Abstract
Novel 1,1'-(5,5'-(1,4-phenylene)bis(3-aryl-1H-pyrazole-5,1-(4H,5H)-diyl))diethanones 7-12 were tested for their antimicrobial activity by disc diffusion and twofold serial dilution method against the tested bacterial and fungal strains. Compounds 7 against Micrococcus luteus, 8 against β-Heamolytic streptococcus, M. luteus, Klebsiella pneumonia, Microsporum gypseum, 9 against Staphylococcus aureus, Shigella flexneri, Vibreo cholerae, Pseudomonas aeruginosa, Aspergillus flavus, Mucor indicus, 10 against Salmonella typhii, S. flexneri, M. gypseum, 11 against K. pneumonia, M. gypseum, 12 against K. pneumonia, and M. gypseum show superior zone of inhibitions and exhibited excellent antibacterial and antifungal activities at a MIC value of 6.25 μg/mL. Moreover, all the tested compounds 7-12 revealed promising antitubercular activity against Mycobacterium tuberculosis H37Rv and INH-resistant M. tuberculosis. Compounds 8 against M. tuberculosis and 11 against INH-resistant M. tuberculosis exhibited the percentage of reduction in RLU at 89 and 85%, respectively.
Keywords: bisacetylated pyrazoles, in situ acetylation, antibacterial activity, antifungal activity; antitubercular activity
1. Introduction
Mycobacterium tuberculosis (MTB) is a pathogenic bacterial species in the genus Mycobacterium and is the causative agent of most cases of tuberculosis. Tuberculosis is a common and often deadly infectious disease in humans [1,2]. Tuberculosis is the most common opportunistic disease in persons infected with human immunodeficiency virus [3]. The genome of MTB is rich in lipid-metabolizing and P450 enzymes. The cell envelope of MTB is unique and is associated with its pathogenicity [4]. Mycolic acids are the major constituents of the protective barrier of cell envelope of MTB and are essential for survival, virulence, and antibiotic resistance [5]. Inhibitors of mycolic acid biosynthesis, such as isoniazid (INH), ethambutol (EMB), and pyrazinamide (PZA), are still in the frontline of antitubercular drugs [6].
The discovery of the norfloxacin plays an important role in structure-activity relationships analysis of the fluoroquinolonic nucleus A-D, (Scheme 1) which led to the development of new derivatives with better solubility, higher antimicrobial activity, prolonged serum half-life, fewer adverse side effects, and both oral and parenteral routes of administration [7-9]. Naturally occurring bacterial DNA gyrase inhibitor such as novobiocin, a coumarin derivative E (Scheme 1), is known as antibacterial agents [10]. The coumarin drug inhibits ATPase activity of DNA gyrase by competing with ATP for binding to the subunit B of the enzyme. Owing to side effects, no pharmaceutically useful drug has been derived from the coumarins [11]. Although huge efforts have been dedicated to find a potent antibacterial agents that can overcome bacterial resistance, promising lead structures of DNA gyrase and topoisomerase IV enzyme inhibitors with novel mechanisms of action have not been found [12]. This reflects the inherent difficulties associated with the discovery and clinical testing of new candidates and the lack of significant pharmaceutical industry research in this area. Hence, the discovery and development of new drugs that effectively combat TB are accorded a great importance. In recent years, interest in pyrazoles has increased significantly because of their proven usefulness as intermediates in the preparation of new pharmaceuticals and agrochemicals [13-15]. Also, pyrazole derivatives F and G (Scheme 1) were identified as a new class of DNA gyrase and topoisomerase IV enzyme inhibitors [16]. Besides these, amides are well known for their therapeutic values since the amide group is an important pharmacophore. Antibiotics such as penicillins and cephalosporins have an amide group. The resistance toward available drugs is rapidly becoming a major worldwide problem. The necessity to design new compounds to overcome this resistance has become one of the most important areas of research today. Owing to our interest in synthesizing fascinating biologically active structurally diverse heterocycles [17-20], we recently reported the clean production of 1,1'-(5,5'-(1,4-phenylene)bis(3-aryl-1H-pyrazole-5,1-(4H,5H)-diyl))diethanones, a novel series of bis pyrazole derivatives using sodium acetate/acetic anhydride triggered by ultrasound irradiation [21], which accelerated the chemical reaction and mass transferred via the process of acoustic cavitation [22]. Extending the research in this area, we decided to investigate the antibacterial, antifungal, and antitubercular activities of the target compounds with the hope to develop some promising antimicrobial and antimycobacterial agents.
Scheme 1.
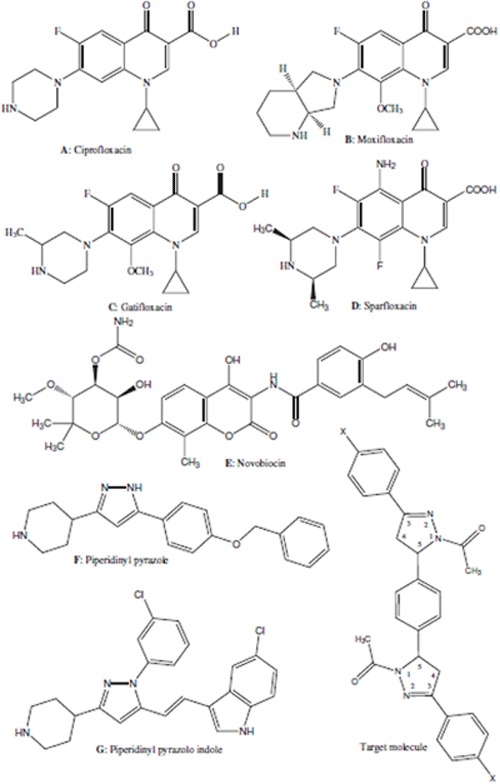
Structure of novel antitubercular agents.
2. Experimental
2.1 Chemistry
Performing TLC assessed the reactions and the purity of the products. All the reported melting points are taken in open capillaries and were uncorrected. Sonication is performed on a Life Care-Fast Ultrasonic system (Life Care Equipments Pvt. Ltd., Mumbai, India) operating at a frequency of 45 kHz. The reaction flask is located in the maximum energy area in the bath and the addition or removal of water controlled the temperature of the water bath. IR spectra are recorded in KBr (pellet forms) on a Thermo Nicolet-Avatar-330 FT-IR spectrophotometer (Thermo Fisher Scientific Inc., Waltham, MA, US) and note worthy absorption values (cm-1) alone are listed. 1H and 13C NMR spectra are recorded at 400 and 100 MHz, respectively, on Bruker AMX 400 NMR spectrometer (Bruker Biospin International, Ag, Aegeristrasse, Switzerland) using CDCl3 as solvent. The ESI +ve MS spectra are recorded on a Varian Saturn 2200 MS spectrometer (Varian Inc., Palo Alto, USA). Satisfactory microanalyses are obtained on Carlo Erba 1106 CHN analyzer (Thermo Fisher Scientific Inc., Waltham, MA, US). By adopting the literature precedent, bis chalcones 1-6 [23] and 1,1'-(5,5'-(1,4-phenylene)bis(3-aryl-1H-pyrazole-5,1-(4H, 5H)-diyl))diethanones 7-12 [21] are prepared.
2.2. Microbiology
All the clinically isolated bacterial strains namely Staphylococcus aureus, β-Heamolytic streptococcus, Micrococcus luteus, Bacillus subtilis, Salmonella typhii, Shigella flexneri, Vibreo cholerae, Escherichia coli, Pseudomonas aeruginosa, Klebsiella pneumonia, MTB H37Rv, INH-resistant MTB and fungal strains namely Aspergillus flavus, Aspergillus niger, Mucor indicus, Rhizopus arrhizus, and Microsporum gypsuem are obtained from the Faculty of Medicine, Annamalai University, Annamalainagar 608 002, Tamil Nadu, India.
2.3. In vitro antibacterial and antifungal activity by disc diffusion method
The in vitro activities of the compounds were tested in Sabourauds dextrose broth (SDB) (Hi-media, Mumbai) for fungi and nutrient broth (NB) (Hi-media, Mumbai) for bacteria by the disc diffusion method following the reported method [24]. The respective hydrochlorides of the test compounds 7-12 were dissolved in water to obtain 1 mg/mL stock solution and the different concentrations (100, 200, 500 ppm) were prepared from the stock solution. Seeded broth (broth-containing microbial spores) was prepared in NB from 24-h-old bacterial cultures on nutrient agar (Hi-media, Mumbai) at 37 ± 1°C while fungal spores from 1 to 7-day-old Sabourauds agar (Hi-media, Mumbai) slant cultures were suspended in SDB. Sterile paper disc of 5-mm diameter was saturated with the three different concentrations and such discs were placed in each seeded agar plates. The petri plates were incubated in BOD incubator (Sigma Instruments, Chennai, India) at 37°C for bacteria and at 28°C for fungi. The zone of inhibition was recorded by visual observations after 24 h of inhibition for bacteria and after 72-96 h of inhibition for fungi. Moreover, the zone of inhibition was measured by excluding the diameter of the paper disc. Ciprofloxacin was used as standard for bacteria and fluconazole as standard for fungi under analogous conditions.
2.4. In vitro antibacterial and antifungal activity by twofold serial dilution method
MIC in μg/mL values was carried out by twofold serial dilution method [25]. The respective test compounds 7-12 were dissolved in dimethyl sulphoxide (DMSO) to obtain 1 mg/mL stock solution. Seeded broth (broth-containing microbial spores) was prepared in NB from 24-h-old bacterial cultures on nutrient agar (Hi-media, Mumbai) at 37 ± 1°C while fungal spores from 1 to 7-day-old Sabourauds agar (Hi-media, Mumbai) slant cultures were suspended in SDB. The colony forming units (cfu) of the seeded broth were determined by plating technique and adjusted in the range of 104-105 cfu/mL. The final inoculums size was 105 cfu/mL for antibacterial assay and 1.1-1.5 × 102 cfu/mL for antifungal assay. Testing was performed at pH 7.4 ± 0.2 for bacteria (NB) and at a pH 5.6 for fungi (SDB). Exactly 0.4 mL of the solution of test compound was added to 1.6 mL of seeded broth to form the first dilution. One milliliter of this was diluted with a further 1 mL of seeded broth to give the second dilution and so on till six such dilutions were obtained. A set of assay tubes containing only seeded broth was kept as control. The tubes were incubated in BOD incubators at 37 ± 1°C for bacteria and 28 ± 1°C for fungi. MICs were recorded by visual observations after 24 h (for bacteria) and 72-96 h (for fungi) of incubation. Ciprofloxacin was used as standard for bacteria studies and fluconazole was used as standard for fungal studies.
2.5. In vitro antitubercular activity by luciferase reporter phage assay method
The preliminary antitubercular activity screening was conducted against M. tuberculosis H37Rv, INH-resistant M. tuberculosis by luciferase reporter phage assay method [26] at two different concentrations (1.00 and 2.00 mg/mL). Fifty microliter bacterial suspension equivalent to MacFarlands No. 2 standard was added to 400 mL of G7H9 with and without the test compound. For each sample, two drug-free controls and two drug concentrations were prepared and this setup was incubated for 72 h at 37°C. After incubation, 50 mL of the high titer Luciferase reporter phage (PhAE129) and 40 mL of 0.1 M CaCl2 were added to all the vials and this setup was incubated at 37°C for 4 h. After incubation, 100 mL of the mixture was taken from each tube into a luminometer cuvette and equal amount of working D-Luciferin (0.3 mM in 0.05 M sodium citrate buffer, pH 4.5) solution was added. The RLU was measured after 10 s of integration in the Luminometer (Monolight 2010, Pegasus Scientific Inc., Rockvillae, USA). Duplicate readings were recorded for each sample and the mean was calculated. The percentage reduction in the RLU was calculated for each test sample and compared with control. The experiment was repeated when the mean RLU of the control was less than 1,000.
3. Results and discussion
3.1. Chemistry
Synthesis of 1,1'-(5,5'-(1,4-phenylene)bis(3-aryl-1H-pyrazole-5,1-(4H,5H)-diyl))diethanones 7-12 is carried out in excellent yields (Scheme 2 and Table 1) by the reaction of bis chalcones 1-6 with hydrazine hydrate catalyzed by anhydrous sodium acetate/acetic anhydride under ultrasonic irradiation method at 45°C within 10-20 min. It has been observed in the traditional classical method, the reaction mixture of bis chalcones 1-6 with hydrazine hydrate catalyzed by anhydrous sodium acetate in refluxing acetic anhydride for 5-8 h yield compounds 7-12 in moderate yields. However, when this reaction is performed under sonication method [27], the reaction takes place rapidly within 10-20 min with excellent yields (Table 1). In this study, acetic anhydride is the best solvent for the facile synthesis of bis pyrazoles, 7-12 in excellent yields without any solubility problem. In addition, in situ acetylation occurs in the course of the reaction because of solvent, acetic anhydride under the reaction conditions. The structures of the synthesized 1,1'-(5,5'-(1,4-phenylene)bis(3-aryl-1H-pyrazole-5,1-(4H,5H)-diyl))diethanones 7-12 are confirmed by FT-IR, MS, 1H NMR, and 13C NMR spectral studies and elemental analysis [21].
Table 1.
Physical and analytical data of compounds 7-12
| Compounds | X | Time Δ (h)/sonication (min) | Yield (%)Δ/sonication | m.p. (°C) | Elemental analysis (%) | m/z (M)+.molecular formula | ||
|---|---|---|---|---|---|---|---|---|
| C Found (calculated) | H Found (calculated) | N Found (calculated) | ||||||
| 7 | H | 7/15 | 65/95 | 261 | 74.55 (74.65) | 5.69 (5.82) | 12.31 (12.44) | 450 C28H26N4O2 |
| 8 | F | 7/15 | 70/94 | 233 | 69.02 (69.12) | 4.77 (4.97) | 11.41 (11.52) | 486 C28H24F2N4O2 |
| 9 | Cl | 8/20 | 55/88 | 260 | 64.52 (64.74) | 4.52 (4.66) | 10.66 (10.79) | 518, 520 C28H24Cl2N4O2 |
| 10 | Br | 7/15 | 60/95 | 262 | 55.13 (55.28) | 3.82 (3.98) | 9.11 (9.21) | 606, 608 C28H24Br2N4O2 |
| 11 | CH3 | 5/10 | 65/98 | 258 | 75.13 (75.29) | 6.22 (6.32) | 11.60 (11.71) | 478 C30H30N4O2 |
| 12 | OCH3 | 5/10 | 65/95 | 202 | 70.43 (70.57) | 5.86 (5.92) | 10.85 (10.97) | 510 C30H30N4O4 |
Scheme 2.
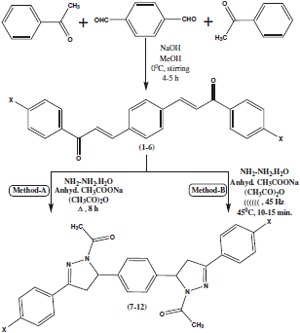
Synthesis of 1,1'-(5,5'-(1,4-phenylene)bis(3-aryl-1H-pyrazole-5,1-(4H,5H)-diyl))diethanones under thermal and sonication methods using anhydrous sodium acetate/acetic anhydride.
3.2. Antimicrobial activity of 1,1'-(5,5'-(1,4-phenylene)bis(3-aryl-1H-pyrazole-5,1-(4H, 5H)-diyl))diethanones by disc diffusion method 7-12
An array of biolabile 1,1'-(5,5'-(1,4-phenylene)bis(3-aryl-1H-pyrazole-5,1-(4H,5H)-diyl))diethanones 7-12 is tested for its antimicrobial activity by disc diffusion method against tested bacterial and fungal strains and the results are presented in Table 2. The use of 1,1'-(5,5'-(1,4-phenylene)bis(3-phenyl-1H-pyrazole-5,1-(4H,5H)-diyl))diethanone 7 shows good zone of inhibitions against M. luteus. Excellent zone of inhibitions is noted against S. aureus, β-H. streptococcus, M. luteus, B. subtilis, V. cholerae, E. coli, K. pneumonia, A. niger, and M. gypseum by compound 8 which has electron withdrawing fluoro substituent at the para position of the phenyl ring. The usage of compound 9 which have electron withdrawing chloro substituent at the para position of the phenyl ring exhibits good zone of inhibitions against all the tested microorganisms except S. typhii, E. coli, and M. gypseum. Compound 10, which have electron withdrawing bromo substituent at the para position of the phenyl ring exhibits fine zone of inhibitions against all the tested bacterial strains except K. pneumonia. Excellent zone of inhibition is noticed by compound 10 against M. gypseum. The use of compound 11 which have electron-donating methyl substituent at the para position of the phenyl ring exhibits superior zone of inhibitions against S. flexneri, K. pneumonia, R. arrhizus, and M. gypseum. Also, the use of compound 12 which have electron donating methoxy substituent at the para position of the phenyl ring exerts higher zone of inhibitions against K. pneumonia, A. niger, and M. gypseum.
Table 2.
In vitro antibacterial and antifungal activities of compounds 7-12 by disc diffusion method
| Microorganisms | Compound 7 (ppm) | Compound 8 (ppm) | Compound 9 (ppm) | Compound 10 (ppm) | Compound 11 (ppm) | Compound 12 (ppm) | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 100 | 200 | 500 | 100 | 200 | 500 | 100 | 200 | 500 | 100 | 200 | 500 | 100 | 200 | 500 | 100 | 200 | 500 | |
| Staphylococcus aureus | ++ | +++ | +++ | - | ++ | +++ | ++ | ++++ | ++++ | ++ | +++ | +++ | + | ++ | ++ | - | ++ | +++ |
| β-Heamolytic streptococcus | ++ | ++ | +++ | ++ | +++ | ++++ | ++ | +++ | +++ | ++ | +++ | +++ | ++ | +++ | +++ | ++ | ++ | ++ |
| Micrococcus luteus | ++ | +++ | ++++ | ++ | +++ | ++++ | ++ | +++ | +++ | ++ | ++ | +++ | - | ++ | ++ | ++ | ++ | ++ |
| Bacillus subtilis | ++ | +++ | +++ | ++ | ++++ | ++++ | ++ | +++ | +++ | - | ++ | +++ | - | ++ | +++ | - | ++ | ++ |
| Salmonella typhii | ++ | ++ | ++ | ++ | +++ | +++ | ++ | +++ | ++++ | ++ | +++ | ++++ | - | ++ | +++ | - | ++ | +++ |
| Shigella flexneri | - | ++ | ++ | ++ | ++ | ++ | ++ | ++++ | ++++ | ++ | +++ | ++++ | ++ | ++ | ++ | - | ++ | +++ |
| Vibreo cholerae | - | ++ | ++ | - | +++ | +++ | ++ | ++++ | ++++ | ++ | +++ | ++++ | - | ++ | +++ | ++ | ++ | +++ |
| Escherichia coli | ++ | ++ | +++ | ++ | +++ | +++ | - | +++ | +++ | ++ | ++ | +++ | ++ | ++ | +++ | ++ | ++ | ++ |
| Pseudomonas aeruginosa | ++ | ++ | ++ | ++ | +++ | +++ | ++ | +++ | ++++ | ++ | ++ | +++ | ++ | ++ | +++ | + | ++ | ++ |
| Klebsiella pneumonia | ++ | ++ | +++ | +++ | +++ | ++++ | ++ | +++ | ++++ | ++ | ++ | ++ | ++ | +++ | ++++ | ++ | ++++ | ++++ |
| Aspergillus flavus | - | ++ | +++ | ++ | +++ | +++ | ++ | +++ | ++++ | ++ | ++ | ++ | ++ | ++ | +++ | - | ++ | ++ |
| Aspergillus niger | + | ++ | ++ | ++ | +++ | +++ | ++ | +++ | +++ | + | + | ++ | ++ | ++ | +++ | ++ | +++ | ++++ |
| Mucor indicus | - | ++ | ++ | + | ++ | ++ | ++ | +++ | ++++ | ++ | ++ | +++ | ++ | ++ | +++ | + | ++ | ++ |
| Rhizopus arrhizus | - | ++ | ++ | + | ++ | ++ | ++ | ++ | +++ | ++ | ++ | ++ | ++ | ++ | +++ | ++ | ++ | +++ |
| Microsporum gypseum | ++ | ++ | +++ | ++ | +++ | ++++ | ++ | ++ | ++ | ++ | +++ | ++++ | ++ | +++ | ++++ | ++ | +++ | ++++ |
(-) = inactive, (+) = weakly active(12-16 mm), (+)(+) = moderately active(17-21 mm), (+)(+)(+) = strong active(22-29 mm), (+)(+)(+)(+) = highly active(30-33 mm). At 500 ppm concentration, standard antibacterial drug, ciprofloxacin exhibits 30 ± 0.5 mm zone of inhibition against all the test bacteria and standard antifungal drug, fluconazole exhibits 20 ± 0.5 mm zone of inhibition against all the test fungi.
3.3. Antimicrobial activity of 1,1'-(5,5'-(1,4-phenylene)bis(3-aryl-1H-pyrazole-5,1-(4H, 5H)-diyl))diethanones by twofold serial dilution method 7-12
In vitro antimicrobial results by the twofold serial dilution method (Table 3) of 1,1'-(5,5'-(1,4-phenylene)bis(3-aryl-1H-pyrazole-5,1-(4H,5H)-diyl))diethanones 7-12 show that compound 7 exhibits good activities against M. luteus at a MIC value of 6.25 μg/mL. Admirable activities against β-H. streptococcus, M. luteus, K. pneumonia, and M. gypseum are displayed by compound 8 at a MIC value of 6.25 μg/mL, whereas it displays modest activities against S. aureus and B. subtilis at a MIC value of 12.5 μg/mL. The use of compound 9 displays higher activities against S. aureus, S. flexneri, V. cholerae, P. aeruginosa, A. flavus, and M. indicus at a MIC value of 6.25 μg/mL. Excellent antimicrobial activities are exhibited by compound 10 against S. typhii, S. flexneri, and M. gypseum at a MIC value of 6.25 μg/mL, whereas it exhibits superior activities against V. cholerae, E. coli, and P. aeruginosa at a MIC value of 12.5 μg/mL. The use of compound 11, which has electron donating methyl group at the para position of the phenyl ring, exhibits greater activities against K. pneumonia and M. gypseum at a MIC value of 6.25 μg/mL. Modest activities are displayed by compound 12 against A. niger at a MIC value of 12.5 μg/mL, whereas it exhibits strong activities against K. pneumonia and M. gypseum at a MIC value of 6.25 μg/mL.
Table 3.
In vitro antibacterial and antifungal activities of compounds 7-12 by twofold serial dilution method
| Microorganisms | Minimum inhibitory concentration (MIC) (μg/mL) | |||||||
|---|---|---|---|---|---|---|---|---|
| 7 | 8 | 9 | 10 | 11 | 12 | Ciprofloxacin | Fluconazole | |
| Staphylococcus aureus | 50 | 12.5 | 6.25 | 25 | 200 | 100 | 25 | - |
| β-Heamolytic streptococcus | 50 | 6.25 | 25 | 25 | 100 | 200 | 25 | - |
| Micrococcus luteus | 6.25 | 6.25 | 25 | 25 | 200 | 200 | 12.5 | - |
| Bacillus subtilis | 50 | 12.5 | 25 | 25 | 200 | 200 | 12.5 | - |
| Salmonella typhii | 100 | 50 | 50 | 6.25 | 100 | 200 | 25 | - |
| Shigella flexneri | 200 | 100 | 6.25 | 6.25 | 25 | 100 | 12.5 | - |
| Vibreo cholerae | 100 | 25 | 6.25 | 12.5 | 50 | 100 | 25 | - |
| Escherichia coli | 50 | 25 | 50 | 12.5 | 50 | 100 | 25 | - |
| Pseudomonas aeruginosa | 25 | 100 | 6.25 | 12.5 | 100 | 50 | 25 | - |
| Klebsiella pneumonia | 25 | 6.25 | 25 | 200 | 6.25 | 6.25 | 12.5 | - |
| Aspergillus flavus | 50 | 50 | 6.25 | 200 | 50 | 100 | - | 12.5 |
| Aspergillus niger | 200 | 25 | 25 | 100 | 50 | 12.5 | - | 12.5 |
| Mucor indicus | 100 | 100 | 6.25 | 100 | 100 | 200 | - | 25 |
| Rhizopus arrhizus | 100 | 100 | 25 | 100 | 25 | 100 | - | 25 |
| Microsporum gypseum | 50 | 6.25 | 200 | 6.25 | 6.25 | 6.25 | - | 12.5 |
3.4. Antitubercular activity of 1,1'-(5,5'-(1,4-phenylene)bis(3-aryl-1H-pyrazole-5, 1-(4H, 5H)-diyl))diethanones by luciferase reporter phage assay method 7-12
In vitro antitubercular activity screening was evaluated against M. tuberculosis H37Rv, INH-resistant M. tuberculosis by luciferase reporter phage assay method at two different concentrations (1.00 and 2.00 mg/mL). The observed percentage inhibitions are summarized in Table 4. A compound is considered to possess antimycobacterial activity if 50% reduction in the relative light units (RLU) is observed when compared to the control using a luminometer. In vitro antitubercular activity results of 7-12 show that all the synthesized compounds exhibited good activity against the tested two M. tuberculosis bacterial strains, namely, M. tuberculosis H37Rv and INH-resistant M. tuberculosis. It is observed from Table 4 that the activity of compounds get increased as the concentration of compound increases from 1.00 to 2.00 μg/mL. The percentage of reduction in RLU for the synthesized compounds is in the range of 74-88% against the tested bacterial strain M. tuberculosis H37Rv and 73-85% against the tested bacterial strain INH-resistant M. tuberculosis. Among the synthesized compounds, compound 8 exhibited excellent antitubercular activity against M. tuberculosis H37Rv and the percentage of reduction in RLU for 8 is 89%. Similarly, compound 11 exhibited excellent antitubercular activity against INH-resistant M. tuberculosis and the percentage of reduction in RLU for fluorine-substituted compound 8 is 85%. Also, fluorine substitution is commonly used in contemporary medicinal chemistry to improve metabolic stability, bioavailability, and protein-ligand interactions [28-30].
Table 4.
In vitro antitubercular activity of compounds 7-12 by luciferase reporter phage assay method
| Compounds | M. Tuberculosis H37Rv | INH resistant M. tuberculosis | ||
|---|---|---|---|---|
| 1.00 (μg/mL) | 2.00 (μg/mL) | 1.00 (μg/mL) | 2.00 (μg/mL) | |
| 7 | 74 | 78 | 73 | 77 |
| 8 | 85 | 89 | 79 | 82 |
| 9 | 82 | 85 | 79 | 82 |
| 10 | 79 | 81 | 78 | 80 |
| 11 | 81 | 83 | 82 | 85 |
| 12 | 82 | 84 | 80 | 84 |
| Isoniazid | 99 | 99 | 90 | 96 |
4. Conclusion
A clean, efficient, convenient, and economical synthesis of 1,1'-(5,5'-(1,4-phenylene)bis(3-aryl-1H-pyrazole-5,1-(4H,5H)-diyl))diethanones using ultrasound irradiation is described. The microbiological screening studies carried out to evaluate the antibacterial and antifungal potencies of the synthesized 1,1'-(5,5'-(1,4-phenylene)bis(3-aryl-1H-pyrazole-5,1-(4H,5H)-diyl))diethanones 7-12 were clearly known from Tables 2 and 3. In vitro antibacterial and antifungal activities profile of substituted aromatics (X = F, Cl, Br) are more active than non-substituted aromatic ring system (X = H) of novel target compounds exerted strong antibacterial and antifungal activity against all the tested bacterial strains. Among all the tested compounds, electron withdrawing-substituted compounds 8, 9, and 10 exerted moderate antimicrobial activity and the range of MIC values of 8-10 are 200-6.25 μg/mL. Among the synthesized compounds, compound 8 against M. tuberculosis and compound 11 against INH-resistant M. tuberculosis exhibited the percentage of reduction in RLU at 89 and 85%, respectively. Further development of this group of 1,1'-(5,5'-(1,4-phenylene)bis(3-aryl-1H-pyrazole-5,1-(4H,5H)-diyl))diethanones may lead to compounds with better pharmacological profile than standard antibacterial, antifungal, and antitubercular drugs that are under progress.
Competing interests
The authors declare that they have no competing interests.
Contributor Information
Vijayakumar Kanagarajan, Email: drkanagarajan@gmail.com.
Muthuvel Ramanathan Ezhilarasi, Email: maxichem@yahoo.co.in.
Mannathusamy Gopalakrishnan, Email: profmgk@yahoo.co.in.
Acknowledgements
The authors wish to thank the NMR Research Centre, Indian Institute of Science, Bangalore, India, for recording spectra. V. Kanagarajan is grateful to the Council of Scientific and Industrial Research (CSIR), New Delhi, India, for providing financial support in the form of CSIR-Senior Research Fellowship (SRF) in Organic Chemistry. M. R. Ezhilarasi is thankful to Cavin Kare Research Centre, Chennai, for providing financial support in the form of Junior Research Fellowship.
References
- Kumar V, Abbas AK, Fausto N, Mitchell RN. Robbins basic pathology. 8. ednSaunders Elsevier; 2007. pp. 516–522. [Google Scholar]
- Ryan KJ, Ray CG. Sherris medical microbiology. 4. McGraw Hill; 2004. [Google Scholar]
- Pereira M, Tripathy S, Inamdar V, Ramesh K, Bhavsar M, Date A, Iyyer R, Acchammachary A, Mehendale S, Risbud A. Drug resistance pattern of Mycobacterium tuberculosis in seropositive and seronegative HIV-TB patients in Pune, India. Indian J Med Res. 2005;121:235–239. [PubMed] [Google Scholar]
- Takayama K, Wang C, Besra GS. Pathway to synthesis and processing of mycolic acids in Mycobacterium tuberculosis. Clin Microbiol Rev. 2005;18:81–101. doi: 10.1128/CMR.18.1.81-101.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barry CE, Lee RE, Mdluli K, Sampson AE, Schroeder BG, Slayden RA, Yuan Y. Mycolic acids: structure, biosynthesis and physiological functions. Progress Lipid Res. 1998;37:143–179. doi: 10.1016/S0163-7827(98)00008-3. [DOI] [PubMed] [Google Scholar]
- Zhang M, Yue J, Yang YP, Zhang HM, Lei JQ, Jin RL, Zhang XL, Wang XX. Detection of mutations associated with isoniazid resistance in Mycobacterium tuberculosis isolates from China. J Clin Microbiol. 2005;43:5477–5482. doi: 10.1128/JCM.43.11.5477-5482.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Souza MVN. New fluoroquinolones: a class of potent antibiotics. Mini-Rev Med Chem. 2005;5:1009–1017. doi: 10.2174/138955705774575246. [DOI] [PubMed] [Google Scholar]
- Anquetin G, Greiner J, Mahmoud N, Gozalbes R, Farhati K, Derouin F, Aubry A, Cambau E, Vierling P. Design, synthesis and activity against Toxoplasma gondii, Plasmodium spp., and Mycobacterium tuberculosis of new 6-fluoroquinolones. Eur J Med Chem. 2006;41:1478–1493. doi: 10.1016/j.ejmech.2006.07.003. [DOI] [PubMed] [Google Scholar]
- De Souza MVN, Vasconcelos TA, Cardoso SH, De Almeida MV. Fluoroquinolones: an important class of antibiotics against tuberculosis. Curr Med Chem. 2006;13:455–463. doi: 10.2174/092986706775527965. [DOI] [PubMed] [Google Scholar]
- Kim OK, Ohemeng KA. Patents on DNA gyrase inhibitors: January 1995 to March 1998. Expert Opin Ther Pat. 1998;8:959–969. doi: 10.1517/13543776.8.8.959. [DOI] [Google Scholar]
- Maxwell A. The interaction between coumarin drugs and DNA Gyrase. Mol Microbiol. 1993;9:681–686. doi: 10.1111/j.1365-2958.1993.tb01728.x. [DOI] [PubMed] [Google Scholar]
- Boehm H, Boehringer M, Bur D, Gmuender H, Hunber W, Klaus W, Kostrewa D, Kuehne H, Luebbers T, Meunier-Keller N, Mueller F. Novel inhibitors of DNA gyrase: 3D structure based biased needle screening, hit validation by biophysical methods, and 3D guided optimization. A promising alternative to random screening. J Med Chem. 2000;43:2664–2674. doi: 10.1021/jm000017s. [DOI] [PubMed] [Google Scholar]
- Kim M, Sim C, Shin D, Suh E, Cho K. Residual and sublethal effects of fenpyroximate and pyridaben on the instantaneous rate of increase of Tetranychus urticae. Crop Prot. 2006;25:542–548. doi: 10.1016/j.cropro.2005.08.010. [DOI] [Google Scholar]
- Fustero S, Roman R, Sanz-Cervera JF, Simon-Fuentes A, Bueno J, Villanova S. Synthesis of new fluorinated tebufenpyrad analogs with acaricidal activity through regioselective pyrazole formation. J Org Chem. 2008;73:8545–8552. doi: 10.1021/jo801729p. [DOI] [PubMed] [Google Scholar]
- Fustero S, Roman R, Sanz-Cervera JF, Simon-Fuentes A, Cunat AC, Villanova S, Murguia M. Improved regioselectivity in pyrazole formation through the use of fluorinated alcohols as solvents: synthesis and biological activity of fluorinated tebufenpyrad analogs. J Org Chem. 2008;73:3523–3529. doi: 10.1021/jo800251g. [DOI] [PubMed] [Google Scholar]
- Tanitame A, Oyamada Y, Ofuji K, Fujimoto M, Iwai N, Hiyama Y, Suzuki Ito H, Terauchi H, Kawasaki M, Nagai K, Wachi M, Yamagishi J. Synthesis and antibacterial activity of a novel series of potent DNA gyrase inhibitors. Pyrazole derivatives J Med Chem. 2004;47:3693–3696. doi: 10.1021/jm030394f. [DOI] [PubMed] [Google Scholar]
- Thanusu J, Kanagarajan V, Gopalakrishnan M. Synthesis, spectral characterization, in vitro antibacterial and antifungal activities of novel 1,3-thiazine-2-amines comprising morpholino nucleus. J Enzyme Inhib Med Chem. 2010;25:756–764. doi: 10.3109/14756360903389898. [DOI] [PubMed] [Google Scholar]
- Thanusu J, Kanagarajan V, Nagini S, Gopalakrishnan M. Chemopreventive potential of 3-[2,6-bis(4-fluorophenyl)-3-methylpiperidin-4-ylideneamino]-2-thioxoimidazolidin-4-one on 7,12-dimethylbenz[a]anthracene (DMBA) induced hamster buccal pouch carcinogenesis. J Enzyme Inhib Med Chem. 2010;25:836–843. doi: 10.3109/14756361003724786. [DOI] [PubMed] [Google Scholar]
- Kanagarajan V, Thanusu J, Gopalakrishnan M. Synthesis and in vitro microbiological evaluation of an array of biolabile 2-morpholino-N-(4,6-diarylpyrimidin-2-yl)acetamides. Eur J Med Chem. 2010;45:1583–1589. doi: 10.1016/j.ejmech.2009.12.068. [DOI] [PubMed] [Google Scholar]
- Gopalakrishnan M, Thanusu J, Kanagarajan V, Govindaraju R. Synthesis, antibacterial and antifungal activities of biolabile (E)-1-4-morpholinophenyl)-3-aryl-prop-2-en-1-ones. Med Chem Res. 2009;18:341–350. doi: 10.1007/s00044-008-9131-2. [DOI] [PubMed] [Google Scholar]
- Kanagarajan V, Ezhilarasi MR, Gopalakrishnan M. 'One-pot' ultrasound irradiation promoted synthesis and spectral characterization of an array of novel 1,1'-(5,5'-(1,4-phenylene)bis(3-aryl-1H-pyrazole-5,1(4H,5H)-diyl))diethanones a bis acetylated pyrazoles derivatives. Spectrochem Acta A Mol Biomol Spec. 2011;78:635–639. doi: 10.1016/j.saa.2010.11.038. [DOI] [PubMed] [Google Scholar]
- Mason TJ. Practical sonochemistry. Ellis Harwood Limited, New York; 1991. [Google Scholar]
- Guthrie W, Wang XP. The aldol condensation of acetophenone with acetone. Can J Chem. 1991;69:339–344. doi: 10.1139/v91-052. [DOI] [Google Scholar]
- Maruzella JC, Percival AH. Antibacterial activity of essential oil vapors. J Am Pharm Assoc. 1958;47:471–473. doi: 10.1002/jps.3030470421. [DOI] [PubMed] [Google Scholar]
- Dhar MH, Dhawan BN, Mehrotra BN, Ray C. Screening of Indian plants biological activity. Part I Indian J Exp Biol. 1968;6:232–247. [PubMed] [Google Scholar]
- Sivakumar PM, Prabu Seenivasan S, Vanaja Kumar, Doble M. Synthesis, antimycobacterial activity evaluation and QSAR studies of chalcone derivatives. Bioorg Med Chem Lett. 2007;17:1695–1700. doi: 10.1016/j.bmcl.2006.12.112. [DOI] [PubMed] [Google Scholar]
- Mason TJ, Lorimer JP. Applied sonochemistry: the uses of power ultrasound in chemistry and processing. Wiley-VCH, Weinheim; 2002. [Google Scholar]
- Begue JP, Bonnet-Delpon D, (eds) Bioorganic and medicinal chemistry of fluorine. Wiley, Hoboken, NJ; 2008. [Google Scholar]
- Lipinski CA, Lombardo F, Dominy BW, Feeney PJ. Experimental and computational approaches to estimate solubility and permeability in drug discovery and development settings. Adv Drug Deliv Rev. 1997;23:3–25. doi: 10.1016/S0169-409X(96)00423-1. [DOI] [PubMed] [Google Scholar]
- Purser S, Moore PR, Swallow S, Gouverneur V. Fluorine in medicinal chemistry. Chem Soc Rev. 2008;37:320–330. doi: 10.1039/b610213c. [DOI] [PubMed] [Google Scholar]


