Abstract
Background
Acute Respiratory Distress Syndrome (ARDS) patients require mechanical ventilation (MV) for breathing support. Patient-specific PEEP is encouraged for treating different patients but there is no well established method in optimal PEEP selection.
Methods
A study of 10 patients diagnosed with ALI/ARDS whom underwent recruitment manoeuvre is carried out. Airway pressure and flow data are used to identify patient-specific constant lung elastance (Elung) and time-variant dynamic lung elastance (Edrs) at each PEEP level (increments of 5cmH2O), for a single compartment linear lung model using integral-based methods. Optimal PEEP is estimated using Elung versus PEEP, Edrs-Pressure curve and Edrs Area at minimum elastance (maximum compliance) and the inflection of the curves (diminishing return). Results are compared to clinically selected PEEP values. The trials and use of the data were approved by the New Zealand South Island Regional Ethics Committee.
Results
Median absolute percentage fitting error to the data when estimating time-variant Edrs is 0.9% (IQR = 0.5-2.4) and 5.6% [IQR: 1.8-11.3] when estimating constant Elung. Both Elung and Edrs decrease with PEEP to a minimum, before rising, and indicating potential over-inflation. Median Edrs over all patients across all PEEP values was 32.2 cmH2O/l [IQR: 26.1-46.6], reflecting the heterogeneity of ALI/ARDS patients, and their response to PEEP, that complicates standard approaches to PEEP selection. All Edrs-Pressure curves have a clear inflection point before minimum Edrs, making PEEP selection straightforward. Model-based selected PEEP using the proposed metrics were higher than clinically selected values in 7/10 cases.
Conclusion
Continuous monitoring of the patient-specific Elung and Edrs and minimally invasive PEEP titration provide a unique, patient-specific and physiologically relevant metric to optimize PEEP selection with minimal disruption of MV therapy.
Keywords: ARDS, ALI, Elastance, Compliance, PEEP, Critical care, Mechanical Ventilation
1 Introductions
Acute respiratory distress syndrome (ARDS) and acute lung injury (ALI), occurs due to severe inflammatory response of the lung, resulting in direct alveolar injury, pulmonary oedema and alveolar collapse [1,2]. The lung injury greatly impairs the patients breathing, reducing alveolar gas exchange, resulting in possible mortality and morbidity if not given a proper treatment. ALI/ARDS patients are associated with high morbidity, mortality up to 60% [3] and significant medical cost [4].
Patients diagnosed with ALI/ARDS are mechanically ventilated for breathing support [5,6]. Various mechanical ventilation (MV) modes have been introduced to clinicians for the support of patients with ALI/ARDS [7]. However, the fundamentals of MV remains in selecting an optimal positive end-expiratory pressure (PEEP) to maximise patients' lung recruitment, prevent alveoli collapse, and avoid ventilator induced lung injury (VILI) [8]. The heterogeneity of the disease and patients' variable response to MV, encourages PEEP treatment to be patient-specific and individualised. However, there is no gold standard method in PEEP selection; consequently, optimising patient-specific PEEP in MV remains a challenge for clinicians [9-11].
Model-based and patient-specific approaches offer the ability to identify intra- and inter-patients variability and thus, potential to guide MV therapy based on patient's condition and needs [12,13]. This approach provides the opportunity to balance risk of lung injury and lung function support and reduce work of breathing [14] during MV. However, to date, only a few have been tested [15-17] and their potential in critical care is not yet validated.
This research presents several model-based approaches to identify patient-specific disease state and patient-specific response to MV therapy using patient-specific, constant lung elastance (Elung) [16,18] with comparison of dynamic lung elastance (Edrs) in ALI/ARDS. Dynamic lung elastance (Edrs) is a time-variant lung elastance during each breath in MV. Elung and Edrs are thus proposed for guiding PEEP selection. By monitoring both the identified parameters (Elastance = 1/Compliance) through limited PEEP titration, it is possible to identify PEEP settings that maximize recruitment, minimize work of breathing without inducing lung injury.
2 Methods
2.1 Study Design
Ten patients in the Intensive Care Unit (ICU), Christchurch Hospital, New Zealand, diagnosed with ALI or ARDS (PaO2/FiO2 (PF ratio) between 150-300 mmHg), underwent a modified protocol-based recruitment manoeuvre (RM) [17]. PEEP is increased with increments of 5cmH2O from zero PEEP (ZEEP) until peak airway pressure reaches a limit of 45cmH2O [19]. Patients were sedated and paralyzed with muscle relaxants to prevent spontaneous breathing efforts. All patients were ventilated using Puritan Bennett PB840 ventilators (Covidien, Boulder, CO, USA) with volume control (tidal volume, Vt = 400~600ml), synchronized intermittent mandatory ventilation (SIMV) mode, throughout the trial. The clinical trials and the use of the data were approved by the New Zealand, South Island Regional Ethics Committee.
A heated-pneumotachometer with Hamilton Medical flow sensor (Hamilton Medical, Switzerland) connected to the ventilator circuit Y-piece is used to record patient's airway pressure and flow data. A Dell™ (Dell, Austin, TX, USA) laptop was used in conjunction with National Instruments USB6009 and Labview Signal Express (National Instruments, Austin, TX, USA) to obtain measurements at a sampling rate of 100 Hz. Analysis was performed using MATLAB (The Mathworks, Natick, Massachusetts, USA).
2.2 Model-based Analysis
The model-based approach incorporates a physiologically relevant and validated recruitment model [17,20] with the use of a single compartment linear lung model that captures fundamental lung mechanics and properties in real-time to identify patient-specific constant lung elastance (Elung) and dynamic lung elastance (Edrs) during MV. The model uses transpulmonary pressure (Ptp), volume (V) and flow (Q) and offset pressure (P0), to identify lung elastance (Elung) and resistance (Rlung). Patient-specific lung elastance, Elung reflects the lung stiffness (1/Compliance). Therefore, a lower Elung is a more compliant lung. Elung is identified from measured data using an integral-based method [21]. The model is defined:
| (1) |
Airway pressure is related to transpulmonary pressure (Ptp) and pleural pressure (Ppl) by:
| (2) |
When the patient is sedated and fully dependant on the ventilator to breathe, it can be assumed that there is no chest wall activity, allowing Ppl to be omitted in this case. Equation 1 is then further modified to eliminate Ppl, yielding:
| (3) |
Patient-specific dynamic lung elastance, Edrs, is identified as a time-variant lung elastance and Equation (3) is defined:
| (4) |
To ensure that the identified parameters of constant Elung and time-variant Edrs (Edrs(t)) are valid, the absolute percentage error between the identified model and measured clinical pressure data is reported.
2.3 Model-Based PEEP Selection
During each breathing cycle, as PEEP rises, lung elastance (Elung) falls as new lung volume is recruited faster than the pressure build-ups in the lung. If little or no recruitment occurs, Elung rises with PEEP indicating that pressure above that PEEP level was unable to recruit significant new lung volume and is, instead, beginning to stretch already recruited lung [22]. Hence, recruitment and potential lung injury can be balanced by selecting PEEP at minimum Elung.
Compared to a single, constant Elung value at each PEEP, identifying time-variant Edrs allows this change to be seen dynamically within each breath as pressure increases thus allowing a more detail view of patient's lung physiological condition. Three model-based approaches based on patient-specific Elung and Edrs trajectory in a patient's breath at different PEEP levels are used to optimize PEEP selection.
Minimum Edrs and Elung: locates the point where minimum Edrs or Elung occurs over all PEEP values (and pressure for Edrs) during the recruitment manoeuvre.
Minimum Edrs Area: Edrs Area is obtained by integrating Edrs over time during the patient's breathing cycle at each PEEP. Edrs Area is more clinically relevant than median or mean Edrs throughout each breath and can be shown to be proportional to patient-specific work of breathing.
Inflection Method: This method detects the inflection in the Edrs Area-PEEP and Elung-PEEP curves. Inflection is defined here at the PEEP value with Edrs value 5-10% above (before) minimum Edrs Area or Elung (105~110% of minimum Edrs Area or Elung). PEEP is selected where inflection occurs, as a point of diminishing returns.
The overall approach implies that as long as Edrs falls during each breath, as PEEP level increases, that recruitment of new volume outweighs lung stretching as flow and volume follow a path of lesser or least resistance. These methods are thus attempts to maximize recruitment (Minimum Edrs and Minimum Edrs Area) and also ensure safety from excessive pressure (Inflection Method). These metrics are three of many possibilities to demonstrate the concept.
2.4 Edrs Area and Work of Breathing
These approaches were also compared with selecting PEEP using the identified minimum or inflection of constant Elung, for comparison to other similar work [23]. Patient-specific Elung and Edrs are only analyzed during inspiration and not during the expiratory cycle. This choice was made because increases in pressure induce lung damage as it passes a limit and thus expiration (decreasing pressure) should not be used to guide PEEP selection.
A higher resolution of the trend changes in Edrs can be observed using Edrs Area. Edrs Area is obtained through integration of Edrs with time. It is also known that the work of breathing (WOB) [24,25] for a patient is proportional to lung elastance. In general, more work is required to fill a given lung volume with higher elastance. WOB is defined:
| (5) |
Substituting Paw from Equation (3) into Equation (5) and using P0 = 0, (atmospheric).
| (6) |
From Equation (6), work of breathing can be divided into work to overcome lung elastance (WOBE = ElungV2) and work to overcome airway resistance (WOBR = RlungQV). Substitution of dynamic lung elastance, Edrs, for constant Elung enables a derivation for WOBE:
| (7) |
Edrs Area in Equation (8) is the integral of Equation (7), yielding the relation of Edrs to the work of breathing required to overcome lung elastance at a given level of PEEP and mode of MV.
| (8) |
2.5 Analysis and Comparisons
In this study, Elung and median Edrs are compared using Pearson's linear correlation coefficients to relate these metrics. Elung and Edrs Area are also compared to median Edrs and WOBE to ensure there was no loss of information for each patient at different PEEP values, and to show the validity of Equation (7) and using Edrs Area. Finally, clinically selected PEEP is compared to the value determined by proposed model-based metrics.
3 Results
Table 1 shows the clinical details of the 10 patients recruited with their clinical diagnostics, and PF ratios. Table 2 shows the median [Inter-quartile Range (IQR)] Edrs for each patient and PEEP, and absolute percentage fitting error. Median absolute percentage fitting error (APEEdrs(t)) across all patients and PEEP is 0.9% [IQR: 0.5-2.4]. Median Edrs at each PEEP is 32.2cmH2O/l [IQR: 26.1-46.6]. Median [IQR] Edrs decreases with increasing PEEP until the minimum Edrs. Patients who suffer from COPD (Patients 1, 4, 5, 9 and 10) have significantly higher Edrs than others (P < 0.0001), as expected clinically. Table 3 shows the constant lung elastance (Elung) at each PEEP with median = 32.2cmH2O/l [IQR: 25.0-45.9], and absolute percentage fitting (APEElung) at 5.6% [IQR: 1.8-11.3]. Table 4 shows the Edrs Area at each PEEP with median [IQR] of 34.0cmH2Os/l [IQR: 24.7-48.5].
Table 1.
Patient demography.
| Patients | Sex | Age (year) | Clinical Diagnostic | PF Ratio |
|---|---|---|---|---|
| 1 | F | 61 | Peritonitis, COPD | 214 |
| 2 | M | 22 | Trauma | 180 |
| 3 | M | 55 | Aspiration | 222 |
| 4 | M | 88 | Pneumonia, COPD | 165 |
| 5 | M | 59 | Pneumonia, COPD | 285 |
| 6 | M | 69 | Trauma | 280 |
| 7 | M | 56 | Legionnaires | 265 |
| 8 | F | 54 | Aspiration | 302 |
| 9 | M | 37 | H1N1, COPD* | 182 |
| 10 | M | 56 | Legionnaires, COPD | 237 |
*Chronic Obstructive Pulmonary Disease
Table 2.
Patient-specific dynamic lung elastance (Edrs) at each PEEP level.
| Patient | Dynamic Lung Elastance, Edrs (cmH2O/l) Median [IQR] |
Edrs (cmH2O/l)
Median [IQR] |
APE*
(%) Median [IQR] |
||||||
|---|---|---|---|---|---|---|---|---|---|
| PEEP (cmH2O) | |||||||||
| 0 | 5 | 10 | 15 | 20 | 25 | 30 | |||
| 1 | 63.1 [46.9-114.9] |
53.8 [43.0-80.2] |
43.6 [38.4-54.5] |
35.0 [33.3-39.4] |
33.4 [32.0-34.2] |
31.1 [32.0-32.4] |
PEEP 27 32.2 [31.9-32.6] |
35.0 [32.5-51.2] |
1.1 [0.5-4.1] |
| 2 | 30.8 [26.3-45.1] |
26.4 [23.7-31.4] |
23.1 [22.0-24.3] |
22.1 [22.0-22.6] |
22.5 [22.4-22.6] |
PEEP 22 23.1 [22.9-23.2] |
23.1 [22.5-26.4] |
0.7 [0.6-2.4] |
|
| 3 | 26.9 [22.6-36.9] |
22.1 [20.2-25.6] |
18.3 [18.0-19.0] |
17.3 [17.2-17.4] |
17.5 [17.1-17.5] |
17.8 [17.4-18.7] |
PEEP 28 19.2 [17.9-19.7] |
18.3 [17.6-21.4] |
0.6 [0.5-1.3] |
| 4 | 73.2 [50.4-144.4] |
70.4 [49.9-126.9] |
54.5 [41.7-82.3] |
36.8 [30.6-43.9] |
28.5 [25.6-31.4] |
25.9 [21.6-28.4] |
23.1 [19.4-25.5] |
36.8 [26.6-66.4] |
3.4 [0.9-5.4] |
| 5 | 105.7 [80.6-199.8] |
97.8 [77.5-166.8] |
89.3 [74.3-143.4] |
79.4 [68.6-107.3] |
67.3 [61.4-79.4] |
52.3 [52.0-55.8] |
84.4 [67.3-97.8] |
3.2 [0.9-6.0] |
|
| 6 | 30.4 [25.9-39.1] |
26.2 [25.5-27.2] |
23.3 [22.4-23.5] |
21.6 [21.5-21.8] |
21.8 [21.3-22.5] |
23.3 [22.6-23.9] |
23.3 [21.8-26.2] |
0.8 [0.6-1.2] |
|
| 7 | 49.3 [46.1-62.4] |
42.2 [41.5-43.1] |
44.3 [41.8-47.7] |
53.6 [48.8-59.7] |
PEEP 16 52.4 [50.3-57.6] |
49.3 [43.8-52.7] |
1.6 [1.3-2.0] |
||
| 8 | 45.7 [37.9-67.8] |
37.2 [32.9-43.0] |
31.8 [29.9-33.5] |
28.8 [28.0-29.8] |
27.4 [27.1-27.9] |
26.8 [26.3-27.0] |
27.0 [26.8-27.5] |
28.8 [27.1-35.9] |
0.8 [0.5-2.2] |
| 9 | 58.1 [47.1-100.8] |
40.5 [36.4-52.8] |
39.9 [35.8-48.7] |
31.2 [30.2-33.6] |
28.3 [27.9-29.0] |
26.3 [26.3-26.5] |
26.2 [25.8-26.5] |
31.2 [26.8-40.4] |
0.8 [0.4-2.1] |
| 10 | 54.4 [48.1-76.2] |
45.2 [41.9-51.8] |
39.4 [38.4-41.7] |
35.9 [35.7-36.0] |
33.9 [33.7-34.1] |
33.9 [33.4-34.6] |
PEEP 27 33.9 [33.2-34.8] |
35.9 [33.9-43.8] |
0.4 [0.4-0.9] |
|
Median [IQR] |
51.9 [30.8-63.1] |
41.4 [26.4-53.8] |
39.7 [23.3-44.3] |
33.1 [22.1-36.8] |
28.4* [22.5-33.9] |
26.3* [23.1-32.2] |
26.6* [23.1-32.2] |
32.2 [26.1-46.6] |
0.9 [0.5-2.4] |
*APE - Absolute Percentage Fitting Error (%)
*Values presented include value from different PEEP.
Table 3.
Patient-specific constant lung elastance (Elung) at different PEEP.
| Patient | Constant Lung Elastance, Elung (cmH2O/l) |
Elung (cmH2O/l)
Median [IQR] |
APE
(%) Median [IQR] |
||||||
|---|---|---|---|---|---|---|---|---|---|
| PEEP (cmH2O) | |||||||||
| 0 | 5 | 10 | 15 | 20 | 25 | 30 | |||
| 1 | 53.8 | 47.0 | 41.2 | 32.8 | 32.8 | 32.1 | PEEP 27 32.2 |
34.7 [32.4-45.5] |
7.2 [1.7-19.0] |
| 2 | 27.7 | 25.3 | 22.8 | 22.3 | 22.6 | PEEP 22 23.1 |
23.0 [22.6-25.3] |
2.5 [1.1-7.7] |
|
| 3 | 24.0 | 21.6 | 18.3 | 17.3 | 17.4 | 18.1 | PEEP 28 19.1 |
18.3 [17.6-20.9] |
4.2 [1.6-6.6] |
| 4 | 60.2 | 59.7 | 50.1 | 35.1 | 27.8 | 25.3 | 22.5 | 35.1 [25.9-57.3] |
17.7 [15.4-32.1] |
| 5 | 87.4 | 84.0 | 81.2 | 74.3 | 65.7 | 53.1 | 77.8 [65.7-84.0] |
15.7 [9.2-19.8] |
|
| 6 | 27.1 | 25.5 | 22.8 | 21.6 | 21.8 | 23.4 | 23.1 [21.8-25.5] |
2.7 [2.2-4.2] |
|
| 7 | 47.7 | 42.5 | 45.5 | 55.7 | PEEP 16 55.3 |
47.7 [44.8-55.4] |
6.2 [5.0-7.7] |
||
| 8 | 41.7 | 35.5 | 31.2 | 28.7 | 27.5 | 26.6 | 27.0 | 28.7 [27.2-34.4] |
2.9 [1.3-8.7] |
| 9 | 51.3 | 39.1 | 38.2 | 31.1 | 28.2 | 26.2 | 26.1 | 29.7 [26.2-38.7] |
3.1 [1.0-10.8] |
| 10 | 51.0 | 44.1 | 39.2 | 35.8 | 33.9 | 34.0 | PEEP 27 34.2 |
35.8 [34.1-42.9] |
2.0 [1.0-5.6] |
| Median [IQR] | 49.4 [27.7-53.8] |
40.8 [25.5-47.0] |
38.7 [22.8-45.5] |
31.9 [22.3-35.5] |
28.0* [22.6-33.9] |
26.2* [23.3-32.6] |
26.6* [22.5-32.2] |
32.2 [25.0-45.9] |
5.6 [1.8-11.3] |
Eg. PEEP 16 is included in PEEP 20 Median [IQR]
*Values presented include value from different PEEP. Eg. PEEP 16 is included in PEEP 20 Median [IQR]
Table 4.
Patient-specific Edrs Area at different PEEP.
| Patient | Edrs Area (mH2Os/l) |
Edrs Area (cmH2Os/l) Median [IQR] |
||||||
|---|---|---|---|---|---|---|---|---|
| PEEP (cmH2O) | ||||||||
| 0 | 5 | 10 | 15 | 20 | 25 | 30 | ||
| 1 | 84.6 | 49.5 | 37.1 | 28.9 | 26.6 | 25.7 | PEEP 27 25.7 |
28.9 [25.9-46.4] |
| 2 | 34.0 | 24.8 | 21.0 | 20.2 | 20.3 | PEEP 22 20.7 |
20.9 [20.3-24.8] |
|
| 3 | 37.7 | 27.6 | 22.2 | 20.8 | 19.1 | 19.7 | PEEP 28 18.9 |
20.8 [19.3-26.3] |
| 4 | 102.2 | 91.2 | 61.7 | 37.9 | 31.7 | 48.1 | 47.5 | 48.1 [40.3-83.8] |
| 5 | 118.7 | 99.9 | 89.1 | 70.6 | 75.7 | 42.9 | 82.4 [70.6-99.9] |
|
| 6 | 29.4 | 23.8 | 20.8 | 21.6 | 19.5 | 20.8 | 21.2 [20.8-23.8] |
|
| 7 | 37.6 | 33.8 | 31.3 | 37.9 | PEEP 16 32.1 |
33.8 [31.9-37.7] |
||
| 8 | 55.1 | 38.5 | 32.0 | 29.0 | 27.5 | 24.1 | 24.3 | 29.0 [25.1-36.9] |
| 9 | 106.5 | 55.2 | 51.3 | 38.3 | 34.1 | 31.6 | 31.3 | 38.4 [32.2-54.2] |
| 10 | 74.7 | 52.6 | 44.0 | 39.5 | 37.3 | 37.2 | PEEP 27 37.3 |
39.5 [37.3-50.5] |
| Median [IQR] | 64.9 [37.6-102.2] |
44.0 [27.6-55.2] |
34.6 [22.2-51.3] |
33.5 [21.6-38.4] |
29.6* [20.3-34.1] |
25.7* [20.8-38.6] |
28.5* [24.3-37.3] |
34.0 [24.7-48.5] |
*Values presented include value from different PEEP. Eg. PEEP 16 is included in PEEP 20 Median [IQR]
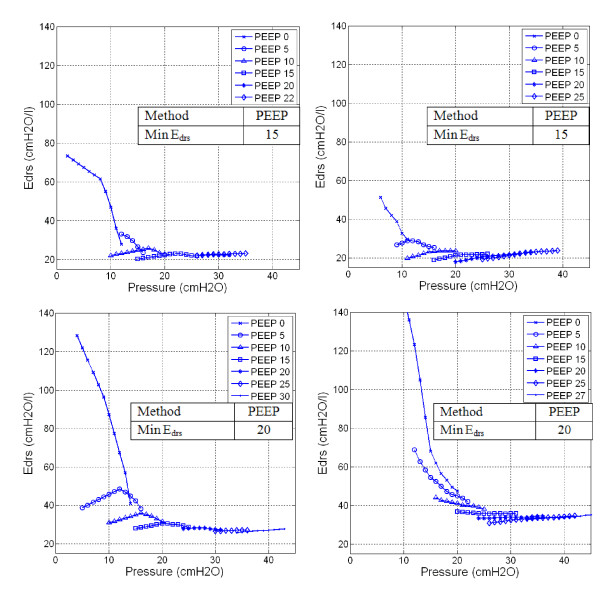
Figure 1 shows patient-specific time-varying Edrs at each PEEP level for Patients 2, 6, 8 and 10. Edrs decreases as pressure increases at each PEEP. However, at higher PEEP, this trend can reverse indicating stretching exceeding recruitment of new lung volume. The optimal PEEP derived by minimum Edrs is indicated.
Figure 1.
Dynamic lung elastance (Edrs)-Pressure-PEEP plot. Top Left Panel: Patient 2, Top Right Panel: Patient 6. Both patients show significant Edrs drop from lower zero PEEP to PEEP 15cmH2O. Further increase of PEEP to 20cmH2O shows increase of overall Edrs. Bottom Left Panel: Patient 8, Bottom Right Panel: Patient 10. Both patients show a consistent drop in overall Edrs with increasing of PEEP and overall Edrs did not rise with PEEP for the entire ranged considered.
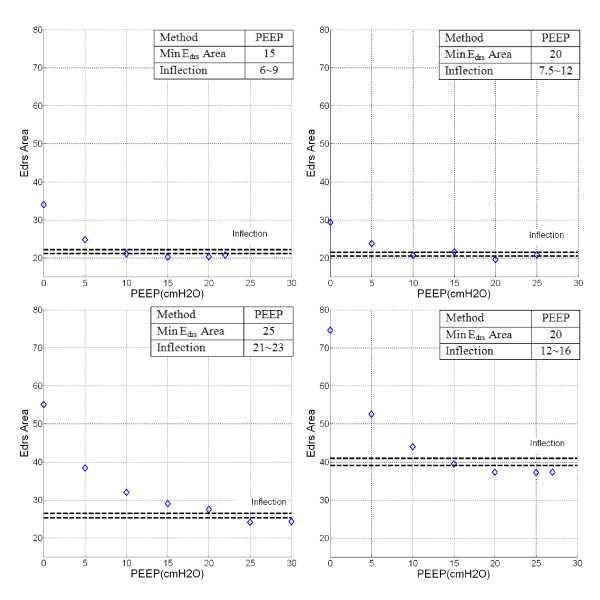
Figure 2 shows patient-specific Edrs Area for Patients 2, 6, 8 and 10 with PEEP. The optimal PEEP is derived using minimum Edrs Area and Inflection method with the band of 5-10% above minimum Edrs Area shown by the dashed-lines.
Figure 2.
Edrs Area-PEEP plot. Top Left Panel: Patient 2, Top Right Panel: Patient 6. Bottom Left Panel: Patient 8, Bottom Right Panel: Patient 10. Severe COPD or patients with similar clinical features (e.g. Patient 10) showed significantly higher Edrs Area compared to other patients. PEEP selection is based on minimum Edrs-Area and the inflection method with PEEP increase.
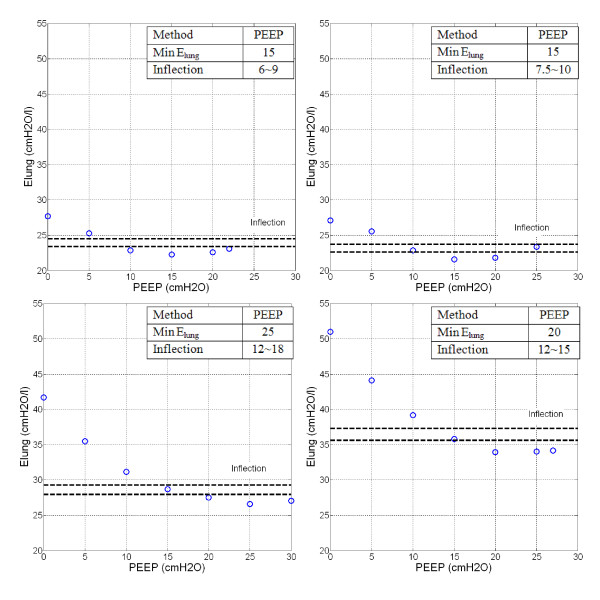
Figure 3 shows patient-specific constant lung elastance (Elung) with increasing PEEP for Patients 2, 6, 8 and 10. Elung decreases with PEEP and the trend is similar to the Edrs Area-PEEP plot of Figure 2, as expected from the high correlation. The optimal PEEP using minimum Elung and Inflection Elung (Dashed-lines) are also indicated.
Figure 3.
Elung-PEEP plot. Top Left Panel: Patient 2, Top Right Panel: Patient 6. Bottom Left Panel: Patient 8, Bottom Right Panel: Patient 10. PEEP derived from Minimum Elung and Inflection method are as indicated.
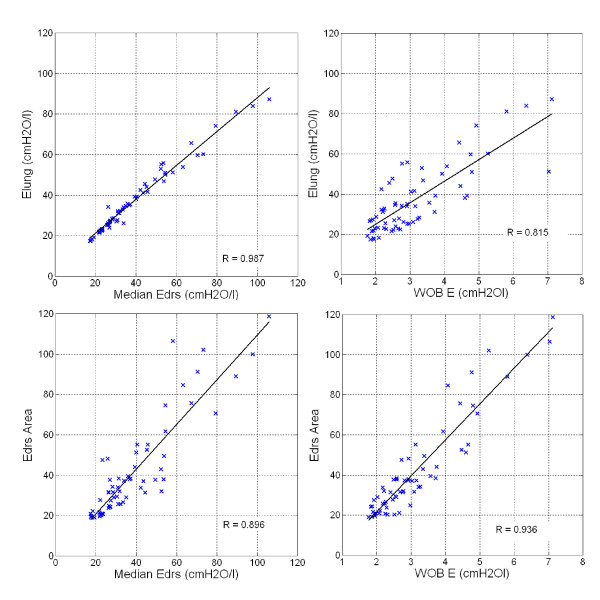
Across all 10 patients, patient-specific constant lung elastance (Elung) can be represented by the median of dynamic lung elastance (Edrs) with correlation R = 0.987. Correlation of Elung and WOBE is R = 0.815. Edrs Area and median Edrs are also closely correlated with R = 0.896. Hence, Edrs can be represented with Edrs Area, where Edrs Area captures all Edrs values in a given breath and thus, is a more physiologically representative metric. Finally, validating Equation (2), Edrs Area is correlated to the work to overcome lung elastance, WOBE, as expected, with R = 0.936. The correlations are shown in Figure 4.
Figure 4.
Pearson's Correlation. Top Left Panel: Elung-Median Edrs, R = 0.987. Top Right Panel: Elung-WOBE, R = 0.815. Bottom Left Panel: Edrs Area-Median Edrs, R = 0.896. Bottom Right Panel: Edrs Area-WOBE, R = 0.936.
Table 5 compares clinically selected PEEP during MV therapy with PEEP selected using Minimum Edrs, and Minimum Edrs Area and the Inflection method. The clinical values are set over a much narrower range, both higher and lower than those selected using Edrs. Minimum Edrs Area always selects a higher PEEP, by definition, than the Inflection method. However, Minimum Edrs Area selects PEEP similar to or higher than Minimum Edrs, where it also thus adds consideration of the reduction in overall WOBE in selecting PEEP. PEEP derived from minimum Elung and Inflection Elung are also indicated.
Table 5.
PEEP (cmH2O) selection in clinical and model-based approach.
| Patients | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Selection Methods | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 |
| Clinical | 10 | 12 | 10 | 10 | 12 | 11 | 7.5 | 12 | 10 | 10 |
| Minimum Edrs | 20 | 15 | 15 | 25 | 25 | 15 | 5 | 20 | 15 | 20 |
| Minimum Edrs Area | 25 | 15 | 20 | 20 | 25 | 20 | 10 | 25 | 25 | 20 |
|
Inflection Edrs Area |
14~16 | 6~9 | 15~17 | 16~18 | 22~24 | 7.5~12 | 5~7.5 | 21~23 | 20~23 | 12~16 |
| Minimum Elung | 25 | 15 | 15 | 30 | 25 | 15 | 5 | 25 | 30 | 20 |
|
Inflection Elung |
13~17 | 6~9 | 8~10 | 26~27 | 21~24 | 7.5~10 | 5 | 12~18 | 19~22 | 12 ~15 |
4 Discussion
4.1 Model-based PEEP Selection
Median fitting error for time-variant Edrs in Table 2 is less than 1%, showing that a single compartment lung model can be used for time-varying Edrs estimation. The wide range of patient-specific Edrs across all patients and PEEP shown in Table 2 reflects the heterogeneity of ALI/ARDS patient condition and response to PEEP that makes standardising and PEEP selection difficult [26]. Compared to the estimation of Elung in Table 3, median fitting error is 5.6% and in specific cases, fitting error can be as high as 15.7-17.7% (Patients 4 and 5). This latter result indicates that a first order model can be used to estimate most patient-specific constant Elung, but, in several cases, the model may not accurately represent patients' physiological condition. Time-varying Edrs provides a better model fit across all patients and also provides a clearer insight into the patient's physiological condition, and is thus the better model-based metric.
Figures 1 and Figure 2 shows Edrs-Pressure-PEEP curves and Edrs Area decrease with increasing PEEP, lung pressure, and volume over each breath. In the beginning of the recruitment manoeuvre, at zero end-expiratory pressure (ZEEP), Edrs is relatively very high for all patients with median 51.9cmH2O/l [IQR: 30.8-63.1]. In particular, chronic obstructive pulmonary disease (COPD) patients or patients with similar clinical features [27] (Patients 1, 4, 5, 9 and 10) have initially the highest Edrs median, as expected, from 63.1cmH2O/l [IQR: 57.2-81.3] versus 30.8cmH2O/l [IQR: 29.5-46.6] for the other patients (p = 0.0079). As PEEP rises, it is observed that Edrs curves drop at patient-specific rates. High constant lung elastance, Elung at ZEEP and decreasing elastance as PEEP increments are also observed in Figure 3 for Patient 10.
In all cases, patient-specific Edrs and Elung decrease to a patient-specific minimum before increasing at higher PEEP. Minimum Edrs and Elung suggest the point where the lung is most compliant, if ventilated at that PEEP level. Further increases in PEEP and pressure thus lead to increased Elung or Edrs, and thus increase detrimental effects. In particular, increases in Elung or Edrs can be associated with overstretching of the patient's lung [16,28]. However, the heterogeneity of ALI/ARDS means there is a possibility of overstretching of healthy lung units even at low PEEP and airway pressures [10]. Thus, Minimum or, perhaps preferably, Inflection Edrs and Elung can provide a potentially higher resolution metric.
Patients 2 and 6 (Figure 1, 2, 3: Top panels) are examples where patient-specific Edrs, Edrs Area and Elung increase after descending to a minimum. Results suggest that further increases of PEEP and inflation pressures will stretch lung units causing possible damage, as seen by increasing Edrs at higher PEEP. The rise of Edrs occurs at relatively low PEEP and pressure 15-20cmH2O in these two patients.
In contrast, Patients 8 and 10 (Figure 1, 2, 3: Bottom panels) never see Edrs or Elung rising even at the maximum PEEP used in this study. However, the Edrs range at higher PEEP for Patients 8 and 10 (PEEP 15~30cmH2O) is relatively small with median Edrs = 31.3cmH2O/l, [IQR = 27.2-33.9]. This outcome indicates that further increases of PEEP from 15 to 30cmH2O has no added advantage in reducing Edrs, suggesting PEEP selection should be made at using the Inflection method.
Table 2 shows median [IQR] Edrs for every patient and PEEP. The IQR range drops significantly for every patient as PEEP increases. This range also indicates lung status or condition with the influence of pressure. A small IQR range indicates that the lung is ventilated at a PEEP level where maximal lung recruitment occurs over a narrow pressure range as tidal volume, Vt is fixed in the MV mode used. A high IQR range shows the opposite. Hence, the lengths along pressure in Figure 1 also indicate how readily the patient was recruited and that easiest recruitment occurs at minimum Edrs [29].
Table 4 shows the patient-specific Edrs Area at each PEEP. It is found that Edrs Area is closely related to median Edrs, as shown in Figure 4. Edrs Area at lower PEEP with median 64.9 cmH2Os/l [IQR: 37.6-102.2] is observed and as PEEP increases, Edrs Area decreases. Upon reaching minimum Edrs Area, patient-specific Edrs Area increase with PEEP (Patients 2, 4, 6, 7 and 10). This trend is similar to the trend observed in patient-specific dynamic Edrs (Table 2) and constant Elung (Table 3). Optimal PEEP derived using minimum or inflection method in Edrs Area is similar to minimum patient-specific Edrs but different as Edrs Area considers the whole inspiration and the effect of WOBE. It is also found that Edrs Area is closely correlated to work in overcoming the lung elastic properties (WOBE). This means that Edrs Area provides combined information of patients-specific lung physiological conditions as well as work of breathing.
Table 5 shows the model-based approaches to PEEP selection compared to clinically selected PEEP. For 9 of 10 patients, the PEEP value selected using Minimum Edrs and Edrs Area results in a value higher than the clinically selected PEEP. This latter result suggests that these patients could be treated at PEEP levels higher than clinically selected PEEP. When Minimum Edrs or Edrs Area metrics are compared with Minimum Elung [16], they result in selecting similar PEEP. However, selecting PEEP is a trade off in minimizing lung pressure and potential damage, versus maximizing recruitment. Hence, the Inflection method offers similar recruitment at a lower PEEP and may be a safer choice, although its selected values are still higher than clinically selected in 7 of 10 cases. Overall, these results reflect the heterogeneity of the ALI/ARDS lung and the need for patient-specific approaches to select PEEP.
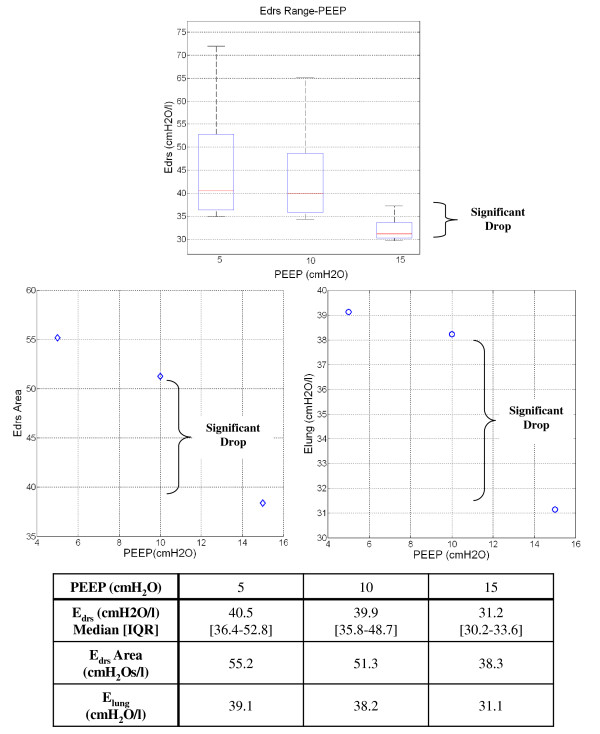
Patient 9 is an interesting case which illustrates the model's potential to capture unique patient-specific lung recruitment and condition as it occurs in a clinically and physiologically relevant manner. When the patient is ventilated from PEEP of 5 to 10cmH2O, median Edrs only decreases by less than 1.0cmH2O/l. However, when PEEP is increased to 15cmH2O, the median Edrs drops significantly, as shown in Figure 5. This smaller Edrs drop suggests that only minimal lung volume is recruited from PEEP of 5 to 10 cmH2O. The significant drop in Edrs at PEEP 15cmH2O indicates that PEEP 15 cmH2O has overcome recruitment resistance and additional new lung volume is recruited. Patient 9 was diagnosed with H1N1 and high PEEP for lung recruitment has proven to be beneficial for these patients [30]. Similar trends can be observed in Figure 5 bottom panels with the Edrs Area-PEEP plot and Elung-PEEP-plot.
Figure 5.
Patient 9 Edrs, Edrs Area and Elung change with PEEP. Top Panel: Box-and-whisker diagram for Patient 9 Edrs when PEEP increase from 5 to 10cmH2O. The Edrs drops significantly when PEEP is increase from 10 to 15cmH2O. Bottom Left Panel: Edrs Area-PEEP plot for Patient 9. Bottom Right Panel: Elung-PEEP for Patient 9.
4.2 Limitations
In this research, the lung model used to identify patient-specific Edrs comprised a single compartment lung model. It was initially proposed for simple computational analysis and neglects the effect of nonlinear flow [31]. However, this analysis is based predominantly on trend comparisons, where the patient is their own reference. In addition, the model is simple and capable of capturing the fundamental lung mechanics, which varies intra- and inter- patients. Hence, this limitation should be minimal in this case, but should be confirmed with direct prospective clinical studies.
During the clinical trials, the patients were sedated and paralyzed using muscle relaxants. It is assumed that after sedation, the patient will be fully dependant on mechanical ventilation and not have spontaneous breathing effort. This assumption thus assumes the patient's pleural pressure (Ppl) after sedation is zero and allows Ppl in Equation (3) to be omitted, which may not be entirely valid [32]. However, this assumption is made for the first step study to prove the concept within a simpler situation. Otherwise, the terms Elung and Edrs would represent a respiratory system elastance [31] and time-variant dynamic respiratory system elastance. However, given the low fitting errors observed, this issue should have little impact in this research.
During the course of estimating patient-specific Elung or Edrs, respiratory system resistance, R, is assumed overall constant within a physiological range [33] as PEEP increases. This assumption may not be entirely valid in some cases [33,34]. However, continuous measurements of respiratory resistance are not typically available and the effect of this resistive term is limited mathematically in its impact. Equally, trend comparison, as used here, across PEEP values will reduce the impact.
The identification of Elung, Edrs and Edrs Area during MV is presented as a method to select PEEP, but there is currently no conclusive, optimum overall Edrs or Edrs Area in patients. Edrs range varies depending on patient disease state and thus will also change over time. However, this trial includes only 10 patients, and there is not yet enough clinical data to indicate an optimum Elung, Edrs or Edrs Area value for a specific patient or group. On-going, prospective trials with more specific patient groups should develop more conclusive outcomes, relating specific set values of Edrs metrics to effective patient-specific treatments and clinical outcome.
In particular, the time-varying Edrs value and its change over a given breathing cycle, provides additional insight to guide ventilation that is not investigated here. For example, changes in ventilator pattern or mode to modify the Edrs trajectory could also be used with this data to guide therapy choice. However, this study does not have the numbers or design to provide that advice, or specific Edrs values associated with specific decrease state or lung damage.
5 Conclusions
The model-based approach presented provides patient-specific, physiological insight not directly measurable without additional invasive, disruptive and clinically intensive test manoeuvres. This method can be directly implemented using modern ventilators with minimal, limited PEEP titrations, and thus without significant interruption to ongoing therapy. In particular, the full manoeuvres used here would not be required for clinical use, and only modest PEEP changes (3-8cmH2O) would be required to determine if Edrs was decreasing at a different PEEP. Edrs offers higher resolution in patients' response to change of pressure and PEEP, which is potentially, a better metric compared to existing constant lung elastance estimation. Thus, the overall method is readily generalisable and clinical practicable. It is able to capture patient-specific condition and responsiveness to PEEP and recruitment accurately, and as clinically expected. Hence, the approach presented offers significant potential to improve clinical insight and delivery of mechanical ventilation, and should be prospectively tested.
6 List of Abbreviations
ALI: Acute lung injury; APE: Absolute percentage error; ARDS: Acute respiratory distress syndrome; COPD: Chronic Obstructive Pulmonary Disease; Elung: Patient-specific constant lung elastance; Edrs: Patient-specific dynamic lung elastance; FiO2: Fraction of Inspired Oxygen; ICU: Intensive care unit; IQR: Interquartile Range; MV: Mechanical ventilation; PaO2: Partial pressure of oxygen in arterial blood; Paw: Airway pressure; Ppl: Pleural pressure; Ptp: Transpulmonary pressure; PEEP: Positive end expiratory pressure; PF Ratio: PaO2/FiO2; P0: Offset pressure; Q: Flow; RM: Recruitment manoeuvre; Rlung: Resistance; SIMV: Synchronized intermittent mandatory ventilation; t: Time; V: Volume; VILI: Ventilation induced lung injury; Vt: Tidal volume; WOB: Work of Breathing; WOBE: Work to overcome respiratory system elastance; WOBR: Work to overcome airway resistance; ZEEP: Zero PEEP
7 Competing Interests
The authors declare that they have no competing interests.
8 Authors Contribution
YSC, JGC, GMS created and defined the model. YSC, JGC and TD had input to analysis of results. GMS, AS implemented trials clinically with input from all others. All authors had input in writing and revising the manuscript. All authors have read and approved the final manuscript.
9 Consent
Written informed consent was obtained from the participant and or relative/friends/family of this study. A copy of written consent is available for review by the Editor-in-Chief of this journal.
Contributor Information
Yeong Shiong Chiew, Email: yeong.chiew@pg.canterbury.ac.nz.
J Geoffrey Chase, Email: geoff.chase@canterbury.ac.nz.
Geoffrey M Shaw, Email: Geoff.Shaw@cdhb.govt.nz.
Ashwath Sundaresan, Email: ashsundaresan@gmail.com.
Thomas Desaive, Email: tdesaive@ulg.ac.be.
References
- Ashbaugh D, Boyd Bigelow D, Petty T, Levine B. ACUTE RESPIRATORY DISTRESS IN ADULTS. The Lancet. 1967;290:319–323. doi: 10.1016/S0140-6736(67)90168-7. [DOI] [PubMed] [Google Scholar]
- Bernard GR, Artigas A, Brigham KL, Carlet J, Falke K, Hudson L, Lamy M, LeGall JR, Morris A, Spragg R. Report of the American-European consensus conference on ARDS: Definitions, mechanisms, relevant outcomes and clinical trial coordination. Intensive Care Medicine. 1994;20:225–232. doi: 10.1007/BF01704707. [DOI] [PubMed] [Google Scholar]
- Phua J, Badia JR, Adhikari NKJ, Friedrich JO, Fowler RA, Singh JM, Scales DC, Stather DR, Li A, Jones A. et al. Has Mortality from Acute Respiratory Distress Syndrome Decreased over Time?: A Systematic Review. Am J Respir Crit Care Med. 2009;179:220–227. doi: 10.1164/rccm.200805-722OC. [DOI] [PubMed] [Google Scholar]
- Dasta JF, McLaughlin TP, Mody SH, Piech CT. Daily cost of an intensive care unit day: The contribution of mechanical ventilation *. Critical Care Medicine. 2005;33:1266–1271. doi: 10.1097/01.CCM.0000164543.14619.00. 1210.1097/1201.CCM.0000164543.0000114619.0000164500. [DOI] [PubMed] [Google Scholar]
- Girard TD, Bernard GR. Mechanical Ventilation in ARDS. Chest. 2007;131:921–929. doi: 10.1378/chest.06-1515. [DOI] [PubMed] [Google Scholar]
- Esteban A, Anzueto A, Frutos F, Alia I, Brochard L, Stewart T, Benito S, Epstein S, Apezteguia S, Nightingale P. et al. Characteristics and outcomes in adult patients receiving mechanical ventilation: a 28-day international study. Jama. 2002;287:345–355. doi: 10.1001/jama.287.3.345. [DOI] [PubMed] [Google Scholar]
- Mireles-Cabodevila E, Diaz-Guzman E, Heresi GA, Chatburn RL. Alternative modes of mechanical ventilation: A review for the hospitalist. Cleveland Clinic Journal of Medicine. 2009;76:417–430. doi: 10.3949/ccjm.76a.08043. [DOI] [PubMed] [Google Scholar]
- Gattinoni L, Carlesso E, Brazzi L, Caironi P. Positive end-expiratory pressure. Current Opinion in Critical Care. 2010;16:39–44. doi: 10.1097/MCC.0b013e3283354723. [DOI] [PubMed] [Google Scholar]
- Ware LB, Matthay MA. The Acute Respiratory Distress Syndrome. N Engl J Med. 2000;342:1334–1349. doi: 10.1056/NEJM200005043421806. [DOI] [PubMed] [Google Scholar]
- Stenqvist O. Practical assessment of respiratory mechanics. British Journal of Anaesthesia. 2003;91:92–105. doi: 10.1093/bja/aeg141. [DOI] [PubMed] [Google Scholar]
- Esteban A, Cook DJ, Anzueto A, Gattinoni L, Chiumello D, Vagginelli F. In: Evidence-Based Management of Patients with Respiratory Failure. Vincent J-L, editor. Springer Berlin Heidelberg; 2005. Management of Patients with Respiratory Failure: An Evidence-based Approach; pp. 21–27. Update in Intensive Care Medicine. [Google Scholar]
- Sundaresan A, Chase JG. Positive end expiratory pressure in patients with acute respiratory distress syndrome - The past, present and future. Biomedical Signal Processing and Control. 2011. in press Corrected Proof.
- Chase JG, Le Compte A, Preiser J-C, Shaw G, Penning S, Desaive T. Physiological modeling, tight glycemic control, and the ICU clinician: what are models and how can they affect practice? Annals of Intensive Care. 2011;1:11. doi: 10.1186/2110-5820-1-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacIntyre NR. Is There a Best Way to Set Positive Expiratory-End Pressure for Mechanical Ventilatory Support in Acute Lung Injury? Clinics in chest medicine. 2008;29:233–239. doi: 10.1016/j.ccm.2008.01.005. [DOI] [PubMed] [Google Scholar]
- Quaglini S, Barahona P, Andreassen S, Rees S, Allerød C, Kjærgaard S, Toft E, Thorgaard P. Artificial Intelligence in Medicine. Vol. 2101. Springer Berlin/Heidelberg; 2001. Diagnosing Patient State in Intensive Care Patients Using the Intelligent Ventilator (INVENT) System; pp. 131–135. Lecture Notes in Computer Science. [DOI] [Google Scholar]
- Carvalho A, Jandre F, Pino A, Bozza F, Salluh J, Rodrigues R, Ascoli F, Giannella-Neto A. Positive end-expiratory pressure at minimal respiratory elastance represents the best compromise between mechanical stress and lung aeration in oleic acid induced lung injury. Critical Care. 2007;11:R86. doi: 10.1186/cc6093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sundaresan A, Chase JG, Shaw G, Chiew Y-S, Desaive T. Model-Based Optimal PEEP in Mechanically Ventilated ARDS Patients in the Intensive Care Unit. BioMedical Engineering OnLine. 2011;10:64. doi: 10.1186/1475-925X-10-64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suarez-Sipmann F, Bohm SH, Tusman G, Pesch T, Thamm O, Reissmann H, Reske A, Magnusson A, Hedenstierna G. Use of dynamic compliance for open lung positive end-expiratory pressure titration in an experimental study. Crit Care Med. 2006. [DOI] [PubMed]
- Gattinoni L, Caironi P, Cressoni M, Chiumello D, Ranieri VM, Quintel M, Russo S, Patroniti N, Cornejo R, Bugedo G. Lung Recruitment in Patients with the Acute Respiratory Distress Syndrome. N Engl J Med. 2006;354:1775–1786. doi: 10.1056/NEJMoa052052. [DOI] [PubMed] [Google Scholar]
- Sundaresan A, Yuta T, Hann CE, Geoffrey Chase J, Shaw GM. A minimal model of lung mechanics and model-based markers for optimizing ventilator treatment in ARDS patients. Computer Methods and Programs in Biomedicine. 2009;95:166–180. doi: 10.1016/j.cmpb.2009.02.008. [DOI] [PubMed] [Google Scholar]
- Hann CE, Chase JG, Lin J, Lotz T, Doran CV, Shaw GM. Integral-based parameter identification for long-term dynamic verification of a glucose-insulin system model. Computer Methods and Programs in Biomedicine. 2005;77:259–270. doi: 10.1016/j.cmpb.2004.10.006. [DOI] [PubMed] [Google Scholar]
- Vieira SR, Puybasset L, Richecoeur J, Lu Q, Cluzel P, Gusman PB, Coriat P, Rouby JJ. A lung computed tomographic assessment of positive end-expiratory pressure-induced lung overdistension. Am J Respir Crit Care Med. 1998;158:1571–1577. doi: 10.1164/ajrccm.158.5.9802101. [DOI] [PubMed] [Google Scholar]
- Carvalho A, Spieth P, Pelosi P, Vidal Melo M, Koch T, Jandre F, Giannella-Neto A, de Abreu M. Ability of dynamic airway pressure curve profile and elastance for positive end-expiratory pressure titration. Intensive Care Medicine. 2008;34:2291–2299. doi: 10.1007/s00134-008-1301-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otis AB, Fenn WO, Rahn H. Mechanics of Breathing in Man. Journal of Applied Physiology. 1950;2:592–607. doi: 10.1152/jappl.1950.2.11.592. [DOI] [PubMed] [Google Scholar]
- Marini JJ, Capps JS, Culver BH. The inspiratory work of breathing during assisted mechanical ventilation. Chest. 1985;87:612–618. doi: 10.1378/chest.87.5.612. [DOI] [PubMed] [Google Scholar]
- Mercat A, Richard J-CM, Vielle B, Jaber S, Osman D, Diehl J-L, Lefrant J-Y, Prat G, Richecoeur J, Nieszkowska A. et al. Positive End-Expiratory Pressure Setting in Adults With Acute Lung Injury and Acute Respiratory Distress Syndrome: A Randomized Controlled Trial. JAMA. 2008;299:646–655. doi: 10.1001/jama.299.6.646. [DOI] [PubMed] [Google Scholar]
- Hoare Z, Lim WS. Pneumonia: update on diagnosis and management. BMJ. 2006;332:1077–1079. doi: 10.1136/bmj.332.7549.1077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carvalho A, Jandre F, Pino A, Bozza F, Salluh J, Rodrigues R, Soares J, Giannella-Neto A. Effects of descending positive end-expiratory pressure on lung mechanics and aeration in healthy anaesthetized piglets. Critical Care. 2006;10:R122. doi: 10.1186/cc5030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amato MBP, Barbas CSV, Medeiros DM, Magaldi RB, Schettino GP, Lorenzi-Filho G, Kairalla RA, Deheinzelin D, Munoz C, Oliveira R. et al. Effect of a Protective-Ventilation Strategy on Mortality in the Acute Respiratory Distress Syndrome. N Engl J Med. 1998;338:347–354. doi: 10.1056/NEJM199802053380602. [DOI] [PubMed] [Google Scholar]
- Ramsey CD, Funk D, Miller RRI, Kumar A. Ventilator management for hypoxemic respiratory failure attributable to H1N1 novel swine origin influenza virus. Critical Care Medicine. 2010;38:e58–e65. doi: 10.1097/CCM.0b013e3181cde600. [DOI] [PubMed] [Google Scholar]
- Bates JHT. Lung Mechanics: An Inverse Modeling Approach. Cambridge University Press; 2009. [Google Scholar]
- Fernandes CR. A importância da pressão pleural na avaliação da mecânica respiratória. Revista Brasileira de Anestesiologia. 2006;56:287–303. doi: 10.1590/S0034-70942006000300009. [DOI] [PubMed] [Google Scholar]
- Mols G, Kessler V, Benzing A, Lichtwarck-Aschoff M, Geiger K, Guttmann J. Is pulmonary resistance constant, within the range of tidal volume ventilation, in patients with ARDS? British Journal of Anaesthesia. 2001;86:176–182. doi: 10.1093/bja/86.2.176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guérin C, Fournier G, Milic-Emili J. Effects of PEEP on inspiratory resistance in mechanically ventilated COPD patients. European Respiratory Journal. 2001;18:491–498. doi: 10.1183/09031936.01.00072001. [DOI] [PubMed] [Google Scholar]