Abstract
Background
The association between metabolic syndrome and electrocardiographic (ECG) abnormalities is not well established.
Methods
ECG tracings of 6,765 men and women aged 45–84 years, free of clinical cardiovascular disease, from the Multi-Ethnic Study of Atherosclerosis were obtained (2000–2002) and classified as normal or having major or minor abnormalities. We evaluated the associations of metabolic syndrome and its components with ECG abnormalities, adjusting for age, ethnicity, and gender and testing for effect modification by ethnicity and gender.
Results
The associations of metabolic syndrome, hypertension, and high triglycerides with ECG abnormalities varied significantly by gender. In males, metabolic syndrome and hypertension were significantly associated with major ECG abnormality [1.69 (1.33–2.13), and 2.22 (1.72–2.86), respectively] after adjusting for ethnicity and gender. Hypertension was also associated significantly with minor ECG abnormality in males after adjusting for age and ethnicity. In females, metabolic syndrome and hypertension were significantly associated with major [1.84 (1.44–2.37), and 1.68 (1.27–2.22), respectively] and minor [1.38 (1.19–1.59), and 1.53 (1.32–1.79), respectively] ECG abnormalities after adjusting for age and ethnicity. High triglycerides were only significantly associated with major ECG abnormality in females after adjusting for age and ethnicity. After adjusting for age, ethnicity, and gender, central obesity and high fasting blood glucose were significantly associated with major and minor ECG abnormalities, whereas low high-density lipoprotein cholesterol was significantly associated with major ECG abnormality only.
Conclusions
Metabolic syndrome and its components are associated with major and/or minor ECG abnormalities. The relationship of metabolic syndrome, hypertension, and high triglycerides with ECG abnormalities varied according to gender.
Introduction
Metabolic syndrome refers to the coexistence of multiple metabolic abnormalities and is associated with an increased risk of nonfatal cardiovascular disease (CVD) as well as cardiovascular and all-cause mortality.1–6 The components of metabolic syndrome constitute a group of underlying, major, and emerging CVD risk factors.7 Similarly, major and minor abnormalities detected on resting electrocardiograms (ECG) have been identified as significant predictors of all-cause, coronary heart disease (CHD) and CVD mortality independent of traditional risk factors.2,8–13
Although the prevalence and prognostic significance of ECG abnormalities have been studied in other populations,8,14,15 the prevalence and associations of ECG abnormalities in persons with metabolic syndrome have not been investigated thoroughly, especially in non-white populations. If ECG abnormalities are more common in persons with metabolic syndrome, their presence might enable the identification of persons with metabolic syndrome who are at especially high risk for a CVD event and who might therefore benefit from more intensive risk factor management. Due to the high prevalence of metabolic syndrome and its components in the adult U.S. population,16 it is important from a public health perspective to determine appropriate approaches to identify those individuals at especially increased cardiovascular risk.
In this study, we examined the prevalence and associations of ECG abnormalities in persons with and without metabolic syndrome and its individual components. We also evaluated for the presence of a progressive relationship between the number of components of metabolic syndrome (the metabolic syndrome score) with major and minor ECG abnormalities. We hypothesized that metabolic syndrome and its individual components are associated with major and minor ECG abnormalities. We also hypothesized that there is a progressive relationship in the association of metabolic syndrome score with major and minor ECG abnormalities.
Methods
Design and sample
The Multi-Ethnic Study of Atherosclerosis (MESA) is a population-based sample of 6,814 men and women from four ethnic groups (38% white, 28% African-American, 22% Hispanic, and 12% Chinese) aged 45–84 years at baseline (2000–2002), without clinical CVD prior to recruitment. Details regarding MESA's design and objectives have been published.17 The cohort was selected from six regions in the United States: Forsyth County, North Carolina; north Manhattan and the Bronx, New York; Baltimore City and Baltimore County, Maryland; St. Paul, Minnesota; Chicago, Illinois; and Los Angeles County, California. The protocol was approved by the institutional review boards of all participating sites, and informed consent was obtained from the participants. Our report is based on cross-sectional data collected during the MESA baseline exam and involving participants who had ECGs at baseline. Participants were excluded from these analyses if they did not have ECGs or if they had unreadable ECGs at baseline.
Baseline measurements
Demographic information on age, gender, and ethnicity was obtained using standard questionnaires. Questionnaires were also used to assess information on medical history of hypertension or diabetes and medication usage. Waist circumference was measured in centimeters. Three measurements of resting blood pressure were obtained from the right arm using a Dinamap automated blood pressure device. The average of the second and third values was used in analyses. Fasting blood samples obtained by venipuncture were collected and transported to the collaborative studies clinical laboratory at Fairview-University Medical Center (Minneapolis, MN) for analysis of fasting blood glucose, triglycerides and high-density lipoprotein-cholesterol (HDL-C). Quality control activities were conducted at the field centers, coordinating centers, laboratories, and ECG reading centers to ensure data quality.
Definition of the metabolic syndrome
Metabolic syndrome is defined according to the modified National Cholesterol Education Program Adult Treatment Panel III (NCEP ATP III) guidelines as consisting of three or more of the following metabolic abnormalities: central obesity (waist circumference >102 cm in men and >88 cm in women), high triglycerides (≥150 mg/dL), low HDL-C (<40 mg/dL in men and <50 mg/dL in women), high blood pressure (≥130/85 mmHg) or being on antihypertensive medications and high fasting blood glucose (≥100 mg/dL) or being on antidiabetic medications.6,18 The metabolic syndrome score is defined as the number of coexisting metabolic abnormalities.
Electrocardiographic measures
A resting 12-lead ECG was obtained from fasting participants using a GE/Marquette MAC 1200 electrocardiograph. Participants with atrial flutter, atrial fibrillation, or the presence of a pacemaker at baseline on locally read ECGs were excluded from further participation in the MESA study. All ECGs were centrally read and coded at the Epidemiological Cardiology Research Center (EPICARE), Wake Forest School of Medicine (Winston-Salem, NC). The Minnesota19 coding system was used to classify ECG tracings as having a major or minor abnormality. Participants with only minor ECG abnormalities were classified as having “any minor abnormalities,” and participants with major ECG abnormalities with or without coexisting minor ECG abnormalities were classified as having “any major ECG abnormalities.” Major ECG abnormalities included: Major ventricular conduction defect; definite myocardial infarction (defined as the presence of major Q wave abnormalities); possible myocardial infarction (defined as the presence of minor Q/-QS wave plus major ST/-T abnormalities); major isolated -ST/-T abnormalities; left ventricular hypertrophy; major atrioventricular conduction abnormalities; major QT prolongation (QT≥116%), pacemaker, and other major arrhythmias. Minor ECG abnormalities included: Minor isolated Q/-QS waves; minor isolated ST/-T abnormalities; high R waves; ST segment elevation; incomplete (left and right) bundle branch block; minor QT prolongation (QTi≥112%); short PR interval; left axis deviation; right axis deviation; frequent ventricular premature beats; and other minor arrhythmias. Detailed descriptions of major and minor ECG abnormalities by the Minnesota code classification system are provided online as Supplementary Tables 1 and 2 (Supplemental Data are available at www.liebertonline.com/met).
Statistical analysis
Baseline characteristics were presented as means and standard deviations for continuous variables and proportions for discrete variables. The prevalence of major and minor ECG abnormalities across groups with and without metabolic syndrome and its components was calculated. We conducted unadjusted and multivariable adjusted multinomial logistic regression analyses to model our outcomes of major and minor ECG abnormalities by metabolic syndrome, components of metabolic syndrome, and other covariates (age, gender and ethnicity). The homogeneity of the association across different gender and ethnic groups was tested in multinomial logistic regression models at a 5% significance level. In model 1, we evaluated the metabolic syndrome or any of its individual components independently and adjusted for age, gender, and ethnicity. In model 2, we jointly evaluated all components of the metabolic syndrome concurrently and adjusted for age, ethnicity, and gender. Odds ratios for major and minor ECG abnormalities between metabolic syndrome and non-metabolic syndrome participants were estimated along with their 95% confidence intervals. We also tested for a trend in the association of metabolic syndrome score with major and minor ECG abnormalities. Statistical analysis was performed using SAS version 9.2 (SAS Institute Inc., Cary, NC).
Sensitivity analysis
In defining criteria for central obesity, Asian-specific criteria employ a threshold for waist circumference >90 cm in men and >80 cm in women.2,20 Twelve percent of the MESA population is Asian (predominantly of Chinese descent). In a sensitivity analysis, we applied the Asian-specific threshold to the diagnosis of central obesity and metabolic syndrome in the Asian-American population of the MESA cohort.
Results
From the original cohort of 6,814, 49 participants were excluded due to poor quality or missing ECGs at baseline, leaving an eligible sample size of 6,765. Demographic, clinical, and metabolic characteristics of study participants are presented in Table 1. Participants included a diverse group of middle-aged and older men and women. Ethnicity- and gender-specific prevalences of major and minor ECG abnormalities are presented according to metabolic syndrome in Table 2. Among participants with metabolic syndrome, the prevalence of major and minor ECG abnormalities was 17.8% and 47.3% respectively, in males and 11.7% and 38.8%, respectively, in females. In all gender-ethnic groups, the prevalence of total (sum of major and minor) ECG abnormalities was greater in persons with metabolic syndrome than in those without.
Table 1.
Characteristics of Study Participants by Gender
| Variables | Male N=3,193 | Female N=3,572 |
|---|---|---|
| Age (years) | 62.2±10.2 | 62.1±10.3 |
| Race | ||
| White, % | 38.9 | 37.7 |
| Chinese, % | 12.2 | 11.6 |
| African-American, % | 26.3 | 29.2 |
| Hispanic, % | 22.5 | 21.6 |
| Waist circumference (cm) | 99.3±12.3 | 97.1±16.0 |
| Systolic blood pressure (mmHg) | 126.1±19.3 | 127.1±23.2 |
| Diastolic blood pressure (mmHg) | 72.1±9.4 | 69.1±10.2 |
| Fasting blood glucose (mg/dL) | 100.2±32.9 | 94.9±27.6 |
| Triglycerides (mg/dL) | 135.3±95.5 | 128.1±82.3 |
| High-density lipoprotein cholesterol (mg/dL) | 45.1±11.8 | 56.3±15.3 |
| Diabetes, % | 14.0 | 11.5 |
| Hypertension, % | 42.8 | 46.6 |
| Metabolic syndrome, % | 32.5 | 39.1 |
| Major electrocardiographic abnormality, % | 13.4 | 8.4 |
| Minor electrocardiographic abnormality, % | 49.0 | 35.2 |
Values are expressed as means±standard deviation (SD) unless otherwise indicated. Variables may contain missing data, and the total sum for each variable may not equal sample size. The percentage of missing values is less than 1% for all variables.
Table 2.
Ethnicity- and Gender-Specific Prevalences of Major and Minor Electrocardiographic Abnormalities According to Metabolic Syndrome Status
| |
Male |
Female |
||
|---|---|---|---|---|
| |
Metabolic syndrome |
Metabolic syndrome |
||
| ECG findings | Present | Absent | Present | Absent |
| White, N | 379 | 861 | 443 | 897 |
| Major abnormality, % | 17.4 | 12 | 11.3 | 6.5 |
| Minor abnormality, % | 47.5 | 51.5 | 36.8 | 32.4 |
| Normal ECG, % | 35.1 | 36.6 | 51.9 | 61.1 |
| Chinese, N | 92 | 297 | 137 | 276 |
| Major abnormality, % | 15.2 | 8.1 | 13.1 | 7.3 |
| Minor abnormality, % | 41.3 | 43.4 | 32.1 | 24.6 |
| Normal ECG, % | 43.5 | 48.5 | 54.7 | 68.1 |
| African American, N | 285 | 552 | 427 | 610 |
| Major abnormality, % | 23.2 | 15.2 | 11.5 | 6.6 |
| Minor abnormality, % | 46.3 | 52.2 | 45.2 | 38.5 |
| Normal ECG, % | 30.5 | 32.6 | 43.3 | 54.9 |
| Hispanic, N | 281 | 438 | 390 | 381 |
| Major abnormality, % | 13.9 | 6.9 | 10 | 6.6 |
| Minor abnormality, % | 49.8 | 47 | 36.4 | 29.7 |
| Normal ECG, % | 36.3 | 46.1 | 53.6 | 63.8 |
| Entire sample, N | 1037 | 2148 | 1397 | 2164 |
| Major abnormality, % | 17.8 | 11.2 | 11.7 | 6.6 |
| Minor abnormality, % | 47.3 | 49.6 | 38.8 | 32.7 |
| Normal ECG, % | 34.9 | 39.2 | 50 | 60.7 |
Total number of eligible participants is 6,765; 19 participants had missing values on metabolic syndrome.
Values are expressed as n for number of participants in each gender, ethnicity, and metabolic syndrome group and % for subclassification by ECG findings in each group.
ECG, electrocardiogram.
In unadjusted analyses, high fasting blood glucose and central obesity were significantly associated with major ECG abnormality [odds ratio (OR) (95% confidence interval, CI)=1.98 (1.67–2.35), and 1.28 (1.09–1.50), respectively] and high fasting blood glucose with minor ECG abnormalities [OR (95% CI)=1.34 (1.19–1.50)]. Low HDL-C and central obesity were not associated with minor ECG abnormalities. Low HDL-C was also not associated with major ECG abnormalities. Adjusted ORs of the associations of metabolic syndrome and its components with major and minor ECG abnormalities are presented in Table 3. Data are shown within strata of gender for metabolic syndrome, hypertension, and high triglycerides in models 1 and 2 because the interactions of “metabolic syndrome with gender,” “hypertension with gender,” and “high triglyceride with gender” were significant at the 5% level. There were no significant interactions between ethnicity and metabolic syndrome or any of its individual components at the 5% level. After adjusting for age, ethnicity, and gender (model 1), the associations of central obesity and low HDL-C became stronger while the association of high fasting blood glucose became weaker for major and minor ECG abnormalities. The simultaneous examination of all components of metabolic syndrome while adjusting for age, gender, ethnicity, interaction terms of “high triglyceride with gender,” and “hypertension with gender” in model 2, to evaluate the relative impact of cardiometabolic risk factors resulted in a weakening of the associations observed in model 1. Metabolic syndrome was not included in model 2.
Table 3.
Adjusted Odds Ratios of the Associations of the Metabolic Syndrome and Its Components with Major and Minor Electrocardiographic Abnormalities
| |
|
Model 1a |
Model 2b |
||
|---|---|---|---|---|---|
| Risk factor | ECG abnormality | ORc | 95% CI | ORc | 95% CI |
| Metabolic syndrome | Major | ||||
| Male | 1.69 | 1.33–2.13 | |||
| Female | 1.84 | 1.44–2.37 | |||
| Minor | |||||
| Male | 1.03 | 0.87–1.21 | |||
| Female | 1.38 | 1.19–1.59 | |||
| Hypertension | Major | ||||
| Male | 2.22 | 1.72–2.86 | 2.05 | 1.58–2.65 | |
| Female | 1.68 | 1.27–2.22 | 1.44 | 1.09–1.92 | |
| Minor | |||||
| Male | 1.31 | 1.12–1.54 | 1.31 | 1.11–1.53 | |
| Female | 1.53 | 1.32–1.79 | 1.49 | 1.27–1.74 | |
| High triglycerides | Major | ||||
| Male | 1.03 | 0.81–1.32 | 0.84 | 0.65–1.09 | |
| Female | 1.84 | 1.41–2.39 | 1.54 | 1.17–2.02 | |
| Minor | |||||
| Male | 0.85 | 0.72–1.01 | 0.79 | 0.66–0.94 | |
| Female | 1.11 | 0.95–1.31 | 1.01 | 0.86–1.20 | |
| Central obesity | Major | 1.67 | 1.39–2.01 | 1.45 | 1.19–1.75 |
| Minor | 1.22 | 1.09–1.37 | 1.16 | 1.03–1.31 | |
| High FBG | Major | 1.60 | 1.34–1.92 | 1.33 | 1.11–1.61 |
| Minor | 1.19 | 1.06–1.35 | 1.11 | 0.98–1.26 | |
| Low HDL-C | Major | 1.26 | 1.06–1.50 | 1.07 | 0.89–1.29 |
| Minor | 1.03 | 0.93–1.15 | 1.00 | 0.89–1.13 | |
Model 1: Metabolic syndrome or its individual components, adjusted for age, gender, and ethnicity. Interactions of “metabolic syndrome with gender” for metabolic syndrome, “high triglyceride with gender” for high triglyceride, and “hypertension with gender” for hypertension was included.
Model 2: All components of the metabolic syndrome concurrently adjusted for age, gender, ethnicity, interaction terms of “high triglyceride with gender” and “hypertension with gender”.
Odds ratios were estimated using multinomial logistic regression with normal ECG as reference.
ECG, electrocardiogram; OR, odds ratio; CI, confidence interval; FBG, fasting blood glucose; HDL–C, high-density lipoprotein cholesterol.
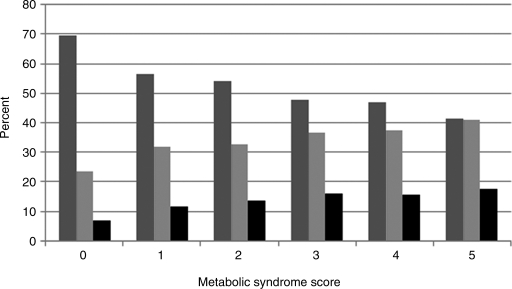
In unadjusted analysis, there was a higher prevalence of both major and minor ECG abnormalities with increasing metabolic syndrome score as shown in Fig. 1. For each additional component present, major ECG abnormalities [OR, 1.31 (1.24–1.39); P<0.001] and minor ECG abnormalities [OR, 1.09 (1.05–1.13); P<0.001] were more common.
FIG. 1.
The relationship of the metabolic syndrome score with major and minor electrocardiogram (ECG) abnormalities. (medium gray) Normal ECG; (light gray) minor ECG abnormality; (black) major ECG abnormality.
When we applied the Asian-specific criteria for waist circumference to our analysis, the general pattern of the results was unchanged; hence, we reported results in which we used the modified NECP ATP III criteria. Results in which we applied the Asian-specific criteria are available online as Supplementary Table 3.
Discussion
Our cross-sectional analysis has shown associations of metabolic syndrome with major and minor ECG abnormalities. Because ECG abnormalities are known predictors of cardiovascular mortality,2, 8–13 our finding agrees with previous studies that have demonstrated associations of metabolic syndrome with CVD morbidity and mortality.1–6 Although, not examined in this cross-sectional analysis, the presence of ECG abnormalities may be an indicator of increased CVD risk in patients with metabolic syndrome. The presence of gender interactions may suggest varying risks associated with ECG abnormalities in men and women with metabolic syndrome.
When individual components were examined, central obesity and high fasting blood glucose (FBG) were significantly associated with major and minor ECG abnormalities after adjusting for age, ethnicity, and gender. Hypertension was significantly associated with major and minor ECG abnormalities after adjusting for age and ethnicity, and the association with major ECG abnormalities was stronger in men than in women. Our findings agree with numerous previous studies that have shown hypertension,21 central obesity,22,23 and high FBG24 to be associated with the greater risk of cardiovascular morbidity and mortality.
In individuals with metabolic syndrome who share a common metabolic background, there are inconsistent data on the role of triglycerides as a CVD predictor.25 Our study demonstrates gender variability in the associations of high triglycerides with major and minor ECG abnormalities, with the association being stronger in women than in men, perhaps because some components of metabolic syndrome predict CVD morbidity and mortality unequally within both genders5 and the association between ECG abnormalities and components of metabolic syndrome may also vary by gender.2 When we accounted for coexisting cardiometabolic risk factors, we failed to observe any significant associations of low HDL-C with major and minor ECG abnormalities. The pathophysiological processes that result in high triglycerides and low HDL-C are similar and may hamper statistical analyses25 if both factors are not considered concurrently.
The prevalence of major and minor ECG abnormalities was higher with increasing metabolic syndrome score. Hence, the number of components of metabolic syndrome may be more useful than the presence of metabolic syndrome per se1,26 in predicting the likelihood of ECG abnormalities and CVD risk. Because not all components of metabolic syndrome resulted in an increased risk of ECG abnormalities, we speculate that individual components may act synergistically1 to result in ECG abnormalities. This speculation is supported by the findings of Dekker et al., which showed that that the number of constituent factors provided a graded assessment of CVD risk.5
Pathophysiological mechanisms
Changes in the electrical status and structure of the heart manifest collectively as ECG abnormalities. The mechanisms by which various components of metabolic syndrome mediate cardiac changes, although interrelated, are likely variable. In hypertension, increases in cardiac afterload result in increased left ventricular (LV) wall stress, which stimulates myocardial hypertrophy27 and is detected on ECG as left ventricular hypertrophy (LVH). The mechanisms by which other metabolic abnormalities result in ECG changes may be even more complex. Central obesity is associated with excess visceral adiposity, which is related to an atherogenic, prothrombotic, and inflammatory profile.23 Increasing myocardial ischemia (resulting from atherosclerosis) leads to scarring and LV dysfunction,27 which promotes ECG abnormalities. The effects of central obesity may be partly mediated by an increased predisposition to hypertension, and LVH itself is closely related to systemic atherosclerosis.27 Thus, LVH may represent the accumulated effects of hypertension and other cardiovascular risk factors over time.27 High blood glucose is associated with co-morbidities such as hypertension, hyperlipidemia, and a prothrombotic state, which interact synergistically23 to promote cardiac changes that result in ECG abnormalities. The mechanisms underlying gender differences in ECG findings among individuals with high triglycerides and hypertension as shown by our study are unclear and require further study.
Strengths and limitations
Our study has multiple strengths. MESA was conducted in six geographic locations in the United States and involved a large number of participants with diverse ethnic and gender representation. Data collection procedures (including ECG) were done under highly standardized conditions. This is the first study to evaluate compositely the association of ECG abnormalities with metabolic syndrome among gender and ethnic subgroups in a U.S. population.
Our study also has limitations. Atrial fibrillation was an exclusion criterion for MESA participants and is not included among the ECG abnormalities evaluated in this study. Because the study was cross-sectional, we cannot make inferences about the time course of ECG abnormalities in persons with metabolic syndrome. Our study participants were aged 45–84 years, and our findings may not apply to a younger population with metabolic syndrome. Although ECG abnormalities are predictive of CVD morbidity and mortality, we cannot make definite extrapolations and require future studies to correlate baseline ECG findings with cardiovascular outcomes in metabolic syndrome.
Conclusions
Our results have shown that metabolic syndrome and its components are associated with major and/or minor ECG abnormalities. The relationship of metabolic syndrome, hypertension, and high triglyceride with major and minor ECG abnormalities varied according to gender. A higher metabolic syndrome score is associated with greater prevalence of major and minor ECG abnormality. Because metabolic syndrome and ECG abnormalities are both associated with an increased risk of cardiovascular morbidity and mortality, the presence of ECG abnormalities possibly identifies individuals with metabolic abnormalities at especially high risk of CVD. Current guidelines recommend routine ECG screening for risk assessment in individuals with hypertension.28,29 Although our results suggest that screening ECGs obtained in individuals with metabolic syndrome may help identify those at higher risk, future trials are needed to assess whether care guided by the results of routine ECG screening improves outcomes in individuals with metabolic syndrome.
Supplementary Material
Acknowledgments
The authors thank the other investigators, the staff, and the participants of the MESA study for their valuable contributions. A full list of participating MESA investigators and institutions can be found at www.mesa-nhlbi.org. We thank Charles N. Campbell, Jr., at Wake Forest University School of Medicine for assistance with ECG coding. The MESA study was supported by contracts N01-HC-95159 through N01-HC-95169 from the National Heart, Lung and Blood Institute.
Author Disclosure Statement
No competing financial interests exist.
References
- 1.Kim J-Y. Mun H-S. Lee BK, et al. Impact of metabolic syndrome and its individual components on the presence and severity of angiographic coronary artery disease. Yonsei Med J. 2010;51:676–682. doi: 10.3349/ymj.2010.51.5.676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kim H-K. Kim C-H. Ko K-H, et al. Variable association between components of the metabolic syndrome and electrocardiographic abnormalities in Korean adults. Korean J Intern Med. 2010;25:174–180. doi: 10.3904/kjim.2010.25.2.174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lakka H-M. Laaksonen DE. Lakka TA, et al. The metabolic syndrome and total and cardiovascular disease mortality in middle-aged men. JAMA. 2002;288:2709–2716. doi: 10.1001/jama.288.21.2709. [DOI] [PubMed] [Google Scholar]
- 4.Isomaa B. Almgren P. Tuomi T, et al. Cardiovascular morbidity and mortality associated with the metabolic syndrome. Diabetes Care. 2001;24:683–689. doi: 10.2337/diacare.24.4.683. [DOI] [PubMed] [Google Scholar]
- 5.Dekker JM. Girman C. Rhodes T, et al. Metabolic syndrome and 10-year cardiovascular disease risk in the Hoorn Study. Circulation. 2005;112:666–673. doi: 10.1161/CIRCULATIONAHA.104.516948. [DOI] [PubMed] [Google Scholar]
- 6.Ford ES. Prevalence of the metabolic syndrome defined by the International Diabetes Federation among adults in the US. Diabetes Care. 2005;28:2745–2749. doi: 10.2337/diacare.28.11.2745. [DOI] [PubMed] [Google Scholar]
- 7.Grundy SM. Brewer HB. Cleeman JI, et al. Definition of the metabolic syndrome: Report of the National Heart, Lung, and Blood Institute/American Heart Association Conference on Scientific Issues Related to Definition. Circulation. 2004;109:433–438. doi: 10.1161/01.CIR.0000111245.75752.C6. [DOI] [PubMed] [Google Scholar]
- 8.Walsh JA. Prineas R. Daviglus ML, et al. Prevalence of electrocardiographic abnormalities in a middle aged biracial population: Coronary artery risk development in young adults study. J Electrocardiol. 2010;43:385.e1–385.e9. doi: 10.1016/j.jelectrocard.2010.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.DeBacquer D. Pereria LSM. DeBacker G, et al. The predictive value of electrocardiographic abnormalities for total and cardiovascular disease mortality in men and women. Eur Heart J. 1994;15:1604–1610. doi: 10.1093/oxfordjournals.eurheartj.a060441. [DOI] [PubMed] [Google Scholar]
- 10.Machado DB. Crow RS. Boland LL, et al. Electrocardiographic findings and incident coronary jeart disease among participants in the Atherosclerosis Risk in Communities (ARIC) Study. AM J Cardiol. 2006;97:1176–1181. doi: 10.1016/j.amjcard.2005.11.036. [DOI] [PubMed] [Google Scholar]
- 11.Sutherland S. Gazes P. Keil J, et al. Electrocardiographic abnormalities and 30-year mortality among white and black men of the Charleston Heart Study. Circulation. 1993;88:2685–2692. doi: 10.1161/01.cir.88.6.2685. [DOI] [PubMed] [Google Scholar]
- 12.Denes P. Larson JC. Llyoyd-Jones DM, et al. Major and minor ECG abnormalities in asymptomatic women and risk of cardiovascular events and mortality. JAMA. 2007;297:978–985. doi: 10.1001/jama.297.9.978. [DOI] [PubMed] [Google Scholar]
- 13.DeBacquer D. DeBacker G. Kornitzer M, et al. Prognostic value of ECG findings for total, cardiovascular disease, and coronary heart disease death in men and women. Heart. 1998;80:570–577. doi: 10.1136/hrt.80.6.570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Vitelli LL. Crow RS. Shahar E, et al. Electrocardiographic findings in a healthy biracial population. Am J Cardiol. 1998;81:453–459. doi: 10.1016/s0002-9149(97)00937-5. [DOI] [PubMed] [Google Scholar]
- 15.Ashley EA. Raxwal V. Froelicher V. An evidence-based review of the resting electrocardiogram as a screening technique for heart disease. Progr Cardiovasc Dis. 2001;44:55–67. doi: 10.1053/pcad.2001.24683. [DOI] [PubMed] [Google Scholar]
- 16.Ervin RB. National Health Statistics Report. Vol. 13. Hyattsville, MD: National Center for Health Statistics; 2009. Prevalence of metabolic syndrome among adults 20 years of age and over, by sex, age, race and ethnicity, and body mass indices: United States, 2003-2006. [PubMed] [Google Scholar]
- 17.Bild DE. Bluemke DA. Burke GL, et al. Multi-Ethnic Study of Atherosclerosis: Objectives and Design. Am J Epidemiol. 2002;156:871–881. doi: 10.1093/aje/kwf113. [DOI] [PubMed] [Google Scholar]
- 18.Assmann G. Guerra R. Fox G, et al. Harmonizing the Definition of the Metabolic Syndrome: Comparison of the Criteria of the Adult Treatment Panel III and the International Diabetes Federation in United States American and European Populations. Am J Cardiol. 2007;99:541–548. doi: 10.1016/j.amjcard.2006.08.045. [DOI] [PubMed] [Google Scholar]
- 19.Prineas RJ. Crow RS. Blackburn HW. The Minnesota Code Manual of Electrocardiographic Findings: Standards and Procedures for Measurement and Classification. Boston, MA: John Wright-PSG; 1982. pp. 203–229. [Google Scholar]
- 20.The World Health Organization Western Pacific Region and the International Obesity Task Force. The Asian-Pacific Perspective: Redefining Obesity and its Treatment. Melbourne: Health Communications Australia Printing Limited; 2000. [Google Scholar]
- 21.Psaty BM. Arnold AM. Olson J, et al. Association between levels of blood pressure and measures of subclinical disease: Multi-Ethnic Study of Atherosclerosis. Am J Hypertens. 2006;19:1110–1117. doi: 10.1016/j.amjhyper.2006.04.002. [DOI] [PubMed] [Google Scholar]
- 22.Balkau B. Deanfield JE. Despres J-P, et al. International Day for the Evaluation of Abdominal Obesity (IDEA): A Study of Waist Circumference, Cardiovascular Disease, and Diabetes Mellitus in 168 000 Primary Care Patients in 63 Countries. Circulation. 2007;116:1942–1951. doi: 10.1161/CIRCULATIONAHA.106.676379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fox KAA. Despres J-P. Richard A-J, et al. Does abdominal obesity have a similar impact on cardiovascular disease and diabetes? A study of 91 246 ambulant patients in 27 European countries. Eur Heart J. 2009;30:3055–3063. doi: 10.1093/eurheartj/ehp371. [DOI] [PubMed] [Google Scholar]
- 24.Melbin LG. Anselimo M. Ryden L. Diabetes, prediabetes and cardiovascular risk. Eur J Cardiovasc Prev Rehabil. 2010;17:s9–s14. doi: 10.1097/01.hjr.0000368192.24732.2f. [DOI] [PubMed] [Google Scholar]
- 25.Harchaoui KEL. Visser ME. Kastelein JJP, et al. Triglycerides and cardiovascular risk. Curr Cardiol Rev. 2009;5:216–222. doi: 10.2174/157340309788970315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Solymoss BC. Bourassa MG. Campeau L, et al. Effect of increasing metabolic syndrome score on atherosclerotic risk profile and coronary artery disease angiographic severity. Am J Cardiol. 2004;93:159–164. doi: 10.1016/j.amjcard.2003.09.032. [DOI] [PubMed] [Google Scholar]
- 27.Krauser DG. Devereux RB. Ventricular hypertrophy and hypertension. Prognostic elements and implications for management. Herz. 2006;31:305–316. doi: 10.1007/s00059-006-2819-5. [DOI] [PubMed] [Google Scholar]
- 28.Scheltens T. De Beus MF. Hoes AW, et al. The potential yield of ECG screening of hypertensive patients: The Utrecht Health Project. J Hypertens. 2010;28:1527–1533. doi: 10.1097/HJH.0b013e328339f95c. [DOI] [PubMed] [Google Scholar]
- 29.Chobanian AV. Bakris GL. Black HR, et al. Seventh report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure. Hypertension. 2003;42:1206–1252. doi: 10.1161/01.HYP.0000107251.49515.c2. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.