5A, an immunoreactive band of ∼145 kDa was detected in immunoprecipitates of Tg(WT) and Tg(PG14) but not in Prnp0/0 mice, or when the immunoprecipitation was done in the absence of the anti-PrP antibody. After deglycosylation with PNGaseF, this band shifted to an apparent molecular weight of 107 kDa, as expected for the α2 polypeptide (Figure S5A) (Davies et al., 2006).
Figure 5.
PrP and α2δ-1 Are Coimmunoprecipitated from Brain Extracts
(A) Cerebellar protein extracts (500 μg) from Tg(WT), Tg(PG14), and Prnp0/0 (KO) mice were incubated with uncoated magnetic beads (lanes 1–3) or magnetic beads coated with anti-PrP monoclonal antibody 94B4 (lanes 4–6). The immunoprecipitated proteins were analyzed by western blot with an anti-α2δ-1 antibody (upper panel) or anti-PrP polyclonal antibody P45-66 (lower panels). The input (lanes 7–9) is 25 μg of total protein.
(B) Cerebellar protein extracts (500 μg) from the same animals as in (A) were incubated with uncoupled magnetic beads (lanes 1–3) or magnetic beads coated with anti-α2δ-1 polyclonal antibody (lanes 4–6). The immunoprecipitated proteins were analyzed by western blot with an anti-α2δ-1 monoclonal antibody (upper panel) or anti-PrP monoclonal antibody 12B2 (lower panels). The input (lanes 7–9) is 25 μg of total protein.
The interaction was confirmed in the reverse experiment in which α2δ-1 was immunoprecipitated from cerebellar extracts and PrP detected by immunoblot (Figure 5B), and was also seen in primary cultured CGNs (Figure S5B) and transiently transfected HeLa cells (Figure S5C). HC-deleted PrP molecules coimmunoprecipitated with α2δ-1 (Figure S5C), indicating that PrP region 114–121 was not essential for the interaction.
Next, we tested whether the distribution of α2δ-1 was altered in cells expressing PG14 PrP. HeLa cells were cotransfected with plasmids encoding the CaVα1A, CaVβ4, and α2δ-1 subunits, and either wild-type or PG14 PrP-EGFP fusion proteins, and analyzed by confocal microscopy after immunofluorescent staining of α2δ-1. Consistent with previous localization of nonfluorescent and EGFP-fused PrPs (Biasini et al., 2010; Fioriti et al., 2005; Ivanova et al., 2001), the majority of wild-type PrP localized on the cell surface (Figures 6A and 6J), whereas PG14 PrP was mostly found in intracellular compartments (Figures 6D and 6J). In cells expressing wild-type PrP, α2δ-1 was efficiently expressed on the plasma membrane where it colocalized with PrP (Figures 6B, 6C, and 6K). In contrast, α2δ-1 was weakly expressed on the surface of PG14 PrP-expressing cells, and was mostly found in perinuclear patches where it colocalized with PrP (Figures 6E, 6F, and 6K), and with ER and Golgi markers (data not shown). This was seen in cells with high or low expression levels, ruling out that the abnormal localization of α2δ-1 was due to overexpression. The CaVα1A pore-forming subunit also accumulated intracellularly in PG14 PrP-expressing cells (Figures S6A–S6H), whereas there was no effect on the localization of 5′ nucleotidase (5′NT), a raft-resident GPI-anchored protein that does not belong to the VGCC complex (Davies et al., 2010) (Figures S6I–S6P). In cells expressing PG14/ΔHC PrP, α2δ-1 was more efficiently delivered to the cell surface, indicating that intracellular retention of mutant PrP played a role in the trafficking defect (Figures 6H, 6I, and 6K).
Figure 6.
α2δ-1 Is Retained in Intracellular Compartments of Cells Expressing PG14 PrP
(A–I) HeLa cells were cotransfected with plasmids encoding wild-type, PG14, or PG14/ΔHC PrP-EGFP fusion protein, and the VGCC subunits CaVα1A, CaVβ4, and α2δ-1 (0.5:3:2:2 ratio). After 48 hr, cells were fixed, permeabilized, stained with anti-α2δ-1 monoclonal antibody followed by Alexa 594 (red)-conjugated anti-mouse IgG secondary antibody, and reacted with DAPI (blue) to stain the nuclei. Scale bar, 20 μm. The fluorescent density of PrP-EGFP (J) and α2δ-1 (K) on the cell surface was measured and expressed as a percentage of the total. Each bar indicates the mean ± SEM of 28 wild-type, 48 PG14, and 8 PG14/ΔHC transfected cells. ∗∗p < 0.01 and ∗∗∗p < 0.001 by one-way ANOVA, Dunn's post hoc test. n.s., not significant.
To demonstrate that intracellular PrP retention was directly responsible, we analyzed the distribution of α2δ-1 in cells expressing PrP molecules artificially targeted to the ER or Golgi. HeLa cells were cotransfected with the CaVα1A, CaVβ4, and α2δ-1 subunits, and with a modified version of wild-type PrP in which the GPI signal had been replaced with the ER retention KDEL motif (PrP-ER) or with the transmembrane domain from the rubella virus envelope glycoprotein E2, which contains a Golgi-targeting signal (PrP-Golgi). Double-immunofluorescent staining of PrP-ER with protein disulfide isomerase, and PrP-Golgi with giantin, confirmed the predicted intracellular localization of these constructs (Figure 7A). In cells expressing PrP-ER or PrP-Golgi, α2δ-1 resided in intracellular compartments, colocalizing with PrP (Figure 7B). Thus, blocking PrP in the ER or Golgi by artificial retention signals resulted in intracellular retention of α2δ-1, as with the PG14 mutation.
Figure 7.
Targeting PrP to the ER or Golgi Induces Intracellular Accumulation of α2δ-1
HeLa cells were cotransfected with plasmids carrying cDNAs encoding PrP-ER or PrP-Golgi and the VGCC subunits α1A, Cavβ4, and α2δ-1 (0.5:3:2:2 ratio).
(A) After 48 hr, cells were fixed, permeabilized, and stained with anti-PrP monoclonal antibody 98A3 (green), and polyclonal antibodies against protein disulfide isomerase (PDI) or giantin to stain the ER or Golgi (red).
(B) Transfected cells were stained with anti-PrP polyclonal antibody R505 (green) and an anti-α2δ-1 monoclonal antibody (red). Cells were reacted with DAPI (blue) to stain the nuclei.
Scale bars, 20 μm.
To investigate whether PG14 PrP expression impaired the cell surface delivery of α2δ-1 in neuronal cells too, we immunostained endogenous α2δ-1 in nonpermeabilized primary CGNs from wild-type and PG14 mice. The immunofluorescent signal was markedly lower in PG14 CGNs than in the wild-type control (Figure S7A); α2δ-1 levels were similar in wild-type and PG14 neurons (Figure S7B), ruling out that the lower α2δ-1 level on the surface of PG14 CGNs was due to reduced α2δ-1 expression. To test whether the synaptic localization of α2δ-1 and CaVα1A was altered in the cerebellum of Tg(PG14) mice, we assessed their levels in purified synaptic membranes by western blot. α2δ-1 and CaVα1A levels were significantly lower in the cerebellar synaptosomal fractions of the mutant mice (Figures S7C and S7D). In addition, immunofluorescent staining of the cerebellar molecular layer showed reduced colocalization of α2δ-1 with VGLUT1 (Figure S7E), and of CaVα1A with the presynaptic marker VAMP2 (Figure S7F) consistent with impaired VGCC transport to synaptic sites.
Another Pathogenic PrP Mutant Alters α2δ-1 Trafficking and Impairs Depolarization-Induced Calcium Influx and Glutamate Release in Primary Neurons and Tg Mice
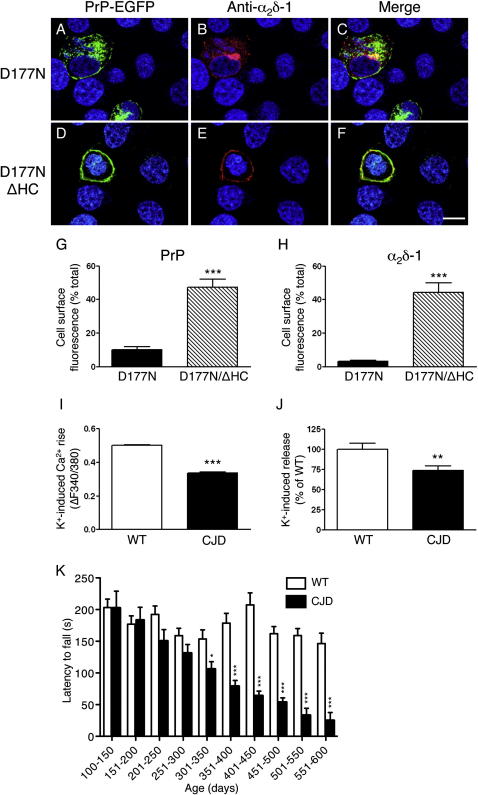
Next, we asked whether another pathogenic PrP mutant affected VGCC trafficking and function. Like PG14 PrP, mouse PrP carrying the D177N/V128 mutation misfolds and accumulates in the ER of CGNs, and in Tg mice it induces a CJD-like syndrome with motor, cognitive, and electroencephalographic abnormalities (Dossena et al., 2008). As was the case for the PG14 mutation, expression of D177N PrP altered α2δ-1 localization in HeLa cells (Figures 8A–8C, 8G, and 8H). This was not seen in cells expressing D177N/ΔHC PrP, which is more efficiently delivered to the cell surface (Biasini et al., 2010) (Figures 8D–8H), confirming that intracellular retention of mutant PrP plays a role in the trafficking defect of α2δ-1. CGNs from Tg(CJD) mice expressing D177N/V128 PrP showed a lower depolarization-induced calcium influx (Figure 8I). Similar to that in Tg(PG14) mice, [3H]D-aspartate release was reduced in cerebellar synaptosomes of Tg(CJD) mice with motor behavioral abnormalities (Figures 8J and 8K).
Figure 8.
The D177N PrP Mutation Alters α2δ-1 Trafficking and Impairs Depolarization-Induced Calcium Influx and Glutamate Release
(A–F) HeLa cells were cotransfected with plasmids encoding D177N or D177N/ΔHC PrP-EGFP fusion protein, and the VGCC subunits CaVα1A, CaVβ4, and α2δ-1 (0.5:3:2:2 ratio). After 48 hr, cells were fixed, permeabilized, stained with anti-α2δ-1 monoclonal antibody followed by Alexa 594 (red)-conjugated anti-mouse IgG secondary antibody, and reacted with DAPI (blue) to stain the nuclei. Scale bar, 20 μm. The fluorescent density of PrP-EGFP (G) and α2δ-1 (H) on the cell surface was measured and expressed as a percentage of the total. Each bar indicates the mean ± SEM of 11 D177N and 19 D177N/ΔHC transfected cells. ∗∗∗p < 0.001 by Mann-Whitney U test.
(I) Primary cultures of CGNs from C57BL/6J (wild-type) and Tg(CJD) mice were preloaded with 10 μM fura-2 AM and depolarized with 30 mM KCl. The K+-induced [Ca2+]i rise was measured in single cells and expressed as ΔF. Values are the mean ± SEM of 540 cells from C57BL/6J and 607 cells from Tg(CJD) mice, from 3 independent experiments. ∗∗∗p < 0.001 by Student's t test.
(J) Depolarization-induced [3H]D-aspartate release was determined in cerebellar synaptosomes from 489- to 520-day-old Tg(WT) and Tg(CJD) mice. Each bar indicates the mean ± SEM of 2 independent analyses (18 superfusion chambers per experimental group) of synaptosomes from 5 Tg(WT) and 5 Tg(CJD) mice. ∗∗p < 0.01 versus the corresponding values of Tg(WT) by Student's t test.
(K) Groups of 9–20 C57BL/6J (wild-type) and 3–12 Tg(CJD) mice were tested on a Rotarod at the ages indicated. Each mouse was tested three times, and the mean latency to fall was calculated. Bars indicate the mean ± SEM latency to fall (s). ∗p < 0.05, ∗∗∗p < 0.001 by Student's t test.
Discussion
The cellular pathways activated by mutant PrP in genetic prion diseases, ultimately leading to neuronal dysfunction and degeneration, are not known. Several mutant PrPs misfold soon after synthesis in the ER and reside longer in transport organelles, suggesting that misfolding and intracellular retention may play a pathogenic role. In the present study we found that early motor behavioral abnormalities in two different Tg mouse models correlate with defective glutamatergic neurotransmission in CGNs. This precedes neurodegeneration and is due to inefficient VGCC-mediated calcium influx in presynaptic terminals. PrP interacts with the VGCC α2δ-1 subunit, which regulates the forward trafficking of the channel. Due to mutant PrP retention in transport organelles, α2δ-1 accumulates intracellularly, resulting in inefficient targeting of the VGCC complex to synaptic sites. These results provide a cell biological explanation for predegenerative cerebellar dysfunction in genetic prion diseases, and suggest a possible physiological role of PrP in VGCC trafficking and activity.
Impaired Cerebellar Glutamatergic Transmission and Early Motor Disturbances
Analysis of motor performance on the Rotarod indicates that motor abnormalities in Tg(PG14) mice emerge at ∼45 days of age, long before kyphosis, foot clasp reflex, and the other neurological signs typical of this model (Chiesa et al., 1998, 2000). At this stage we found no synaptic or granule cell loss in the cerebellar cortex but a significant decrease of glutamate release from presynaptic terminals, suggesting that mutant PrP leads to perturbation of synaptic transmission independently of neuronal death. Consistent with this, glutamate release was impaired in CGNs isolated from Tg(PG14) mice, which remain healthy in primary culture, and in cerebellar synaptosomes of Tg(CJD) mice, which develop motor disease in the absence of granule cell loss (Dossena et al., 2008). Thus, the onset of cerebellar dysfunction and neuron demise are dissociated in mutant PrP mice, as in mouse models of spinocerebellar ataxia type-1 (Duvick et al., 2010).
In the cerebellar cortex, parallel fibers (PFs) from granule neurons transmit excitatory glutamatergic inputs to dendrites of Purkinje cells (PCs), which serve as the output system for motor control (Ghez, 1991). Tg mice in which glutamate exocytosis from PFs is selectively suppressed by conditional expression of tetanus neurotoxin in CGNs develop motor dysfunction that can be rescued by switching off the neurotoxin expression (Yamamoto et al., 2003), indicating a vital role of CGN glutamatergic transmission in sensorimotor function. Because mutant PrP impairs glutamate release in CGNs, as documented by the reduced depolarization-evoked exocytosis and changes in short-term plasticity, the motor deficit in mutant mice is most likely the consequence of inefficient excitatory inputs at the PF-PC synapse.
A number of observations support the idea that synaptic dysfunction induced by abnormal PrP is an important determinant of early behavioral abnormalities in prion diseases. In prion-infected mice defects in presynaptic hippocampal function precede neurodegeneration, and parallel the deficits in hippocampus-dependent spontaneous ethological behaviors, such as recognition memory, burrowing, and nesting (Chiti et al., 2006; Cunningham et al., 2003; Mallucci et al., 2007).
Intracellular Retention of Mutant PrP and VGCC Dysfunction
Our results indicate that defective glutamate release in the cerebellum of Tg(PG14) mice is due to inefficient VGCC function in CGNs, and that mutant PrP is directly responsible. Lentivirus-mediated knockdown of PG14 PrP restored the depolarization-evoked calcium rise, and transfection of a plasmid encoding PG14 PrP impaired the calcium response in wild-type neurons. The latter observation indicates that PG14 PrP alters calcium dynamics with a dominant effect over wild-type PrP, consistent with a gain-of-toxicity mechanism. However, a loss of a putative PrP function in governing VGCC activity (see below) may also be involved.
Previous analyses suggested that accumulation of mutant PrP in the secretory pathway might be critical in neuronal dysfunction, possibly due to interference with transport and delivery of essential cargo molecules to synapses (Dossena et al., 2008; Massignan et al., 2010; Medrano et al., 2008). Here, we found that intracellular retention of mutant PrP was required for perturbing neuronal calcium dynamics, and identified the α2δ-1 subunit of VGCCs as a target molecule. We documented a physical interaction between PrP and α2δ-1 by coimmunoprecipitation, and the two proteins colocalized in transfected cells, consistent with analysis of the native PrP interactome, which identified α2δ subunits as candidate PrP interactors (Rutishauser et al., 2009).
The α2δ subunits play a vital role in intracellular trafficking of the pore-forming CaVα1 subunits of the CaV1 and CaV2 classes, and boost calcium current amplitude by increasing the number of channels on the cell surface (Cantí et al., 2005). α2δ interacts with CaVα1 during biosynthetic maturation, and promotes the transport of the heteromeric channel complex to correct presynaptic sites (Bauer et al., 2010; Cantí et al., 2005; Hendrich et al., 2008; Saheki and Bargmann, 2009). We found that α2δ-1 and CaVα1A were weakly expressed on the cell surface and localized mainly in the ER and Golgi in mutant PrP-expressing cells, suggesting impaired secretory transport. We also found smaller amounts of α2δ-1 and CaVα1A in cerebellar synaptosomal fractions of Tg(PG14) mice, and reduced colocalization with synaptic markers, consistent with inefficient targeting of the channel complex to axonal terminals of granule neurons. Thus, the smaller depolarization-evoked calcium rise in cerebellar synaptosomes and in primary CGNs can be explained by the fact that there are fewer functional channels on the plasma membrane.
We previously found that PG14 and D177N PrP molecules with a deletion in the hydrophobic core between residues 114–121 had less tendency to misfold and were more efficiently delivered to the cell surface than their full-length counterparts, providing a model for assessing the role of intracellular retention in neurotoxicity (Biasini et al., 2010). By comparing the effect of HC-deleted and full-length molecules on α2δ-1 trafficking and calcium dynamics, we provided evidence that VGCC dysfunction depends on intracellular retention of mutant PrP. This, and the fact that PrP interacts physically with the α2δ-1 subunit, suggests a mechanism whereby interaction between mutant PrP and α2δ-1 results in the latter being sequestered in secretory organelles, impairing correct assembly and delivery of the channel complex to synaptic sites.
Although this can readily explain the low levels of VGCCs at presynaptic terminals, an indirect mechanism might also be involved. PrP may participate in cell signaling governing membrane protein transport (Málaga-Trillo et al., 2009) that could be altered by pathogenic mutations. We did in fact find that cells expressing D177N PrP had an impairment in Rab11-dependent trafficking (Massignan et al., 2010), which could potentially affect the endocytic recycling of α2δ-1 (Tran-Van-Minh and Dolphin, 2010).
Our analysis indicates that glutamatergic neurotransmission in Tg(PG14) mice is preferentially impaired in CGNs, in line with the selective expression of α2δ-1 by these cells in the cerebellum (Cole et al., 2005). However, α2δ-1 is also expressed by glutamatergic neurons in other brain regions (Cole et al., 2005). Therefore, there might be defects in α2δ-1 transport and neurotransmission in other neural systems, which could be responsible for additional neurological signs. For example the deficit in spatial working memory in Tg(CJD) mice (Dossena et al., 2008) might depend on abnormal glutamatergic function in the hippocampus.
Three different α2δ subunits are expressed in functionally distinct neurons of the brain, with the α2δ-2 and α2δ-3 isoforms sharing, respectively, 55.6% and 30.3% sequence identity with α2δ-1 (Klugbauer et al., 1999). It will be interesting to see if PrP interacts with α2δ-2 and α2δ-3, and if their cellular trafficking is affected by mutant PrP, as with α2δ-1. It will also be important to see whether VGCC dynamics are perturbed in prion diseases acquired by infection. N-type VGCC function is impaired in prion-infected hypothalamic GT1-1 cells (Sandberg et al., 2004), but it is not clear whether this is due to deficient channel insertion in the plasma membrane.
At an advanced stage of disease, Tg(PG14) mice show synaptic degeneration in the cerebellar molecular layer and apoptosis of granule neurons, raising the possibility that functional impairment of α2δ subunits resulting from sequestration by mutant PrP may eventually lead to synaptic disruption and neuron demise. Consistent with this, targeted deletion or spontaneous mutation of the mouse Cacna2d2 gene encoding α2δ-2, which is primarily present in Purkinje neurons, results in cerebellar ataxia with PC depletion and apoptosis of granule neurons (Barclay et al., 2001; Ivanov et al., 2004). It is striking that mutations in other VGCC subunits also often result in cerebellar degeneration in mice (Pietrobon, 2005), perhaps because cerebellar neurons are more sensitive to changes in calcium levels, or less able to activate compensatory responses than neurons of other brain regions. In this regard the fact that glutamate release was not consistently impaired in the cerebral cortex of Tg(PG14) mice despite high α2δ-1 expression (Cole et al., 2005) may be due to upregulation of cellular pathways that positively affect VGCC trafficking and activity (Simms and Zamponi, 2012).
Possible Physiological Significance of the PrP-α2δ-1 Interaction
Our findings that wild-type PrP and α2δ-1 are coimmunoprecipitated from mouse brain extracts and colocalize in transfected cells suggest a role of PrP in VGCC function. In line with this, cerebellar granules and hippocampal CA1 neurons lacking PrP showed alterations in L-type VGCC-dependent calcium dynamics (Fuhrmann et al., 2006; Herms et al., 2000). In addition, treatment of synaptosomes with recombinant PrP resulted in cytosolic calcium elevation that was inhibited by gadolinium—a nonselective VGCC blocker—and an anti-PrP monoclonal antibody impaired the calcium response to depolarization (Whatley et al., 1995). Finally, exposure of neurons to full-length PrP or N-terminal fragments affected L-type VGCC-mediated calcium entry (Florio et al., 1998; Korte et al., 2003). Although we found no significant deficits in depolarization-evoked calcium influx in cerebellar synaptosomes from PrP-deficient mice, there was a modest but significant decrease in primary CGNs lacking PrP (data not shown), consistent with an effect on somatic channels (predominantly L-type) (Herms et al., 2000).
PrP might regulate VGCC activity through several mechanisms. Interaction with α2δ-1 in the ER might titrate its association with CaVα1A and fine-tune the anterograde transport of the channel complex. Alternatively, PrP may influence the channel activity by associating with α2δ-1 on the plasma membrane, or acting as a scaffold protein to target the channel complex to specific membrane microdomains (Madore et al., 1999). Like other GPI-anchored proteins, α2δ-1 is preferentially located in detergent-resistant lipid rafts (Davies et al., 2006, 2010). This lipid raft localization appears to be independent of the GPI-anchoring motif (Robinson et al., 2011), suggesting that it may rely on interaction with other raft-resident proteins, such as PrP. Finally, the PrP-α2δ-1 interaction may have a physiological significance unrelated to the channel activity. Recent findings, in fact, show that α2δ-1 is involved in synaptogenesis (Eroglu et al., 2009), a function in which PrP has also been involved (Kanaani et al., 2005; Pantera et al., 2009; Santuccione et al., 2005).
Clearly, further studies are required to establish the physiological significance of the PrP-α2δ-1 interaction. It will be critical to identify the protein domain(s) involved in the interaction, and any other interacting partners. In light of the dysfunctional consequences of the mutant PrP association with α2δ-1, disrupting their binding might represent a means for therapeutic intervention.
Experimental Procedures
Mice
The production of Tg mice expressing wild-type, PG14, and D177N/V128 mouse PrPs with an epitope for the monoclonal antibody 3F4 has already been reported by Chiesa et al. (1998) and Dossena et al. (2008). In this study we used Tg mice of the Tg(WT-E1+/+) line, which expresses about four times the endogenous PrP level, referred to throughout the text as Tg(WT); we also used Tg(PG14-A3+/−) and Tg(D177N/V128-A21+/−) mice expressing Tg PrP at approximately one time, referred to as Tg(PG14) and Tg(CJD), respectively. These mice were originally generated on a C57BL/6J X CBA/J hybrid and were then bred with the Zurich I line of Prnp0/0 mice (Büeler et al., 1992) with a pure C57BL/6J background (European Mouse Mutant Archive, Monterotondo, Rome; EM:01723). C57BL/6J mice were purchased from Charles River Laboratories.
All procedures involving animals were conducted according to European Union (EEC Council Directive 86/609, OJ L 358,1; December 12, 1987) and Italian (D.L. n.116, G.U. suppl. 40, February 18, 1992) laws and policies, and were in accordance with the United States Department of Agriculture Animal Welfare Act and the National Institutes of Health Policy on Humane Care and Use of Laboratory Animals. They were reviewed and approved by the Mario Negri Institute Animal Care and Use Committee that includes ad hoc members for ethical issues (18/01-D, 18/01-C). Animal facilities meet international standards and are regularly checked by a certified veterinarian who is responsible for health monitoring, animal welfare supervision, experimental protocols, and review of procedures.
MRI
Animals were anesthetized with 1% isoflurane in a 30%:70% O2:N2O gas mixture and imaged in a horizontal bore 7-Tesla USR preclinical MRI system (BioSpec 70/30; Bruker BioSpin, Germany) with a shielded gradient insert (BGA 12, 400 mT/m; rise time, 110 us). A 7 mm birdcage resonator for RF transmission and a 10 mm diameter single-loop receiver coil were used to receive the signal. T2-weighted anatomical images of the mouse brain were acquired with the following parameters: TR 2500 ms, TE 50 ms, RARE factor 16, FOV 3 × 1.5 × 1.5 cm, Matrix 256 × 102 × 102, voxel 0.147 × 0.117 × 0.147. The scan time was approximately 25 min. The cerebellar volume was quantified using the ImageJ software (http://rsbweb.nih.gov/ij/).
Accelerated Rotarod Test
We used an accelerating Rotarod 7650 model (Ugo Basile). Juvenile mice were tested starting from 19 days of age (P19) for 7 consecutive days. On the first day a training session was done during which each mouse was placed on the Rotarod at a constant speed (4 rpm) for a maximum of 60 s. Then they were assessed in three consecutive test sessions with a 10 min intertrial resting period. They were positioned on the rotating bar and allowed to become acquainted with the environment for 30 s. The rod motor was started initially at 4 rpm and accelerated to 40 rpm at a constant rate of 0.12 rpm/s for a maximum of 300 s. Adult mice were trained three times the week before official testing. The performance was scored as latency to fall, in seconds. Animals were given three trials, and the average was used for statistical analysis.
Synaptosome Preparation
Synaptosomes were isolated from the cerebral cortex and the cerebellum using a discontinuous Percoll gradient as described by Stigliani et al. (2006).
Cells
Primary CGNs were prepared from 6-day-old mice as described by Biasini et al. (2010). HeLa cells were grown in a 1:1 mixture of Dulbecco's modified Eagle's medium (DMEM) and minimal essential medium α (MEM), supplemented with GlutaMAX (Invitrogen), 10% FBS, nonessential amino acids (Sigma-Aldrich), 100 U/ml penicillin, and 100 μg/ml streptomycin (GIBCO), and maintained at 37°C in 5% CO2/95% air. Plasmid transfections and transduction with lentiviral vectors were done as described in the Supplemental Experimental Procedures.
Biochemical Analyses
Detergent insolubility and immunoprecipitation with antibody 15B3 were assayed as described by Biasini et al. (2009) and Chiesa et al. (1998). Coimmunoprecipitation was done as described in the Supplemental Experimental Procedures.
Immunofluorescence Staining
Immunofluorescence staining of cells and brain sections was performed as described in the Supplemental Experimental Procedures.
Antibodies
Antibodies used for western blot, immunoprecipitation, and immunofluorescence are described in the Supplemental Experimental Procedures.
Neurotransmitter Uptake and Release
Neurotransmitter uptake and release from synaptosomes and CGNs were done according to published protocols by Stigliani et al. (2006), and are described more fully in the Supplemental Experimental Procedures.
Calcium Measurements
Calcium levels in synaptosomes were determined spectrofluorimetrically after incubating purified synaptosomes with the calcium-sensitive fluorescent dye fura-2 AM. Synaptosomes washed in Tris/acetate buffer (128 mM NaCl, 5 mM KCl, 1 mM MgSO4, 1.5 mM NaHPO4, 10 mM glucose, 10 mM Tris/HCl [pH 7.4]) were resuspended at a total protein concentration of 1 μg/μl and loaded with fura-2 AM 5 μM at 37°C for 30 min in Tris/acetate buffer plus BSA 1%. After centrifugation at 16,000 × g for 5 min, synaptosomes were resuspended at a final protein concentration of 0.25 μg/μl in Tris/acetate buffer plus 1 mM CaCl2 and aliquoted in 96-well plates (50 μg protein/well). Fluorescence was measured at 37°C for 200 cycles, each cycle alternating excitation at 340 and 380 nm and monitoring emission at 510 nm. Depolarization was induced by injecting 50 mM KCl at cycle 50. The fluorescence ratio F340:380 was measured for each cycle, and data were reported as ΔF340/380, the difference between F340/380 before and after the stimulus, which is proportional to the KCl-induced Ca2+ influx. Single-cell calcium imaging was done as described by Verderio et al. (2004) using an Olympus IX81 inverted microscope equipped with a calcium imaging unit (CellR; Olympus).
Electrophysiology
Whole-cell VGCC currents were recorded from CGNs as described by Condliffe et al. (2010). For evoked and miniature EPSC recordings, CGNs were bathed in an external solution containing 125 mM NaCl, 5 mM KCl, 1.2 mM MgSO4, 2 mM CaCl2, 1.2 mM KHPO4, 25 mM HEPES, 10 mM Glu (pH 7.4). For miniature recordings 1 μM tetrodotoxin was added. Patch pipettes were filled with internal solutions (130 mM KGluc, 1 mM EGTA, 10 mM KCl, 2 mM MgCl2, 10 mM HEPES, 4 mM Mg-ATP, and 0.3 mM Tris-GTP). EPSCs for paired pulse recordings were evoked from monosynaptically coupled neurons by pairs of 2 ms depolarizing pulses from −70 to 30mV (50 ms interpulse interval [20 Hz]) every 5 s. During miniature EPSCs and paired-pulse recordings, cells were held at −70mV (unless otherwise stated). Series resistance ranged from 10 to 20 MΩ and was monitored for consistency during recordings. Cells in culture with leak currents >100 pA were excluded from the analysis. Signals were amplified, sampled at 10 kHz, filtered to 2 or 5 KHz, and analyzed using pClamp 10 data acquisition and analysis program.
Acknowledgments
We thank Giovanna R. Mallucci for the lentiviruses expressing shRNAs targeting PrP, and David Westaway for the pBud-EGFP plasmid. We also thank David A. Harris for the P45-66 antibody and the PrP-ER and PrP-Golgi constructs, Richard Kasksak for the 3F4 antibody, Jan P. Langeveld for the 12B2, 94B4, 98A3 and R505 antibodies, and Alex Raeber from Prionics (Zurich, Switzerland) for the 15B3 antibody. We are grateful to Simona Airaghi, Davide Pozzi and Egidio D'Angelo for participating in the initial phase of this project; to Pietro Veglianese for advice on time-lapse and confocal microscopy; to Elisabetta Menna and Stefano Fumagalli for advice on immunofluorescence quantification; to Luisa Diomede for advice on calcium measurement in synaptosomes; to Alan Zanardi for help with primary CGN culture; and to Michele Sallese for comments on the manuscript. This work was supported by grants from Telethon Italy (TCR08005), “Fondazione Cariplo” (2008-2338 and 2010-0828), the E.C. Network of Excellence NeuroPrion (FOOD-CT-2004-506579), the European Union Seventh Framework Programme under grant agreement HEALTH-F2-2009-241498 (“EUROSPIN” project), and by the Italian Ministry of Health (Malattie Rare RF-INN-2008-1215065). A.S. was supported by an anonymous fellowship grant. R.C. is an Associate Telethon Scientist (Dulbecco Telethon Institute, Fondazione Telethon). This work is dedicated to the memory of Renato Dulbecco (February 22, 1914, Catanzaro, Italy — February 19, 2012, La Jolla, CA, USA).
Published: April 25, 2012
Footnotes
Supplemental Information includes seven figures and Supplemental Experimental Procedures and can be found with this article online at doi:10.1016/j.neuron.2012.02.027.
Supplemental Information
References
- Barclay J., Balaguero N., Mione M., Ackerman S.L., Letts V.A., Brodbeck J., Canti C., Meir A., Page K.M., Kusumi K. Ducky mouse phenotype of epilepsy and ataxia is associated with mutations in the Cacna2d2 gene and decreased calcium channel current in cerebellar Purkinje cells. J. Neurosci. 2001;21:6095–6104. doi: 10.1523/JNEUROSCI.21-16-06095.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bauer C.S., Rahman W., Tran-van-Minh A., Lujan R., Dickenson A.H., Dolphin A.C. The anti-allodynic alpha(2)delta ligand pregabalin inhibits the trafficking of the calcium channel alpha(2)delta-1 subunit to presynaptic terminals in vivo. Biochem. Soc. Trans. 2010;38:525–528. doi: 10.1042/BST0380525. [DOI] [PubMed] [Google Scholar]
- Biasini E., Tapella L., Mantovani S., Stravalaci M., Gobbi M., Harris D.A., Chiesa R. Immunopurification of pathological prion protein aggregates. PLoS One. 2009;4:e7816. doi: 10.1371/journal.pone.0007816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biasini E., Tapella L., Restelli E., Pozzoli M., Massignan T., Chiesa R. The hydrophobic core region governs mutant prion protein aggregation and intracellular retention. Biochem. J. 2010;430:477–486. doi: 10.1042/BJ20100615. [DOI] [PubMed] [Google Scholar]
- Büeler H., Fischer M., Lang Y., Bluethmann H., Lipp H.P., DeArmond S.J., Prusiner S.B., Aguet M., Weissmann C. Normal development and behaviour of mice lacking the neuronal cell-surface PrP protein. Nature. 1992;356:577–582. doi: 10.1038/356577a0. [DOI] [PubMed] [Google Scholar]
- Cantí C., Nieto-Rostro M., Foucault I., Heblich F., Wratten J., Richards M.W., Hendrich J., Douglas L., Page K.M., Davies A., Dolphin A.C. The metal-ion-dependent adhesion site in the Von Willebrand factor-A domain of alpha2delta subunits is key to trafficking voltage-gated Ca2+ channels. Proc. Natl. Acad. Sci. USA. 2005;102:11230–11235. doi: 10.1073/pnas.0504183102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiesa R., Harris D.A. Prion diseases: what is the neurotoxic molecule? Neurobiol. Dis. 2001;8:743–763. doi: 10.1006/nbdi.2001.0433. [DOI] [PubMed] [Google Scholar]
- Chiesa R., Piccardo P., Ghetti B., Harris D.A. Neurological illness in transgenic mice expressing a prion protein with an insertional mutation. Neuron. 1998;21:1339–1351. doi: 10.1016/s0896-6273(00)80653-4. [DOI] [PubMed] [Google Scholar]
- Chiesa R., Drisaldi B., Quaglio E., Migheli A., Piccardo P., Ghetti B., Harris D.A. Accumulation of protease-resistant prion protein (PrP) and apoptosis of cerebellar granule cells in transgenic mice expressing a PrP insertional mutation. Proc. Natl. Acad. Sci. USA. 2000;97:5574–5579. doi: 10.1073/pnas.97.10.5574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiesa R., Piccardo P., Quaglio E., Drisaldi B., Si-Hoe S.L., Takao M., Ghetti B., Harris D.A. Molecular distinction between pathogenic and infectious properties of the prion protein. J. Virol. 2003;77:7611–7622. doi: 10.1128/JVI.77.13.7611-7622.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiesa R., Piccardo P., Dossena S., Nowoslawski L., Roth K.A., Ghetti B., Harris D.A. Bax deletion prevents neuronal loss but not neurological symptoms in a transgenic model of inherited prion disease. Proc. Natl. Acad. Sci. USA. 2005;102:238–243. doi: 10.1073/pnas.0406173102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiti Z., Knutsen O.M., Betmouni S., Greene J.R. An integrated, temporal study of the behavioural, electrophysiological and neuropathological consequences of murine prion disease. Neurobiol. Dis. 2006;22:363–373. doi: 10.1016/j.nbd.2005.12.002. [DOI] [PubMed] [Google Scholar]
- Cole R.L., Lechner S.M., Williams M.E., Prodanovich P., Bleicher L., Varney M.A., Gu G. Differential distribution of voltage-gated calcium channel alpha-2 delta (alpha2delta) subunit mRNA-containing cells in the rat central nervous system and the dorsal root ganglia. J. Comp. Neurol. 2005;491:246–269. doi: 10.1002/cne.20693. [DOI] [PubMed] [Google Scholar]
- Condliffe S.B., Corradini I., Pozzi D., Verderio C., Matteoli M. Endogenous SNAP-25 regulates native voltage-gated calcium channels in glutamatergic neurons. J. Biol. Chem. 2010;285:24968–24976. doi: 10.1074/jbc.M110.145813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cunningham C., Deacon R., Wells H., Boche D., Waters S., Diniz C.P., Scott H., Rawlins J.N., Perry V.H. Synaptic changes characterize early behavioural signs in the ME7 model of murine prion disease. Eur. J. Neurosci. 2003;17:2147–2155. doi: 10.1046/j.1460-9568.2003.02662.x. [DOI] [PubMed] [Google Scholar]
- Daude N., Lehmann S., Harris D.A. Identification of intermediate steps in the conversion of a mutant prion protein to a scrapie-like form in cultured cells. J. Biol. Chem. 1997;272:11604–11612. doi: 10.1074/jbc.272.17.11604. [DOI] [PubMed] [Google Scholar]
- Davies A., Douglas L., Hendrich J., Wratten J., Tran Van Minh A., Foucault I., Koch D., Pratt W.S., Saibil H.R., Dolphin A.C. The calcium channel alpha2delta-2 subunit partitions with CaV2.1 into lipid rafts in cerebellum: implications for localization and function. J. Neurosci. 2006;26:8748–8757. doi: 10.1523/JNEUROSCI.2764-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies A., Kadurin I., Alvarez-Laviada A., Douglas L., Nieto-Rostro M., Bauer C.S., Pratt W.S., Dolphin A.C. The alpha2delta subunits of voltage-gated calcium channels form GPI-anchored proteins, a posttranslational modification essential for function. Proc. Natl. Acad. Sci. USA. 2010;107:1654–1659. doi: 10.1073/pnas.0908735107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dolphin A.C. Calcium channel diversity: multiple roles of calcium channel subunits. Curr. Opin. Neurobiol. 2009;19:237–244. doi: 10.1016/j.conb.2009.06.006. [DOI] [PubMed] [Google Scholar]
- Dossena S., Imeri L., Mangieri M., Garofoli A., Ferrari L., Senatore A., Restelli E., Balducci C., Fiordaliso F., Salio M. Mutant prion protein expression causes motor and memory deficits and abnormal sleep patterns in a transgenic mouse model. Neuron. 2008;60:598–609. doi: 10.1016/j.neuron.2008.09.008. [DOI] [PubMed] [Google Scholar]
- Drisaldi B., Stewart R.S., Adles C., Stewart L.R., Quaglio E., Biasini E., Fioriti L., Chiesa R., Harris D.A. Mutant PrP is delayed in its exit from the endoplasmic reticulum, but neither wild-type nor mutant PrP undergoes retrotranslocation prior to proteasomal degradation. J. Biol. Chem. 2003;278:21732–21743. doi: 10.1074/jbc.M213247200. [DOI] [PubMed] [Google Scholar]
- Drisaldi B., Coomaraswamy J., Mastrangelo P., Strome B., Yang J., Watts J.C., Chishti M.A., Marvi M., Windl O., Ahrens R. Genetic mapping of activity determinants within cellular prion proteins: N-terminal modules in PrPC offset pro-apoptotic activity of the Doppel helix B/B′ region. J. Biol. Chem. 2004;279:55443–55454. doi: 10.1074/jbc.M404794200. [DOI] [PubMed] [Google Scholar]
- Duvick L., Barnes J., Ebner B., Agrawal S., Andresen M., Lim J., Giesler G.J., Zoghbi H.Y., Orr H.T. SCA1-like disease in mice expressing wild-type ataxin-1 with a serine to aspartic acid replacement at residue 776. Neuron. 2010;67:929–935. doi: 10.1016/j.neuron.2010.08.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eroglu C., Allen N.J., Susman M.W., O'Rourke N.A., Park C.Y., Ozkan E., Chakraborty C., Mulinyawe S.B., Annis D.S., Huberman A.D. Gabapentin receptor alpha2delta-1 is a neuronal thrombospondin receptor responsible for excitatory CNS synaptogenesis. Cell. 2009;139:380–392. doi: 10.1016/j.cell.2009.09.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fioriti L., Dossena S., Stewart L.R., Stewart R.S., Harris D.A., Forloni G., Chiesa R. Cytosolic prion protein (PrP) is not toxic in N2a cells and primary neurons expressing pathogenic PrP mutations. J. Biol. Chem. 2005;280:11320–11328. doi: 10.1074/jbc.M412441200. [DOI] [PubMed] [Google Scholar]
- Florio T., Thellung S., Amico C., Robello M., Salmona M., Bugiani O., Tagliavini F., Forloni G., Schettini G. Prion protein fragment 106-126 induces apoptotic cell death and impairment of L-type voltage-sensitive calcium channel activity in the GH3 cell line. J. Neurosci. Res. 1998;54:341–352. doi: 10.1002/(SICI)1097-4547(19981101)54:3<341::AID-JNR5>3.0.CO;2-G. [DOI] [PubMed] [Google Scholar]
- Fremeau R.T., Jr., Troyer M.D., Pahner I., Nygaard G.O., Tran C.H., Reimer R.J., Bellocchio E.E., Fortin D., Storm-Mathisen J., Edwards R.H. The expression of vesicular glutamate transporters defines two classes of excitatory synapse. Neuron. 2001;31:247–260. doi: 10.1016/s0896-6273(01)00344-0. [DOI] [PubMed] [Google Scholar]
- Fuhrmann M., Bittner T., Mitteregger G., Haider N., Moosmang S., Kretzschmar H., Herms J. Loss of the cellular prion protein affects the Ca2+ homeostasis in hippocampal CA1 neurons. J. Neurochem. 2006;98:1876–1885. doi: 10.1111/j.1471-4159.2006.04011.x. [DOI] [PubMed] [Google Scholar]
- Ghez C. The cerebellum. In: Kandel E.R., Schwartz J.H., Jessel T.M., editors. Principles of Neural Science. Third Edition. Appleton 6 Lange; Norwalk, CT: 1991. pp. 626–646. [Google Scholar]
- Hendrich J., Van Minh A.T., Heblich F., Nieto-Rostro M., Watschinger K., Striessnig J., Wratten J., Davies A., Dolphin A.C. Pharmacological disruption of calcium channel trafficking by the alpha2delta ligand gabapentin. Proc. Natl. Acad. Sci. USA. 2008;105:3628–3633. doi: 10.1073/pnas.0708930105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herms J.W., Korte S., Gall S., Schneider I., Dunker S., Kretzschmar H.A. Altered intracellular calcium homeostasis in cerebellar granule cells of prion protein-deficient mice. J. Neurochem. 2000;75:1487–1492. doi: 10.1046/j.1471-4159.2000.0751487.x. [DOI] [PubMed] [Google Scholar]
- Hilber P., Caston J. Motor skills and motor learning in Lurcher mutant mice during aging. Neuroscience. 2001;102:615–623. doi: 10.1016/s0306-4522(00)00509-1. [DOI] [PubMed] [Google Scholar]
- Ivanov S.V., Ward J.M., Tessarollo L., McAreavey D., Sachdev V., Fananapazir L., Banks M.K., Morris N., Djurickovic D., Devor-Henneman D.E. Cerebellar ataxia, seizures, premature death, and cardiac abnormalities in mice with targeted disruption of the Cacna2d2 gene. Am. J. Pathol. 2004;165:1007–1018. doi: 10.1016/S0002-9440(10)63362-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ivanova L., Barmada S., Kummer T., Harris D.A. Mutant prion proteins are partially retained in the endoplasmic reticulum. J. Biol. Chem. 2001;276:42409–42421. doi: 10.1074/jbc.M106928200. [DOI] [PubMed] [Google Scholar]
- Kanaani J., Prusiner S.B., Diacovo J., Baekkeskov S., Legname G. Recombinant prion protein induces rapid polarization and development of synapses in embryonic rat hippocampal neurons in vitro. J. Neurochem. 2005;95:1373–1386. doi: 10.1111/j.1471-4159.2005.03469.x. [DOI] [PubMed] [Google Scholar]
- Klugbauer N., Lacinová L., Marais E., Hobom M., Hofmann F. Molecular diversity of the calcium channel alpha2delta subunit. J. Neurosci. 1999;19:684–691. doi: 10.1523/JNEUROSCI.19-02-00684.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knight R.S., Will R.G. Prion diseases. J. Neurol. Neurosurg. Psychiatry. 2004;75(Suppl 1):i36–i42. doi: 10.1136/jnnp.2004.036137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korte S., Vassallo N., Kramer M.L., Kretzschmar H.A., Herms J. Modulation of L-type voltage-gated calcium channels by recombinant prion protein. J. Neurochem. 2003;87:1037–1042. doi: 10.1046/j.1471-4159.2003.02080.x. [DOI] [PubMed] [Google Scholar]
- Liu S., Friel D.D. Impact of the leaner P/Q-type Ca2+ channel mutation on excitatory synaptic transmission in cerebellar Purkinje cells. J. Physiol. 2008;586:4501–4515. doi: 10.1113/jphysiol.2008.156232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ly C.V., Yao C.K., Verstreken P., Ohyama T., Bellen H.J. straightjacket is required for the synaptic stabilization of cacophony, a voltage-gated calcium channel alpha1 subunit. J. Cell Biol. 2008;181:157–170. doi: 10.1083/jcb.200712152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madore N., Smith K.L., Graham C.H., Jen A., Brady K., Hall S., Morris R. Functionally different GPI proteins are organized in different domains on the neuronal surface. EMBO J. 1999;18:6917–6926. doi: 10.1093/emboj/18.24.6917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Málaga-Trillo E., Solis G.P., Schrock Y., Geiss C., Luncz L., Thomanetz V., Stuermer C.A. Regulation of embryonic cell adhesion by the prion protein. PLoS Biol. 2009;7:e55. doi: 10.1371/journal.pbio.1000055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mallucci G.R., White M.D., Farmer M., Dickinson A., Khatun H., Powell A.D., Brandner S., Jefferys J.G., Collinge J. Targeting cellular prion protein reverses early cognitive deficits and neurophysiological dysfunction in prion-infected mice. Neuron. 2007;53:325–335. doi: 10.1016/j.neuron.2007.01.005. [DOI] [PubMed] [Google Scholar]
- Massignan T., Biasini E., Lauranzano E., Veglianese P., Pignataro M., Fioriti L., Harris D.A., Salmona M., Chiesa R., Bonetto V. Mutant prion protein expression is associated with an alteration of the Rab GDP dissociation inhibitor alpha (GDI)/Rab11 pathway. Mol. Cell. Proteomics. 2010;9:611–622. doi: 10.1074/mcp.M900271-MCP200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mastrianni J.A. The genetics of prion diseases. Genet. Med. 2010;12:187–195. doi: 10.1097/GIM.0b013e3181cd7374. [DOI] [PubMed] [Google Scholar]
- Medrano A.Z., Barmada S.J., Biasini E., Harris D.A. GFP-tagged mutant prion protein forms intra-axonal aggregates in transgenic mice. Neurobiol. Dis. 2008;31:20–32. doi: 10.1016/j.nbd.2008.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mintz I.M., Sabatini B.L., Regehr W.G. Calcium control of transmitter release at a cerebellar synapse. Neuron. 1995;15:675–688. doi: 10.1016/0896-6273(95)90155-8. [DOI] [PubMed] [Google Scholar]
- Pantera B., Bini C., Cirri P., Paoli P., Camici G., Manao G., Caselli A. PrPc activation induces neurite outgrowth and differentiation in PC12 cells: role for caveolin-1 in the signal transduction pathway. J. Neurochem. 2009;110:194–207. doi: 10.1111/j.1471-4159.2009.06123.x. [DOI] [PubMed] [Google Scholar]
- Pietrobon D. Function and dysfunction of synaptic calcium channels: insights from mouse models. Curr. Opin. Neurobiol. 2005;15:257–265. doi: 10.1016/j.conb.2005.05.010. [DOI] [PubMed] [Google Scholar]
- Prusiner S.B. Prions. Proc. Natl. Acad. Sci. USA. 1998;95:13363–13383. doi: 10.1073/pnas.95.23.13363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qian J., Noebels J.L. Presynaptic Ca2+ influx at a mouse central synapse with Ca2+ channel subunit mutations. J. Neurosci. 2000;20:163–170. doi: 10.1523/JNEUROSCI.20-01-00163.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quaglio E., Restelli E., Garofoli A., Dossena S., De Luigi A., Tagliavacca L., Imperiale D., Migheli A., Salmona M., Sitia R. Expression of mutant or cytosolic PrP in transgenic mice and cells is not associated with endoplasmic reticulum stress or proteasome dysfunction. PLoS One. 2011;6:e19339. doi: 10.1371/journal.pone.0019339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson P., Etheridge S., Song L., Shah R., Fitzgerald E.M., Jones O.T. Targeting of voltage-gated calcium channel α2δ-1 subunit to lipid rafts is independent from a GPI-anchoring motif. PLoS One. 2011;6:e19802. doi: 10.1371/journal.pone.0019802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rutishauser D., Mertz K.D., Moos R., Brunner E., Rülicke T., Calella A.M., Aguzzi A. The comprehensive native interactome of a fully functional tagged prion protein. PLoS One. 2009;4:e4446. doi: 10.1371/journal.pone.0004446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saheki Y., Bargmann C.I. Presynaptic CaV2 calcium channel traffic requires CALF-1 and the alpha(2)delta subunit UNC-36. Nat. Neurosci. 2009;12:1257–1265. doi: 10.1038/nn.2383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandberg M.K., Wallén P., Wikström M.A., Kristensson K. Scrapie-infected GT1-1 cells show impaired function of voltage-gated N-type calcium channels (Ca(v) 2.2) which is ameliorated by quinacrine treatment. Neurobiol. Dis. 2004;15:143–151. doi: 10.1016/j.nbd.2003.09.006. [DOI] [PubMed] [Google Scholar]
- Santuccione A., Sytnyk V., Leshchyns'ka I., Schachner M. Prion protein recruits its neuronal receptor NCAM to lipid rafts to activate p59fyn and to enhance neurite outgrowth. J. Cell Biol. 2005;169:341–354. doi: 10.1083/jcb.200409127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simms B.A., Zamponi G.W. Trafficking and stability of voltage-gated calcium channels. Cell. Mol. Life Sci. 2012;69:843–856. doi: 10.1007/s00018-011-0843-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stahl N., Borchelt D.R., Hsiao K., Prusiner S.B. Scrapie prion protein contains a phosphatidylinositol glycolipid. Cell. 1987;51:229–240. doi: 10.1016/0092-8674(87)90150-4. [DOI] [PubMed] [Google Scholar]
- Stigliani S., Zappettini S., Raiteri L., Passalacqua M., Melloni E., Venturi C., Tacchetti C., Diaspro A., Usai C., Bonanno G. Glia re-sealed particles freshly prepared from adult rat brain are competent for exocytotic release of glutamate. J. Neurochem. 2006;96:656–668. doi: 10.1111/j.1471-4159.2005.03631.x. [DOI] [PubMed] [Google Scholar]
- Sudhof T.C. The synaptic vesicle cycle. Annu. Rev. Neurosci. 2004;27:509–547. doi: 10.1146/annurev.neuro.26.041002.131412. [DOI] [PubMed] [Google Scholar]
- Tran-Van-Minh A., Dolphin A.C. The alpha2delta ligand gabapentin inhibits the Rab11-dependent recycling of the calcium channel subunit alpha2delta-2. J. Neurosci. 2010;30:12856–12867. doi: 10.1523/JNEUROSCI.2700-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verderio C., Pozzi D., Pravettoni E., Inverardi F., Schenk U., Coco S., Proux-Gillardeaux V., Galli T., Rossetto O., Frassoni C., Matteoli M. SNAP-25 modulation of calcium dynamics underlies differences in GABAergic and glutamatergic responsiveness to depolarization. Neuron. 2004;41:599–610. doi: 10.1016/s0896-6273(04)00077-7. [DOI] [PubMed] [Google Scholar]
- Whatley S.A., Powell J.F., Politopoulou G., Campbell I.C., Brammer M.J., Percy N.S. Regulation of intracellular free calcium levels by the cellular prion protein. Neuroreport. 1995;6:2333–2337. doi: 10.1097/00001756-199511270-00015. [DOI] [PubMed] [Google Scholar]
- White M.D., Farmer M., Mirabile I., Brandner S., Collinge J., Mallucci G.R. Single treatment with RNAi against prion protein rescues early neuronal dysfunction and prolongs survival in mice with prion disease. Proc. Natl. Acad. Sci. USA. 2008;105:10238–10243. doi: 10.1073/pnas.0802759105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto M., Wada N., Kitabatake Y., Watanabe D., Anzai M., Yokoyama M., Teranishi Y., Nakanishi S. Reversible suppression of glutamatergic neurotransmission of cerebellar granule cells in vivo by genetically manipulated expression of tetanus neurotoxin light chain. J. Neurosci. 2003;23:6759–6767. doi: 10.1523/JNEUROSCI.23-17-06759.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zucker R.S., Regehr W.G. Short-term synaptic plasticity. Annu. Rev. Physiol. 2002;64:355–405. doi: 10.1146/annurev.physiol.64.092501.114547. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.