Abstract
Background
The classical Strecker reaction is one of the simplest and most economical methods for the synthesis of racemic α-aminonitriles (precursor of α-amino acids) and pharmacologically useful compounds.
Results
Indium powder in water is shown to act as a very efficient catalyst for one-pot, three-component synthesis of α-aminonitriles from diverse amines, aldehydes and TMSCN. This general rapid method is applicable to a wide range of amines and aldehydes and produces products in excellent yield.
Conclusions
The present one-pot, three-component environmentally benign procedure for the synthesis of α-aminonitriles will find application in the synthesis of complex biologically active molecules.
Background
Strecker reaction [1], the oldest known synthesis of α-aminonitriles, is one of the most general methods potentially useful for syntheses of amino acids and other bioactive compounds including natural products. In addition, the Strecker reaction represents one of the simplest and most economical methods for the preparation of α-amino acids for both laboratory and industrial scales [2]. Since 1850, a number of publications have appeared on this reaction. Still this reaction is under active investigation. Recently, synthesis of hepatitis C virus NS3 serine protease inhibitors [3], (±)-phthalascidin 622 [4] and novel boron-containing retinoids [5] have been reported following this strategy. A number of new catalysts have also been reported for this reaction which includes mesoporous aluminosilicate (Al-MCM-41) [6], lanthanum(III)-binaphthyl disulfonate [7], nanocrystalline magnesium oxide [8], BINOL-phosphoric acid [9,10], Fe(Cp)2PF6 [11], Jacobsen's thiourea catalyst [12], N-heterocyclic carbene (NHC)-amidate palladium(II) complex [13], Yb(OTf)3-pybox [14], K2PdCl4 [15], gallium (III) triflate [16], bisformamides [17], IBX/TBAB [18], Lewis base e. g. N,N-dimethylcyclohexylamine [19], superparamagnetic iron oxide [20], and ionic liquid [21]. To prepare α-aminonitriles (precursor to α-amino acids) generally an imine is reacted with a cyanide source. Notable among them are HCN [22], KCN [23], (EtO)2P(O)CN [24,25], Et2AlCN [26,27], Bu3SnCN [28,29], and TMSCN [3,4,6-20]. Among these cyanide sources, trimethylsilyl cyanide (TMSCN) is relatively easy to handle and highly soluble in organic solvents. In contrast, many of these reported methods involve the use of expensive reagents, hazardous solvents, longer reaction times and tedious workup procedure. Therefore, it is desirable to develop an efficient and practical method for the Strecker reaction under eco-friendly conditions.
Results
We have been working on the synthesis and biological evaluation of various β-lactams as novel anticancer agents [30-35] over the past several years. The synthesis of β-lactams through imines requires a carbonyl compound and an amine. Our study suggests that carbonyl compounds, amines and TMSCN in the presence of a mild acidic reagent will lead to the synthesis of α-aminonitriles in good to excellent yield. This hypothesis has been tested by reacting several amines with various carbonyl compounds and TMSCN in the presence of indium as catalyst. Recently, organic reactions in water have received much attention in view of green methodologies [36]. First of all, indium and a number of indium salts have been screened using aniline, benzaldehyde and TMSCN as a model reaction at room temperature. The results are shown in Table 1. The reaction was then performed in various solvents using indium as the catalyst to identify the best condition. It suggests that indium is the best catalyst in aqueous medium for the reaction (Table 2). The same reaction was used to optimize the amount of the catalyst. The results show (Table 3) that 10 mol% indium is required to complete the reaction in 30 minutes. Considering the above observations we carried out a series of reaction using various carbonyl compounds, amines and TMSCN in presence of indium (10 mol%) in water as solvent (Figure 1). In all the cases, the reactions were completed within 30 min to 1.5 hr and the products were obtained in excellent yield (Table 4). The products have demonstrated satisfactory spectral and mp data with the reported values.
Table 1.
Three component Strecker reaction using aniline (1 mmol), benzaldehyde (1 mmol) and TMSCN (1.2 mmol) in water (30 min): catalyst optimization
| Entry | Catalyst (10 mol %) |
Yield (%)a |
|---|---|---|
| 1 | Indium | 98 |
| 2 | Indium (II) chloride | 70 |
| 3 | Indium (III) chloride | 82 |
| 4 | Indium (III) bromide | 85 |
| 5 | Indium selenide | 62 |
| 6 | Indium oxide | 48 |
aisolated yield
Table 2.
Three component Strecker reaction using aniline (1 mmol), benzaldehyde (1 mmol) and TMSCN (1.2 mmol) in presence of indium (10 mol%) in various solvents (30 min): solvent optimization
| Entry | Solvent | Yield (%)a |
|---|---|---|
| 1 | Water | 98 |
| 2 | THF | 34 |
| 3 | Ethanol | 56 |
| 4 | Toluene | 60 |
| 5 | Methanol | 68 |
| 6 | Dichloromethane | 61 |
| 7 | DMSO | 76 |
| 8 | THF/H2O (1:1) | 54 |
| 9 | Ethanol/H2O (1:1) | 71 |
aisolated yield
Table 3.
Three component Strecker reaction using aniline (1 mmol), benzaldehyde (1 mmol) and TMSCN (1.2 mmol) in water (30 min): optimization of the amount of the catalyst
| Entry | Indium (mol %) | Yield (%)a |
|---|---|---|
| 1 | 30 | 89 |
| 2 | 25 | 91 |
| 3 | 20 | 88 |
| 4 | 15 | 89 |
| 5 | 10 | 98 |
| 6 | 5 | 67 |
| 7 | 2 | 54 |
| 8 | 1 | 43 |
aisolated yield
Figure 1.

Three component Strecker reaction using amines (1 mmol), carbonyl compounds (1 mmol) and TMSCN (1.2 mmol) in water in presence of indium (10 mol%).
Table 4.
Three component Strecker reaction using amines (1 mmol), carbonyl compounds (1 mmol) and TMSCN (1.2 mmol) in water in presence of indium (10 mol%)
| Entry | Amine | Carbonyl compound | Product | Time (min) | Yield (%)a | Ref. |
|---|---|---|---|---|---|---|
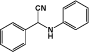
| 1 | 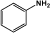 |
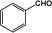 |
 |
30 | 98 | [11] |
| 2 | 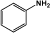 |
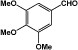 |
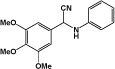 |
75 | 93 | [15] |
| 3 | 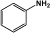 |
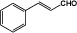 |
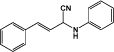 |
75 | 79 | [11] |
| 4 | 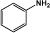 |
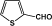 |
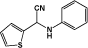 |
45 | 86 | [11] |
| 5 | 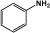 |
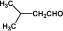 |
 |
60 | 88 | [11] |
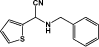
| 6 | 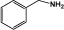 |
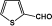 |
 |
75 | 91 | [11] |
| 7 | 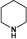 |
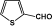 |
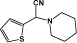 |
90 | 94 | [21] |
| 8 |  |
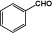 |
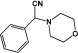 |
75 | 95 | [21] |
| 9 |  |
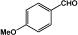 |
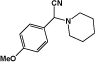 |
90 | 97 | [21] |
| 10 |  |
 |
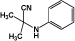 |
45 | 98 | [10] |
aisolated yield
Discussion
A series of α-aminonitriles were synthesized by using diverse amines, aldehydes and TMSCN in the presence of indium metal (10 mol%) as catalyst in water. As shown in Table 4, the reaction proceeded equally well irrespective of the nature of the carbonyl compounds (aliphatic, aromatic, heteroaromatic) or amines (aliphatic, heterocyclic, and aromatic) to afford the corresponding products in excellent yield (79-98%). The catalytic system worked well with acid sensitive heteroaromatic aldehyde (entries 4, 6, 7), α, β unsaturated aldehyde (entry 3), aliphatic aldehyde (entry 5) and ketone (entry 10). Aromatic primary amine (aniline), benzyl amine (entry 6), heterocyclic amines (entries 7, 8 and 9) could effectively undergo Strecker reaction with aldehydes and TMSCN to give the corresponding products in excellent yields (94-97%). For aliphatic amines such as benzyl amine, piperidine and morpholine relatively slower reaction rate was observed.
A plausible mechanism may follow a two-step pathway. In the first step, indium acts as an Lewis acid to facilitate formation of the corresponding imine from the condensation of the amine and aldehyde. In the subsequent step, the imine is further activated due to the presence of indium, to form a more electrophilic C = N intermediate. As a result, an attack of TMSCN to the imine carbon can take place and thus the corresponding α-aminonitriles is formed via hydrolysis in water.
Conclusions
There is growing interest in the one-pot Strecker synthesis of α-aminonitriles from carbonyl compounds, amines and TMSCN, because of the significant importance of α-aminonitriles in preparing a wide variety of amino acids, amides, diamines, and nitrogen containing heterocycles. In summary, we have developed a rapid, convenient and efficient one-pot, three-component environmentally benign Strecker reaction using indium as catalyst at room temperature. A series of α-aminonitriles were obtained in excellent yields. This reaction will be applicable to the synthesis of various organic compounds of medicinal interests.
Methods
General
FT-IR spectra were registered on a Bruker IFS 55 Equinox FTIR spectrophotometer as KBr discs. 1H-NMR (600 MHz) and 13C-NMR (125 MHz) spectra were obtained at room temperature with Bruker-600 equipment using TMS as internal standard and CDCl3 as solvent. Analytical grade chemicals (Sigma-Aldrich Corporation) were used throughout the project. Deionized water was used for the preparation of all aqueous solutions.
General procedure for the one-pot, three-component Strecker reaction
A representative experimental procedure (entry 1) is as follows: In powder (11 mg) was added to a mixture of aniline (1 mmol), benzaldehyde (1 mmol) and TMSCN (1.2 mmol) in water (1 mL). The resulting mixture was stirred at room temperature and the progress of the reaction was monitored by TLC. After completion of the reaction (Table 4) diethyl ether was added and the solution was filtered, washed with brine and water. It was dried over anhydrous sodium sulphate and filtered. A short column of silica gel was used to purify the product 2-phenyl-2-(phenylamino)-acetonitrile in 98% yield.
Competing interests
The authors declare that they have no competing interests.
Contributor Information
Debasish Bandyopadhyay, Email: bandyopad@utpa.edu.
Juliana M Velazquez, Email: julie.velazquez@hotmail.com.
Bimal K Banik, Email: banik@utpa.edu.
Acknowledgements
We gratefully acknowledge the funding support from National Cancer Institute (NIH/NCI-P20, Grant# 5P20CA138022-02).
References
- Strecker A. Ueber die künstliche Bildung der Milchsäure und einen neuen, dem glycocoll homologen. Ann Chem Pharm. 1850;75:27–45. doi: 10.1002/jlac.18500750103. [DOI] [Google Scholar]
- Gröger H. Catalytic enantioselective Strecker reactions and analogous syntheses. Chem Rev. 2003;103:2795–2827. doi: 10.1021/cr020038p. [DOI] [PubMed] [Google Scholar]
- Arasappan A, Venkatraman S, Padilla AI, Wu W, Meng T, Jin Y, Wong J, Prongay A, Girijavallabhan V, Njoroge GF. Practical and efficient method for amino acid derivatives containing β-quaternary center: application toward synthesis of hepatitis C virus NS3 serine protease inhibitors. Tetrahedron Lett. 2007;48:6343–6347. doi: 10.1016/j.tetlet.2007.07.002. [DOI] [Google Scholar]
- Razafindrabe CR, Aubry S, Bourdon B, Andriantsiferana M, Pellet-Rostaing S, Lemaire M. Synthesis of (±)-phthalascidin 650 analogue: new synthetic route to (±)-phthalascidin 622. Tetrahedron. 2010;66:9061–9066. doi: 10.1016/j.tet.2010.08.053. [DOI] [Google Scholar]
- Das BC, Anguiano J, Mahalingam SM. Design and synthesis of α-aminonitrile-functionalized novel retinoids. Tetrahedron Lett. 2009;50:5670–5672. doi: 10.1016/j.tetlet.2009.07.119. [DOI] [Google Scholar]
- Iwanami K, Seo H, Choi J-C, Sakakura T, Yasuda H. Al-MCM-41 catalyzed three-component Strecker-type synthesis of α-aminonitriles. Tetrahedron. 2010;66:1898–1901. doi: 10.1016/j.tet.2010.01.001. [DOI] [Google Scholar]
- Hatano M, Hattori Y, Furuya Y, Ishihara K. Chiral lanthanum(III)-binaphthyldisulfonate complexes for catalytic enantioselective Strecker reaction. Org Lett. 2009;11:2321–2324. doi: 10.1021/ol900680f. [DOI] [PubMed] [Google Scholar]
- Kantam ML, Mahendar K, Sreedhar B, Choudary BM. Synthesis of α-amino nitriles through Strecker reaction of aldimines and ketoimines by using nanocrystalline magnesium oxide. Tetrahedron. 2008;64:3351–3360. doi: 10.1016/j.tet.2008.01.128. [DOI] [Google Scholar]
- Simo'n L, Goodman JM. Mechanism of BINOL-phosphoric acid-catalyzed Strecker beaction of benzyl imines. J Am Chem Soc. 2009;131:4070–4077. doi: 10.1021/ja808715j. [DOI] [PubMed] [Google Scholar]
- Zhang G-W, Zheng D-H, Nie J, Wang T, Ma J-A. Bronsted acid-catalyzed efficient Strecker reaction of ketones, amines and trimethylsilyl cyanide. Org Biomol Chem. 2010;8:1399–1405. doi: 10.1039/b924272d. [DOI] [PubMed] [Google Scholar]
- Khan NH, Agrawal S, Kureshy R, Abdi SHR, Singh S, Suresh E, Jasra RV. Fe(Cp)2PF6 catalyzed efficient Strecker reactions of ketones and aldehydes under solvent-free conditions. Tetrahedron Lett. 2008;49:640–644. doi: 10.1016/j.tetlet.2007.11.136. [DOI] [Google Scholar]
- Pan SC, List B. Catalytic asymmetric three-component acyl-Strecker reaction. Org Lett. 2007;9:1149–1151. doi: 10.1021/ol0702674. [DOI] [PubMed] [Google Scholar]
- Jarusiewicz J, Choe Y, Yoo K, Park CP, Jung KW. Efficient three-component Strecker reaction of aldehydes/ketones via NHC-amidate palladium(II) complex catalysis. J Org Chem. 2009;74:2873–2876. doi: 10.1021/jo900163w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karimi B, Maleki A, Elhamifar D, Clark JH, Hunt AJ. Self-assembled organic-inorganic hybrid silica with ionic liquid framework: a novel support for the catalytic enantioselective Strecker reaction of imines using Yb(OTf)3-pybox catalyst. Chem Commun. 2010;46:6947–6949. doi: 10.1039/c0cc01426e. [DOI] [PubMed] [Google Scholar]
- Karmakar B, Banerji J. K2PdCl4 catalyzed efficient multicomponent synthesis of α-aminonitriles in aqueous media. Tetrahedron Lett. 2010;51:2748–2750. doi: 10.1016/j.tetlet.2010.03.059. [DOI] [Google Scholar]
- Prakash GKS, Mathew T, Panja C, Alconcel S, Vaghoo H, Do C, Olah GA. Gallium (III) triflate catalyzed efficient Strecker reaction of ketones and their fluorinated analogs. Proc Nat Acad Sci USA. 2007;104:3703–3706. doi: 10.1073/pnas.0611316104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wen Y, Xiong Y, Chang L, Huang J, Liu X, Feng X. Chiral bisformamides as effective organocatalysts for the asymmetric one-pot, three-component Strecker reaction. J Org Chem. 2007;72:7715–7719. doi: 10.1021/jo701307f. [DOI] [PubMed] [Google Scholar]
- Fontaine P, Chiaroni A, Masson G, Zhu J. One-pot three-component synthesis of α-iminonitriles by IBX/TBAB-mediated oxidative Strecker reaction. Org Lett. 2008;10:1509–1512. doi: 10.1021/ol800199b. [DOI] [PubMed] [Google Scholar]
- Cruz-Acosta F, Santos-Exposito A, de Armas P, Garcia-Tellado F. Lewis base-catalyzed three-component Strecker reaction on water. An efficient manifold for the direct α-cyanoamination of ketones and aldehydes. Chem Commun. 2009;44:6839–6841. doi: 10.1039/b914151k. [DOI] [PubMed] [Google Scholar]
- Mojtahedi MM, Saeed AM, Alishiri T. Superparamagnetic iron oxide as an efficient catalyst for the one-pot, solvent-free synthesis of α-aminonitriles. Tetrahedron Lett. 2009;50:2322–2325. doi: 10.1016/j.tetlet.2009.02.199. [DOI] [Google Scholar]
- Mojtahedi MM, Abaee MS, Abbasi H. Environmentally friendly room temperature strecker reaction: one-pot synthesis of α-aminonitriles in ionic liquid. J Iran Chem Soc. 2006;3:93–97. [Google Scholar]
- Kato N, Suzuki M, Kanai M, Shibasaki M. Catalytic enantioselective Strecker reaction of ketimines using catalytic amount of TMSCN and stoichiometric amount of HCN. Tetrahedron Lett. 2004;45:3153–3155. doi: 10.1016/j.tetlet.2004.02.077. [DOI] [Google Scholar]
- Gruszecka E, Soroka M, Mastalerz P. Preparation of D,L-phosphinothricin by Strecker reaction. Polish J Chem. 1979;53:937–9. [Google Scholar]
- Reimann E, Dammertz W. Bicyclic α-amino acids. IV: Synthesis of 3-(1-tetralinyl)- and 3-(5,6,7,8-tetrahydro-5-quinolinyl)alanine. Arch Pharm. 1983;316:297–302. doi: 10.1002/ardp.19833160403. [DOI] [Google Scholar]
- Harusawa S, Hamada Y, Shioiri T. New methods and reagents in organic synthesis. 5. Diethyl phosphorocyanidate (DEPC). A novel reagent for the classical Strecker's α-amino nitrile synthesis. Tetrahedron Lett. 1979;48:4663–4666. [Google Scholar]
- Davis FA, Prasad KR, Carroll PJ. Asymmetric synthesis of polyhydroxy α-amino acids with the sulfinimine-mediated asymmetric Strecker reaction: 2-amino 2-deoxy L-xylono-1,5-lactone (Polyoxamic acid lactone) J Org Chem. 2002;67:7802–7806. doi: 10.1021/jo020302e. [DOI] [PubMed] [Google Scholar]
- Kaur P, Pindi S, Wever W, Rajale T, Li G-G. Asymmetric catalytic Strecker reaction of N-phosphonyl imines with Et2AlCN using amino alcohols and BINOLs as catalysts. Chem Commun. 2010;46:4330–4332. doi: 10.1039/c0cc00287a. [DOI] [PubMed] [Google Scholar]
- Xie Z, Li G, Zhao G, Wang J. Strecker-type reaction catalyzed by carboxylic acids in aqueous media. Synthesis. 2009;12:2035–2039. [Google Scholar]
- Ishitani H, Komiyama S, Hasegawa Y, Kobayashi S. Catalytic asymmetric Strecker synthesis. Preparation of enantiomerically pure α-amino acid derivatives from aldimines and tributyltin cyanide or achiral aldehydes, amines, and hydrogen cyanide using a chiral zirconium catalyst. J Am Chem Soc. 2000;122:762–766. doi: 10.1021/ja9935207. [DOI] [Google Scholar]
- Becker FF, Banik BK. Polycyclic aromatic compounds as anticancer agents: synthesis and biological evaluation of some chrysene derivatives. Bioorg Med Chem Lett. 1998;8:, 2877–2880. doi: 10.1016/s0960-894x(98)00520-4. [DOI] [PubMed] [Google Scholar]
- Becker FF, Mukhopadhyay C, Hackfeld L, Banik I, Banik BK. Polycyclic aromatic compounds as anticancer agents: synthesis and biological evaluation of dibenzofluorene derivatives. Bioorg Med Chem. 2000;8:, 2693–2699. doi: 10.1016/s0968-0896(00)00213-3. [DOI] [PubMed] [Google Scholar]
- Banik BK, Becker FF. Polycyclic aromatic compounds as anticancer agents. 4. Structure-activity relationships of chrysene and pyrene derivatives. Bioorg Med Chem. 2001;9:593–605. doi: 10.1016/S0968-0896(00)00297-2. [DOI] [PubMed] [Google Scholar]
- Banik BK, Becker FF. Synthesis, electrophilic substitution and structure-activity relationship studies of polycyclic aromatic compounds towards the development of anticancer agents. Curr Med Chem. 2001;8:1513–1533. doi: 10.2174/0929867013372120. [DOI] [PubMed] [Google Scholar]
- Banik BK, Becker FF, Banik I. Synthesis of anticancer β-lactams: Mechanism of action. Bioorg Med Chem. 2004;12:, 2523–2528. doi: 10.1016/j.bmc.2004.03.033. [DOI] [PubMed] [Google Scholar]
- Banik I, Becker FF, Banik BK. Stereoselective Synthesis of β-Lactams with Polyaromatic Imines: Entry to New and Novel Anticancer Agents. J Med Chem. 2003;46:, 12–15. doi: 10.1021/jm0255825. [DOI] [PubMed] [Google Scholar]
- Li C-J. Organic reactions in aqueous media with a focus on carbon-carbon bond formations. A decade update. Chem Rev. 2005;105:3095–3165. doi: 10.1021/cr030009u. [DOI] [PubMed] [Google Scholar]


