Abstract
Objective
There is widespread evidence that cancer confers an increased risk of deep venous thrombosis (DVT). This risk is thought to vary among different cancer types. The purpose of this study is to better define the incidence of thrombotic complications among patients undergoing surgical treatment for a spectrum of prevalent cancer diagnoses in contemporary practice.
Methods
All patients undergoing one of 11 cancer surgical operations (breast resection, hysterectomy, prostatectomy, colectomy, gastrectomy, lung resection, hepatectomy, pancreatectomy, cystectomy, esophagectomy, and nephrectomy) were identified by Current Procedural Terminology and International Classification of Diseases, Ninth Revision codes using the American College of Surgeons National Surgical Quality Improvement Program database (2007–2009). The study endpoints were DVT, pulmonary embolism (PE), and overall postoperative venous thromboembolic events (VTE) within 1 month of the index procedure. Multivariate logistic regression was utilized to calculate adjusted odds ratios for each endpoint.
Results
Over the study interval, 43,808 of the selected cancer operations were performed. The incidence of DVT, PE, and total VTE within 1 month following surgery varied widely across a spectrum of cancer diagnoses, ranging from 0.19%, 0.12%, and 0.28% for breast resection to 6.1%, 2.4%, and 7.3%, respectively, for esophagectomy. Compared with breast cancer, the incidence of VTE ranged from a 1.31-fold increase in VTE associated with gastrectomy (95% confidence interval, 0.73–2.37; P = .4) to a 2.68-fold increase associated with hysterectomy (95% confidence interval, 1.43–5.01; P = .002). Multivariate logistic regression revealed that inpatient status, steroid use, advanced age (≥60 years), morbid obesity (body mass index ≥35), blood transfusion, reintubation, cardiac arrest, postoperative infectious complications, and prolonged hospitalization were independently associated with increased risk of VTE.
Conclusions
The incidence of VTE and thromboembolic complications associated with cancer surgery varies substantially. These findings suggest that both tumor type and resection magnitude may impact VTE risk. Accordingly, such data support diagnosis and procedural-specific guidelines for perioperative VTE prophylaxis and can be used to anticipate the risk of potentially preventable morbidity.
Deep venous thrombosis (DVT) and pulmonary embolism (PE) constitute the spectrum of venous thromboembolism (VTE) and remain prevalent causes of mortality and morbidity.1 Accordingly, several national medical and surgical organizations, including the American College of Surgeons, have made detection and prevention of VTE a focus for patient safety initiatives.2–4 DVT and PE are currently recorded in the American College of Surgeons (ACS) National Surgical Quality Improvement Program (NSQIP) as postoperative complications, and are considered by many to be an important quality indicator in surgery.2
Currently, there is widespread consensus that certain patient populations are believed to be at higher risk for VTE. Specifically, malignancy has been associated with a six- to sevenfold increased risk of venous thrombosis5,6 and may result in a threefold increase in PE.7 Interestingly, this risk may vary substantially based on the specific associated malignancy type.5,8,9 Deep venous thrombosis rates have been shown to vary from 2% to 34% among patients with various different localized cancers, while metastatic disease appears to confer an increased supplemental risk.8 Among patients undergoing surgical resection for specific malignancies, VTE remains a major cause for mortality10 and may serve as an important predictor of overall survival.9 Accordingly, patients undergoing surgical resection for cancer are at high risk for VTE and require prophylaxis.1
Currently, recommendations for VTE prophylaxis are concordant for most cancer types, despite the variation in associated VTE risk among specific malignancies. The purpose of this study was to better define the incidence and impact of surgical therapy on rates of DVT, PE, and overall VTE among patients with clinically prevalent cancer diagnoses. In addition, we sought to identify specific periprocedural risk factors associated with VTE in this high-risk patient population, to better identify processes of care to reduce the incidence of VTE and potentially improve outcomes in patients undergoing cancer surgery.
METHODS
NSQIP dataset and study population
The American College of Surgeons National Surgical Quality Improvement Program (ACS-NSQIP) is a multi-institutional cohort study of patients undergoing surgery within participating hospitals. Data are recorded on preoperative, operative, and postoperative outcomes for 30 days after the index procedure. Participant hospitals are provided risk-adjusted outcomes for quality improvement purposes. Data abstraction and variable definitions are available from the ACS-NSQIP Web site.11 Patients who underwent breast surgery, gastrectomy, lung resection, prostatectomy, colectomy, pancreatectomy, esophagectomy, hysterectomy, hepatectomy, cystectomy, and nephrectomy were identified using the ACS-NSQIP Participant Use File for 2007–2009. Cases were identified by primary procedural Current Procedural Terminology (CPT-9 CM) codes and International Classification of Diseases, Ninth Revision (ICD-9) codes for malignant disease to identify cases performed for cancer (Appendix A, online only). Cases done for benign diagnoses were excluded from analysis.
Definition of DVT, PE and VTE
Our endpoints for analysis were postoperative DVT, PE, and overall VTE. DVT/phlebitis is currently defined within ACS-NSQIP as the “identification of a new blood clot or thrombus within the venous system, which may be coupled with inflammation within 30 days of the operation. This diagnosis is confirmed by a duplex [ultrasound], venogram or [computed tomography] CT scan. The patient must be treated with anticoagulation therapy or placement of a vena cava filter or clipping of the vena cava.” PE is defined as “lodging of a blood clot in a pulmonary artery with subsequent obstruction of blood supply to the lung parenchyma. PE [is] documented if the patient has a [ventilation/perfusion] V-Q scan interpreted as high probability of pulmonary embolism or a positive CT spiral exam, pulmonary arteriogram, or CT angiogram.”11 VTE is defined as the presence of DVT and/or PE during the postoperative period.
Statistical analysis
Preoperative, operative, and postoperative variables were compared for each endpoint using χ2 for categorical variables. A two-sample t-test or Wilcoxon rank-sum test was used to compare parametric or nonparametric continuous variables, respectively. Variables of clinical significance and those with a P value of <.05 by univariate analysis were included in a backward stepwise multiple logistic regression to identify significant predictors for each outcome. Variables were removed using the likelihood-ratio test. Continuous variables with nonlinear risk were categorized for logistic analysis. Probability values of <.05 were considered significant. All analyses were performed with Stata Release 11 (STATA Corp, College Station, Tex).
RESULTS
Incidence of thromboembolic complications
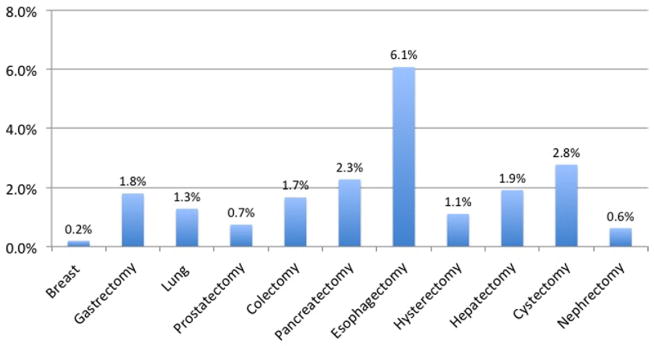
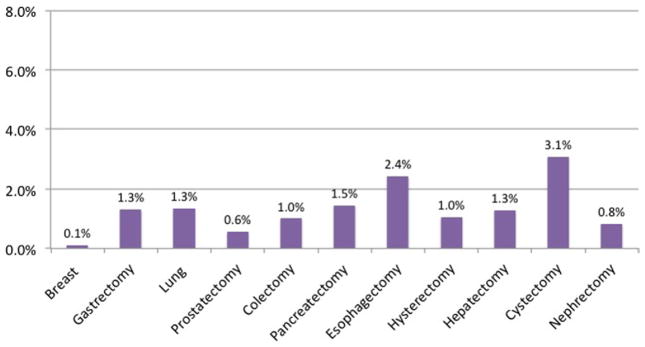
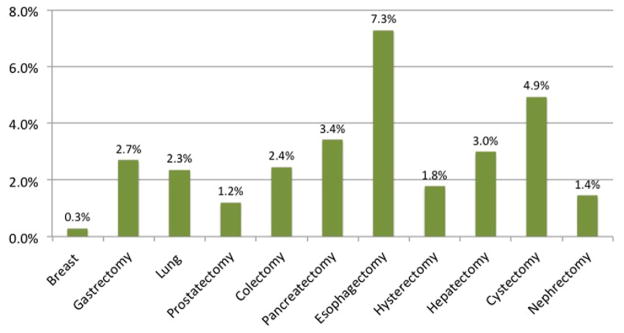
Within the ACS-NSQIP (2007–2009), we identified 43,808 cancer patients undergoing one of 11 surgeries: breast resection (17.7%), gastrectomy (5.4%), lung resection (3.2%), prostatectomy (6.8%), colectomy (36.7%), pancreatectomy (12.6%), esophagectomy (2.5%), hysterectomy (3.7%), hepatectomy (8.5%), cystectomy (0.7%), and nephrectomy (2.2%). The incidence of postoperative DVT, PE, and VTE varied substantially among specific procedures. Associated DVT rates ranged from 0.19% for breast surgery to 6.1% for esophagectomy (Fig 1). The rates of PE ranged from 0.12% for breast surgery to 3.1% for cystectomy (Fig 2). Overall VTE rates ranged from 0.28% for breast surgery to 7.28% for esophagectomy (Fig 3).
Fig 1.
Incidence of deep venous thrombosis (DVT) postoperatively or within 30 days from index cancer operation within National Surgical Quality Improvement Program (NSQIP) (2007–2009).
Fig 2.
Incidence of pulmonary embolism (PE) postoperatively or within 30 days from index cancer operation within National Surgical Quality Improvement Program (NSQIP) (2007–2009).
Fig 3.
Incidence of venous thromboembolism (VTE) postoperatively or within 30 days from index cancer operation within National Surgical Quality Improvement Program (NSQIP) (2007–2009).
Predictors of DVT
Compared with breast resection, major intra-abdominal operations, such as prostatectomy (odds ratio [OR], 2.49, 95% confidence interval [CI], 1.24–4.99), colectomy (OR, 2.12; 95% CI, 1.2–3.75), esophagectomy (OR, 3.64; 95% CI, 1.9–6.95), hysterectomy (OR, 2.95; 95% CI, 1.44–6.03), and hepatectomy (OR, 2.18; 95% CI, 1.16–4.05), had twofold or greater odds of postoperative DVT (Table I). By multivariate analysis, recent steroid use, age (≥60 years), a body mass index (BMI) ≥35 kg/m2, longer operative time, and blood transfusions were associated with increased odds of DVT (Table II). Additionally, postoperative complications, such as wound infection, reintubation, PE, peripheral nerve injury, and postoperative sepsis also increased the odds of DVT. Extended length of stay was associated with a 2.9- to 7.2-times increased odds in DVT compared to shorter lengths of stay (<1 week) (Table II; Appendix B, online only).
Table I.
Adjusted odds ratios of DVT, PE, and overall VTE following selected cancer operations within the American College of Surgeons National Surgical Quality Improvement Program (ACS-NSQIP) (2007–2009)
| Case | DVT
|
PE
|
VTE
|
||||||
|---|---|---|---|---|---|---|---|---|---|
| OR | 95% CI | P | OR | 95% CI | P | OR | 95% CI | P | |
| Breast resection | 1.0 (ref) | 1.0 (ref) | 1.0 (ref) | ||||||
| Gastrectomy | 1.6 | 0.8–3.0 | .17 | 3.0 | 1.4–6.6 | <.01 | 1.3 | 0.7–2.4 | .37 |
| Lung resection | 1.8 | 0.8–3.7 | .12 | 3.9 | 1.7–9.1 | <.01 | 1.8 | 0.9–3.3 | .08 |
| Prostatectomy | 2.5 | 1.2–5.0 | .01 | 3.8 | 1.6–8.9 | <.01 | 2.2 | 1.2–4.0 | .01 |
| Colectomy | 2.1 | 1.2–3.8 | <.01 | 2.8 | 1.4–5.7 | <.01 | 1.7 | 1.0–2.9 | .05 |
| Pancreatectomy | 1.6 | 0.1–2.9 | .15 | 2.9 | 1.4–6.0 | <.01 | 1.3 | 0.8–2.3 | .32 |
| Esophagectomy | 3.6 | 1.9–7.0 | <.01 | 2.2 | 0.9–5.2 | .08 | 2.3 | 1.2–4.1 | <.01 |
| Hysterectomy | 2.9 | 1.4–6.0 | <.01 | 5.5 | 2.4–12.6 | <.01 | 2.7 | 1.4–5.0 | <.01 |
| Hepatectomy | 2.2 | 1.2–4.1 | .01 | 3.8 | 1.8–8.3 | .01 | 1.9 | 1.1–3.4 | .0 |
| Cystectomy | 1.6 | 0.6–4.1 | .29 | 6.4 | 2.4–17.0 | <.01 | 1.8 | 0.9–3.9 | .1 |
| Nephrectomy | 1.3 | 0.5–3.5 | .63 | 4.0 | 1.5–10.8 | <.01 | 1.7 | 0.8–3.5 | .2 |
CI, Confidence interval; DVT, deep vein thrombosis; OR, odds ratio; PE, pulmonary embolism; VTE, venous thromboembolism. Bolded variables indicate P < .05.
Table II.
Multivariate predictors of increased odds of DVT, PE, and VTE
| Variables | OR for DVTa (95% CI, P) | OR for PEb (95% CI, P) | OR for VTEc (95% CI, P) |
|---|---|---|---|
| Recent steroid use | 1.5 (1.0–2.3, .04) | — | 1.4 (1.01–2.0, .04) |
| Recent radiation therapy | — | 1.9 (1.2–3.2,<.01) | — |
| Age >60 years | 1.6 (1.2–2.2,<.01) | — | 1.4 (1.1–1.9, <.01) |
| −1.8 (1.2–2.5, <.01) | |||
| Body mass index ≥35 | 1.5 (1.2–2.0, <.01) | — | 1.3 (1.1–1.7, <.01) |
| Platelets ≥400 K | — | 1.5 (1.1–2.0, .01) | — |
| Transfusion ≥3 units | 1.5 (1.1–2.1, .02) | — | 1.3 (1.0–1.8, .05) |
| −3.4 (1.9–6.3, <.01) | −2.3 (1.3–4.1, <.01) | ||
| Deep infection | 1.5 (1.2–1.9, <.01) | 1.4 (1.0–2.0, .03) | 1.5 (1.2–1.9, <.01) |
| Reintubated | 1.5 (1.2–2.0, <.01) | 2.0 (1.4–2.8, <.01) | 1.7 (1.4–2.2, <.01) |
| PE | 11.5 (8.9–15.0, <.01) | — | — |
| DVT | — | 11.2 (8.6–14.6, <.01) | — |
| Urinary tract infection | — | 1.8 (1.3–2.5, <.01) | — |
| Peripheral nerve injury | 6.9 (2.5–19.2, <.01) | — | — |
| Sepsis postoperation | 1.5 (1.2–2.0, <.01) | — | 1.4 (1.1–1.7, <.01) |
| Shock postoperation | 1.5 (1.1–2.1, <.01) | — | 1.3 (1.0–1.7, .04) |
| Cardiac arrest | — | 3.1 (1.9–5.2, <.01) | 1.9 (1.3–2.8, .02) |
| Operative time (minutes) | 1.0 (1.0–1.001, <.01) | — | — |
| Length of stay >1 week | 2.9 (2.2–3.7, <.01) | 3.0 (2.3–4.0, <.01) | 3.4 (2.8–4.1, <.01) |
| −7.2 (4.4–11.7, <.01) | −6.4 (2.3–14.7, <.01) | −11.7 (7.8–17.5, <.01) |
CI, Confidence interval; DVT, deep venous thrombosis; OR, odds ratio; PE, pulmonary embolism; VTE, venous thromboembolic event.
See Appendix B (online only) for complete DVT multivariate model.
See Appendix C (online only) for complete PE multivariate model.
See Appendix E (online only) for complete VTE multivariate model.
Predictors of PE
Like DVT, the odds of PE varied by operation as well. Compared with breast resection, every operation except for esophagectomy had over a twofold increased odds of PE (Table I). Additionally, hysterectomy (OR, 5.51; 95% CI, 2.41–12.6) and cystectomy (OR, 6.39; 95% CI, 2.4–17.01) conferred a much higher risk of postoperative PE. By multivariate analysis, recent radiation treatment, thrombocytosis (platelets ≥400.000), and postoperative complications such as wound infection, reintubation, DVT, urinary tract infection, and cardiac arrest all were associated with increased odds of PE (Table II). Of note, prolonged hospital length of stay conferred a 3.03 to 6.44 odds of PE compared with shorter in-hospital length of stay (<1 week). Interestingly, low BMI (<25 kg/m2) and postoperative dialysis reduced the odds of PE (Table III, Appendix C, online only).
Table III.
Multivariate predictors of decreased odds of DVT, PE, and VTE
| Variables | OR for DVTa (95% CI, P) | OR for PEb (95% CI, P) | OR for VTEc (95% CI, P) |
|---|---|---|---|
| Body mass index <25 | — | 0.7 (0.5–0.9, <.01) | 0.8 (0.7–0.9, .04) |
| Postoperative dialysis | — | 0.4 (0.2–0.8, .02) | — |
| Outpatient status | — | — | 0.5 (0.2–0.9, .02) |
| Days from admission to surgery | — | — | 0.9 (0.9–0.97, <.01) |
CI, Confidence interval; DVT, deep venous thrombosis; OR, odds ratio; PE, pulmonary embolism; VTE, venous thromboembolic event.
See Appendix B (online only) for complete DVT multivariate model.
See Appendix C (online only) for complete PE multivariate model.
See Appendix E (online only) for complete VTE multivariate model.
Predictors of VTE
Among the 135 patient-level variables recorded in the ACS-NSQIP, 60 perioperative variables were associated with the development of VTE, our composite endpoint, by univariate comparison (Appendix D, online only). Compared with breast resection, prostatectomy (OR, 2.17; 95% CI, 1.17–4.02), esophagectomy (OR, 2.26; 95% CI, 1.24–4.12), hysterectomy (OR, 2.68; 95% CI, 1.43–5.01), and hepatectomy (OR, 1.92; 95% CI, 1.09–3.39) were found to confer a higher risk of VTE. Multivariate predictors of VTE included age (60–79 years), recent steroid use, BMI ≥35 kg/m2, and postoperative complications, including wound infection, reintubation, cardiac arrest, and sepsis (Table II). Longer hospitalizations conferred a 3.4 to 11.16 increased odds of VTE compared with shorter hospitalizations (<1 week; Table II). Conversely, admission prior to surgery, outpatient procedures, and low a BMI (<25 kg/m2) conferred some benefit from VTE (Table III; Appendix E, online only).
DISCUSSION
Vascular surgeons are frequently consulted to optimize hospital care among patients identified with de novo DVT, PE, and VTE. Often, such care features surgical oncology patients having undergone operative resection. Although there is a widespread recognition for associated hypercoaguability among patients with malignancy, less is known about the potential variation in thrombotic complications in this presumptive high-risk group. Moreover, despite potential variations in the risk of VTE among patients with different cancer diagnoses, there are few variations in prophylactic care to prevent these morbid complications. This study demonstrates that the risks of DVT, PE, and concordantly VTE, vary across the spectrum of neoplastic diagnoses. Specifically, these data suggest that patients undergoing prostatectomy, esophagectomy, hysterectomy, and hepatectomy for cancer are at increased risk of VTE. Interestingly, hospital length of stay appears to be one of the greatest risk factors associated with VTE.
Intuitively, patients undergoing cancer surgery remain at a high risk for VTE as they meet criteria for Virchow’s triad of hypercoaguability, stasis, and endothelial injury. Furthermore, cancer has been shown in prior population-based studies to carry a fourfold increased risk of VTE.6,12 The pathophysiology of hypercoagulability in cancer remains complex and likely involves a series of cytokine signaling, abnormal fibrinolysis, and dysfunctional platelet adhesion.13 In addition, surgical resection may carry as much as a 22-fold increased risk of VTE,6 which may still be augmented by varying cancer type, adjuvant chemotherapy, and the requisite need for indwelling catheters.13
Several studies have documented that the risk of VTE varies by the type of cancer.5,8,14 Across studies, pancreatic, brain, lung, stomach, and hematologic malignancies remain associated with a higher risk of VTE. In contrast, head and neck cancers, as well as breast malignancies, although still greater than control, appear to have a lower associated thrombotic risk. Interestingly, although perhaps not surprisingly, the presence of metastatic disease may double the risk of VTE.8 This study demonstrates that the risk of VTE also appears to vary by cancer procedure, in addition to cancer type. Yet our analysis demonstrates that previously perceived lower-risk malignancies such as prostate cancer, esophageal cancer, and liver cancer may actually carry a higher risk of VTE than previously believed. This finding may be due to several differences in our study compared with others. Previous studies have been predominantly epidemiologic in nature5,8 and not specific to patients in the perioperative period. ACS-NSQIP represents a special population of cancer patients, and their risk of VTE likely reflects a combination of both their specific cancer type as well as the magnitude of their surgery. Thus, it is not unexpected for example, that esophageal cancer may carry a more significant increased risk of VTE, as this diagnosis often requires an anatomic resection in both the abdomen and thorax. Therefore, we believe these findings represent a novel analysis of VTE risk among cancer patients.
The current American College of Chest Physicians guidelines recommend pharmacologic VTE prophylaxis for patients with multiple risk factors for thrombotic complications and for those undergoing moderate to major risk surgical procedures. Patients at low risk and undergoing low-risk procedures do not require routine chemical prophylaxis. Ongoing pharmacologic prophylaxis, however, remains recommended for hospitalized cancer patients following surgery, or those who are felt to be medically high risk or bedridden.1 Interestingly, there is currently no variation in method or duration of prophylaxis among cancer types, oncological procedures, or length of stay despite a clear disparity in the risk of sustaining a thrombotic event. Although length of stay alone as a risk factor for the development of a DVT is not well established, the association of prolonged hospitalization, increased cost, and mortality referable to VTE is well known. Specifically, VTE may result in an additional 5 days of hospitalization, an increased cost of $20,000, and a 6% increased mortality on average based on National Inpatient Sample data.15 Based on these findings, this study may assist efforts to reduce VTE events in postoperative cancer patients, by minimizing length of stay, including proper risk stratification, vigilant prophylaxis, minimizing postoperative infectious complications, and minimizing requisite transfusions.
Although Merkow et al previously demonstrated a variation in the incidence of VTE in a similar patient population, the study focused more on associated rates of postdischarge VTE in an effort to recognize the potential need for prolonged prophylaxis.16 This study, in contrast, highlights the perioperative factors that may predispose patients to VTE. Thus, the model incorporated herein utilized additional variables, such as length of stay, which has been shown to increase the risk of DVT.17–19 Length of stay is also a reflection of surgical magnitude and an effect of overall complication rates. As these data point out, prolonged hospital stay is a major risk factor for VTE, which may reflect extended bed rest and associated venous stasis. Thus, our estimates of risk across cancer surgeries are more conservative as we have incorporated these important perioperative variables.
This study has several important limitations. Using these retrospective data, we remain unable to prove causation between specific malignancy resection and thrombotic complications but rather only association. In addition, specific patient-level data remain unavailable within the ACS-NSQIP public use file. Accordingly, we remain unable to identify patients with prior VTE, who may be at higher risk for a second event. Furthermore, specific VTE prophylaxis, such as specific pharmacologic protocols, inferior vena cava filter use, or sequential compression device use, may vary by both surgeon and facility, which could impact the observed rates of variation. Lastly, anatomic features of DVTs (upper vs lower extremity) or the presence of indwelling catheters remain unknown, which may confound these results. Nevertheless, these results do depict variation in thrombotic events associated with various malignancies in real-world contemporary practice. Moreover, this analysis has identified both higher-risk surgeries and independent predictors of DVT, PE, and VTE, which may be considered in an attempt to diminish the incidence of these morbid complications. These results may be used to develop prospective cohort studies designed to validate risk of VTE in patients undergoing various cancer surgeries. This will then be able to overcome the limitations of observational data.
CONCLUSIONS
Patients with cancer remain at increased risk for VTE; however, this appears to vary by malignancy type. In addition, variation in VTE risk among patients undergoing surgical resection may differ not only by cancer but also by the associated resection magnitude. Among procedure types evaluated herein, breast resection appears to have the lowest risk of VTE, while specific intra-abdominal and pelvic cancer resections have the highest risk. These data further identify patients at increased risk of VTE, such as those with increased age, length of stay, BMI, and transfusion rates, as well as those with infectious complications following cancer surgery. Clinicians may use these findings to stratify patient risk for VTE following operative resection in an attempt to minimize the morbidity associated with DVT, PE, and VTE. Additionally, this study may provide the basis for improved diagnosis and procedural-specific guidelines for perioperative DVT and PE prophylaxis.
Supplementary Material
Footnotes
Competition of interest: none.
Presented at the Thirty-eighth Annual Meeting of the New England Society for Vascular Surgery, Providence, RI, September 16–18, 2011.
Additional material for this article may be found online at www.jvascsurg.org.
The editors and reviewers of this article have no relevant financial relationships to disclose per the JVS policy that requires reviewers to decline review of any manuscript for which they may have a competition of interest.
Additional material for this article may be found online at www.jvascsurg.org.
AUTHOR CONTRIBUTIONS
Conception and design: RD, PG, JW, MC, DS
Analysis and interpretation: RD, PG, ES, ER, DW, DS
Data collection: RD, ES, JW, MC
Writing the article: RD, DS
Critical revision of the article: RD, JW, DS
Final approval of the article: RD, PG, JW, MC, ER, DW, DS
Statistical analysis: RD, ES, JW
Obtained funding: Not applicable
Overall responsibility: RD
References
- 1.Geerts WH, Bergqvist D, Pineo GF, Heit JA, Samama CM, Lassen MR, et al. Prevention of venous thromboembolism: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines (8th edition) Chest. 2008;133(6 Suppl):381S–453S. doi: 10.1378/chest.08-0656. [DOI] [PubMed] [Google Scholar]
- 2.AHRQ. AHRQ Quality Indicators. Rockville, MD: Agency for Healthcare Research and Quality; 2006. [Accessed July 2011]. Quality Indicators: Guide to Patient Safety Indicators. Available at: http://www.qualityindicators.ahrq.gov/Modules/psi_resources.aspx. [Google Scholar]
- 3.US Department of Health and Human Services. [Accessed November 30, 2011];The surgeon general’s call to action to prevent deep vein thrombosis and pulmonary embolism. 2008 Available at: http://www.surgeongeneral.gov/topics/deepvein/calltoaction/call-to-action-on-dvt-2008.pdf. [PubMed]
- 4.Association APH. [Accessed November 30, 2011];Coalition to Prevent Deep Venous Thrombosis. 2003 Available at: http://www.apha.org/programs/partnerships/dvt.htm.
- 5.Blom JW, Doggen CJ, Osanto S, Rosendaal FR. Malignancies, prothrombotic mutations, and the risk of venous thrombosis. JAMA. 2005;293:715–22. doi: 10.1001/jama.293.6.715. [DOI] [PubMed] [Google Scholar]
- 6.Heit JA, Silverstein MD, Mohr DN, Petterson TM, O’Fallon WM, Melton LJ., 3rd Risk factors for deep vein thrombosis and pulmonary embolism: a population-based case-control study. Arch Intern Med. 2000;160:809–15. doi: 10.1001/archinte.160.6.809. [DOI] [PubMed] [Google Scholar]
- 7.Kakkar AK, Haas S, Wolf H, Encke A. Evaluation of perioperative fatal pulmonary embolism and death in cancer surgical patients: the MC-4 cancer substudy. Thromb Haemost. 2005;94:867–71. doi: 10.1160/TH04-03-0189. [DOI] [PubMed] [Google Scholar]
- 8.Blom JW, Vanderschoot JP, Oostindiër MJ, Osanto S, van der Meer FJ, Rosendaal FR. Incidence of venous thrombosis in a large cohort of 66,329 cancer patients: results of a record linkage study. J Thromb Haemost. 2006;4:529–35. doi: 10.1111/j.1538-7836.2006.01804.x. [DOI] [PubMed] [Google Scholar]
- 9.Chew HK, Wun T, Harvey D, Zhou H, White RH. Incidence of venous thromboembolism and its effect on survival among patients with common cancers. Arch Intern Med. 2006;166:458–64. doi: 10.1001/archinte.166.4.458. [DOI] [PubMed] [Google Scholar]
- 10.Agnelli G, Bolis G, Capussotti L, Scarpa RM, Tonelli F, Bonizzoni E, et al. A clinical outcome-based prospective study on venous thromboembolism after cancer surgery: the RISTOS project. Ann Surg. 2006;243:89–95. doi: 10.1097/01.sla.0000193959.44677.48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.American College of Surgeons. [Accessed November 30, 2011];National Surgical Quality Improvement Program. Available at: http://www.acsnsqip.org/
- 12.Silverstein MD, Heit JA, Mohr DN, Petterson TM, O’Fallon WM, Melton LJ., 3rd Trends in the incidence of deep vein thrombosis and pulmonary embolism: a 25-year population-based study. Arch Intern Med. 1998;158:585–93. doi: 10.1001/archinte.158.6.585. [DOI] [PubMed] [Google Scholar]
- 13.Osborne NH, Wakefield TW, Henke PK. Venous thromboembolism in cancer patients undergoing major surgery. Ann Surg Oncol. 2008;15:3567–78. doi: 10.1245/s10434-008-0151-4. [DOI] [PubMed] [Google Scholar]
- 14.Levitan N, Dowlati A, Remick SC, Tahsildar HI, Sivinski LD, Beyth R, Remick AA, et al. Rates of initial and recurrent thromboembolic disease among patients with malignancy versus those without malignancy. Risk analysis using Medicare claims data. Medicine (Baltimore) 1999;78:285–91. doi: 10.1097/00005792-199909000-00001. [DOI] [PubMed] [Google Scholar]
- 15.Zhan C, Miller MR. Excess length of stay, charges, and mortality attributable to medical injuries during hospitalization. JAMA. 2003;290:1868–74. doi: 10.1001/jama.290.14.1868. [DOI] [PubMed] [Google Scholar]
- 16.Merkow RP, Bilimoria KY, McCarter MD, Cohen ME, Barnett CC, Raval MV, et al. Post-discharge venous thromboembolism after cancer surgery: extending the case for extended prophylaxis. Ann Surg. 2011;254:131–7. doi: 10.1097/SLA.0b013e31821b98da. [DOI] [PubMed] [Google Scholar]
- 17.Paffrath T, Wafaisade A, Lefering R, Simanski C, Bouillon B, Spanholtz T, et al. Venous thromboembolism after severe trauma: incidence, risk factors and outcome. Injury. 2010;41:97–101. doi: 10.1016/j.injury.2009.06.010. [DOI] [PubMed] [Google Scholar]
- 18.Ambrosetti M, Salerno M, Zambelli M, Mastropasqua F, Tramarin R, Pedretti RF. Deep vein thrombosis among patients entering cardiac rehabilitation after coronary artery bypass surgery. Chest. 2004;125:191–6. doi: 10.1378/chest.125.1.191. [DOI] [PubMed] [Google Scholar]
- 19.Napolitano LM, Garlapati VS, Heard SO, Silva WE, Cutler BS, O’Neill AM, et al. Asymptomatic deep venous thrombosis in the trauma patient: is an aggressive screening protocol justified? J Trauma. 1995;39:651–7. doi: 10.1097/00005373-199510000-00006. Discussion:7–9. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.