Abstract
Background
The influence of moderate-to-vigorous physical activity (MVPA) and general versus central adiposity on pediatric metabolic risk is not well described.
Methods
Secondary analyses on pediatric participants from the National Health and Nutrition Examination Survey, 2003–2006 (n=2,155). MVPA (min/day) and adherence to MVPA recommendations were assessed objectively by accelerometers. Body mass index (BMI) z-score and waist circumference (WC) were measured by standard protocols. The main dependent variables included an overall metabolic risk score and clinical tests related to metabolic risk. A series of linear regression analyses were used to examine BMI z-score versus WC as a mediator of the relationship between MVPA and the metabolic risk score or the individual components, controlling for sociodemographic covariates. All analyses with BMI z-score as an independent variable controlled for WC and vice versa. The product-of-coefficients method was used to test for mediation.
Results
MVPA adherence was inversely associated and WC was positively associated with the metabolic risk score (all P<0.05). MVPA was inversely associated with systolic blood pressure and positively associated with high-density lipoprotein cholesterol (HDL-C) (all P<0.05). WC was inversely associated with HDL-C and positively associated with C-reactive protein (CRP), glycohemoglobin, fasting triglycerides, and fasting insulin (all P<0.05). WC mediated the relationship between MVPA and CRP or HDL-C (both P<0.05).
Conclusions
MVPA correlated with pediatric metabolic risk and this relationship was mediated by central adiposity for CRP and HDL-C. This finding suggests the need for programs to screen for and improve children's MVPA and WC.
Introduction
Physical activity is important for disease prevention throughout the life span. The U.S. Physical Activity Guidelines Advisory Committee Report1 recommended that youth achieve at least 60 min of moderate-to-vigorous physical activity (MVPA) daily. However, only 42% of U.S. youth aged 6–11 years and <8% of 12- to 19-year olds met the recommended amount.2
Among adults, strong inverse associations were reported between MVPA and adiposity, metabolic risk, cardiovascular-related mortality, and all-cause mortality.1 Central adiposity, e.g., waist circumference (WC), was more strongly related to metabolic risk than general adiposity, e.g., body mass index (BMI), among adults, which is reflected by the inclusion of WC as a criterion of the metabolic syndrome.3 Similar pediatric studies reported inverse associations between MVPA and adiposity or metabolic risk. However, these pediatric studies had limitations, such as small sample sizes, narrowly selected populations, failing to control for general and central adiposity when examining metabolic risk, or using subjective methods to measure MVPA.1,4 Whereas many of these previous studies reported inverse relationships between MVPA and metabolic risk, none determined if the relationship was mediated by general or central adiposity, i.e., the effect of MVPA can be explained by differences in adiposity. Both general and central adiposity were determinants of metabolic risk.5–9 No reports used mediating variable analysis10 to examine physical activity, general or central adiposity, and metabolic risk.
Mediating variable analysis allows for the simultaneous consideration of variables hypothesized as influential to the outcome in one model.10 A mediating variable analysis would clarify the role of general versus central adiposity to metabolic risk and if general or central adiposity falls on the pathway between MVPA to pediatric metabolic risk, which in turn would provide guidance for screening and intervention programs to decrease metabolic risk among youth. This study examined: (1) The association between objectively measured MVPA and metabolic risk; (2) whether general or central adiposity is more strongly related to metabolic risk; and (3) whether general or central adiposity mediates the relationship between MVPA and metabolic risk among a nationally representative sample of U.S. youth. The study hypotheses were that: (1) MVPA would be inversely associated with general and central adiposity and metabolic risk; (2) central adiposity would be more strongly related to metabolic risk than general adiposity; and (3) central adiposity, and not general adiposity, would partially mediate the relationship between MVPA and metabolic risk.
Methods
Data source
This study was a secondary data analysis of the National Health and Nutrition Examination Survey (NHANES) conducted by the U.S. Centers for Disease Control and Prevention (CDC). NHANES is a continuous cross-sectional survey of the U.S. noninstitutionalized civilian population and provides a nationally representative sample through its stratified, multistage, probability cluster design.11 NHANES 2003–2004 and 2005–2006 were used because these data releases uniquely measured physical activity objectively by accelerometry in participants 6 years of age and older, as described in greater detail below.
Participants
Nonpregnant participants aged 6–19 years were included. Participants with missing data or insufficient accelerometry data to estimate physical activity (see below) were excluded from analyses. NHANES oversampled Mexican-American and non-Hispanic black participants. We restricted analyses to participants who identified as non-Hispanic white, non-Hispanic black, and Mexican-American to provide sufficient sample size for the analyses; participants identified as “Other race–Including Multiracial” and “Other Hispanic” were dropped due to low numbers, similar to a previous NHANES analysis on pediatric metabolic syndrome.12 The Institutional Review Board of Baylor College of Medicine exempted this secondary analysis on deidentified data from review.
Outcome variables
Blood pressure (systolic and diastolic; SBP and DBP) and anthropometric measurements (height, weight, and WC) were assessed on participants using standardized techniques and equipment in the NHANES Mobile Examination Centers.11 BMI was calculated as weight in kilograms divided by the square of height in meters, and BMI z-scores were determined from the CDC U.S. growth charts.13
Clinical tests were performed per standard protocol11 and selected on the basis of their relationship to metabolic risk (cardiovascular disease or insulin resistance).14,15 The following were obtained with detailed methods reported elsewhere11: C-reactive protein (CRP), total cholesterol, and high-density lipoprotein cholesterol (HDL-C).
Glycohemoglobin was obtained on participants ≥12 years old. Serum triglycerides (TG), plasma glucose, and plasma insulin levels were obtained on a subsample of fasting participants ≥12 years old. Fasting low-density lipoprotein cholesterol (LDL-C) was calculated with the Friedewald equation from the fasting subsample of participants.
A metabolic risk score was calculated, based on the sum of the age- and gender-adjusted z-scores for SBP, DBP, HDL, TG, and fasting glucose.16 The z-score for HDL-C was multiplied by −1. The metabolic risk score was composed of the clinical and laboratory components that comprise the metabolic syndrome14,17,18 but excluded central adiposity (excess WC), because a mediator should not be a component of the outcome variable.10
Main exposure
Physical activity was assessed by accelerometry as previously described.2 Participants wore an accelerometer (Model 7164, Actigraph, LLC; Ft. Walton Beach, FL) for 7 days. The accelerometers measured the volume and intensity of movement in 1-min intervals and provided a valid and reliable objective measure of physical activity in children.19 Accelerometer data were processed following the criteria in a previous NHANES study on physical activity.2 Habitual physical activity was estimated by using participants' data that had at least ≥4 valid days (10 h or more of monitor wear daily). To ensure consistency with previous NHANES accelerometer studies,2,20,21 we used the age-specific thresholds developed by Freedson for MVPA,22 which was set at four metabolic equivalents. Minutes that met or exceeded the threshold were summed for the daily estimate of MVPA minutes.2 This sum was divided by the number of valid days to calculate mean daily minutes of MVPA. While Trost23 has generally recommended using the physical activity intensity thresholds of Evenson24 due to their classification accuracy for all four levels of physical activity, both the Freedson and Evenson thresholds for combined MVPA exhibited “excellent” classification accuracy and were superior to other cut points.23
Because a large number of participants would be excluded on the basis of having <4 valid days of accelerometer data and because only a limited number of participants were assessed for all components of the metabolic risk score, we sought to maximize sample size for analyses involving the metabolic risk score by using a Bayesian approach2,25 to estimate adherence to the physical activity recommendation for children (≥60 min MVPA on 5 of 7 days). The Bayesian approach required ≥1-valid day (compared to ≥4-valid days for the traditional estimate), which increased eligible participants by ≈300 participants. The estimate is based on the probability of a participant obtaining the recommended amount of MVPA on a given day, taking into account the number of days on which the accelerometer was worn and the number of days in which the recommendation was met.25 The Bayesian approach provided an estimate of adherence to the physical activity recommendations, termed MVPA adherence, and was only used for analyses with the metabolic risk score.
Covariates
Several sociodemographic and dietary/lifestyle variables were included as covariates that might confound the relationship between MVPA and adiposity or metabolic risk. The following covariates were included: (1) Age in years, (2) gender, (3) race/ethnicity categorized as non-Hispanic white, non-Hispanic black, and Mexican-American; and (4) income, i.e., the poverty-to-income ratio (PIR). PIR values <1 were below the poverty threshold. PIR was provided by NHANES in the following six categories: <1, ≥1 <2, ≥2 <3, ≥3 <4, ≥4 <5, and ≥5 PIR.11
Two dietary variables were included as covariates: Total dietary energy intake (all participants) and alcohol intake (participants aged 12 years and older) calculated from the mean of two 24-h dietary recalls.26 Alcohol intake was an important covariate because: (1) Up to one-half of adolescents reported consuming alcohol during the past month in the 2007 U.S. Youth Risk Behavior Survey,27 and (2) studies have reported a positive relationship between alcohol intake and the Metabolic Syndrome.28,29
Cigarette smoking was a covariate because it is common among adolescents30 and has been independently associated with the metabolic syndrome.29,31 It was assessed by two methods: (1) Serum cotinine32 (participants ≥3 years old) and (2) the question, “During the past 5 days, did [you/he/she] use any product containing nicotine including cigarettes, pipes, cigars, chewing tobacco, snuff, nicotine patches, nicotine gum, or any other product containing nicotine?” with those who answered “yes” considered as smokers (participants 12–19 years old).33
Analyses
Frequencies, percentages, means, and standard deviations were calculated to describe participant characteristics. Participants with missing data were excluded on an analysis-by-analysis basis. Differences between excluded and included participants were examined using chi-squared tests of independence and analysis of variance.
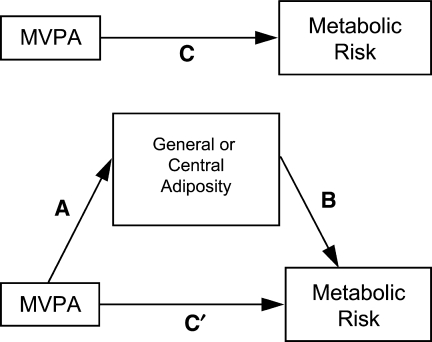
A series of linear regression analyses were conducted that examined the BMI z-score as a potential mediator of the relationship between MVPA adherence and the metabolic risk score (sum of the z-scores for SBP, DBP, HDL-C, TG, and fasting glucose), while controlling for WC. For the first linear regression analyses, termed the “C path” (see Fig. 1), the relationship between MVPA adherence and the metabolic risk score was examined. Covariates included age, gender, race/ethnicity, PIR, total energy intake, total alcohol intake, smoking status, and WC. We next conducted linear regression analyses with MVPA adherence as the independent variable and BMI z-score as the dependent variable to determine if MVPA adherence was inversely associated with BMI z-score (“A path” of Fig. 1). Covariates were identical to those listed above for the first linear regression analysis. The relationship between BMI z-score and the metabolic risk score, controlling for covariates (“B path” of Fig. 1), was also examined. A fourth set of regression analyses was conducted with both MVPA adherence and BMI z-score as independent variables and the metabolic risk score as the dependent variable to determine if BMI z-score mediated any potential relationship between MVPA and the metabolic risk score (“C path” of Fig. 1). The product-of-coefficients method10 was used to assess whether BMI z-score was a mediator using the Sobel formula.10 This method is a refinement10,34 of the original causal steps approach,35 but does not require large sample sizes. In parallel analyses, a series of linear regression analyses was conducted with WC as the potential mediator, while controlling for BMI z-score in analyses of the A, B, C, and C′ paths.
FIG. 1.
Diagram for general or central adiposity mediating the relationship between moderate-to-vigorous physical activity (MVPA) and metabolic risk.
Similar to the mediation analyses examining BMI z-score or WC as mediators for the metabolic risk score, analyses of the A, B, C, and C′ paths were conducted examining BMI z-score or WC as mediators for several individual metabolic risk factors (SBP, DBP, CRP, glycohemoglobin, total cholesterol, HDL, TG, LDL-C, fasting glucose, or fasting insulin). In this series of regression analyses, the mean daily minutes of MVPA value was used as the main independent variable because sample size was not as constrained compared to analyses involving the metabolic risk score.
Analyses were conducted using the survey weights and commands in SAS 9.1.3 (SAS Institute Inc., Cary, NC), e.g., PROC SURVEYREG, to take into account the complex survey design. For analyses involving nonfasting clinical and laboratory variables (CRP, SBP, DBP, and glycohemoglobin), special sampling weights were used for the subsample of participants with ≥4 valid days of accelerometer data, which reweights the data to be nationally representative.2 For analyses involving fasting laboratory variables (LDL-C, TG, glucose, and insulin) and the metabolic risk score, fasting sample weights provided by NHANES were used as recommended to reweight the data to be nationally representative.11 A significance level of 0.05 was chosen. Standardized regression coefficients (std. β) are presented; however, tests of mediation were performed using the unstandardized coefficients.
Results
A total of 2,155 participants were eligible for this study on the basis of inclusion criteria (Table 1); their average age was 14.2±0.1 years and 50.4%±1.2% were male. Overall, 10.9±1.3 were identified as cigarette smokers and among 12- to 19-year-old participants, 7.0%±0.6% reported alcohol intake. Of 6,770 participants aged 6–19 years from NHANES 2003–2006, participants were excluded from analyses due to pregnancy (n=120), unreliable dietary recall data11 per NHANES criteria (n=576), <4 valid days of accelerometer data (n=2,603), missing demographic data (n=1,124), and missing sample weights (n=192). Included participants, termed the full study sample, were older (14.2 vs. 10.1 years, P<0.0001) compared to excluded participants, with no significant differences by gender, race/ethnicity, or income.
Table 1.
Participant Characteristics (n=2,155)
| Normal weight | Overweight | Obese | All | |
|---|---|---|---|---|
| Gendera | ||||
| Male | 50.7 (2.0) | 49.6 (3.1) | 50.0 (3.0) | 50.4 (1.2) |
| Female | 49.3 (2.0) | 50.4 (3.1) | 50.0 (3.0) | 49.6 (1.2) |
| Ethnicity/racea | ||||
| Mexican American | 12.5 (2.0) | 11.7 (2.4) | 14.6 (2.7) | 12.7 (2.0) |
| Non-Hispanic white | 72.5 (2.8) | 73.4 (4.3) | 65.3 (4.1) | 71.3 (3.0) |
| Non-Hispanic black | 15.1 (1.9) | 14.9 (2.5) | 20.2 (2.6) | 16.0 (2.0) |
| Property income ratio (PIR)a | ||||
| PIR 0≥ and <1 | 16.8 (1.5) | 19.6 (3.1) | 20.1 (2.1) | 17.9 (1.6) |
| PIR ≥1 and <2 | 20.4 (1.7) | 17.7 (2.7) | 21.8 (2.9) | 20.1 (1.5) |
| PIR ≥2 and <3 | 15.4 (1.4) | 26.3 (3.1) | 27.0 (3.9) | 19.6 (1.3) |
| PIR ≥3 and <4 | 14.1 (1.1) | 17.3 (2.9) | 12.8 (2.7) | 14.5 (1.2) |
| PIR ≥4 and <5 | 12.8 (1.9) | 5.8 (1.6) | 7.8 (1.8) | 10.5 (1.4) |
| PIR ≥5 | 20.5 (2.0) | 13.4 (3.2) | 10.5 (2.3) | 17.3 (1.7) |
| Smoking statusa | ||||
| Nonsmoker | 89.9 (1.1) | 89.0 (3.0) | 86.5 (2.9) | 89.1 (1.3) |
| Smoker | 10.1 (1.1) | 11.0 (3.0) | 13.5 (2.9) | 10.9 (1.3) |
| Age (years)b | 14.3 (0.2) | 14.0 (0.2) | 13.9 (0.2) | 14.2 (0.1) |
| Body mass index (BMI) z-scoreb | −0.04 (0.0) | 1.3 (0.0) | 2.1 (0.0) | 0.6 (0.0) |
| Waist circumference (cm)b | 71.2 (0.4) | 83.3 (0.6) | 98.9 (0.8) | 78.6 (0.5) |
| Moderate-to-vigorous physical activity (minutes/day)b | 38.9 (1.9) | 36.0 (2.4) | 31.8 (2.0) | 37.0 (1.5) |
| Energy (kcal)b | 2,429 (34.6) | 2,123 (73.0) | 2,066 (53.5) | 2,304 (29.7) |
| Systolic blood pressure (mmHg)b | 106.3 (0.5) | 107.7 (1.2) | 112.6 (0.7) | 107.7 (0.4) |
| Diastolic blood pressure (mmHg)b | 59.5 (0.6) | 56.0 (1.3) | 59.2 (0.9) | 58.8 (0.6) |
| C-reactive protein (mg/dL)b | 0.1 (0.0) | 0.2 (0.0) | 0.4 (0.1) | 0.2 (0.0) |
| Triglycerides (mmol/L)b | 0.9 (0.0) | 1.1 (0.0) | 1.2 (0.1) | 1.0 (0.0) |
| Total cholesterol (mmol/L)b | 4.1 (0.1) | 4.3 (0.1) | 4.3 (0.1) | 4.2 (0.0) |
| High-density lipoprotein cholesterol (mmol/L)b | 1.5 (0.0) | 1.3 (0.0) | 1.2 (0.0) | 1.4 (0.0) |
| Low-density lipoprotein cholesterol (mmol/L)b | 2.3 (0.0) | 2.5 (0.1) | 2.6 (0.1) | 2.4 (0.0) |
| Glycohemoglobin (%)b,c | 5.1 (0.0) | 5.1 (0.0) | 5.1 (0.0) | 5.1 (0.0) |
| Fasting glucose (mmol/L)b,c | 5.1 (0.1) | 5.0 (0.0) | 5.2 (0.1) | 5.1 (0.0) |
| Fasting insulin (pmol/L)b,c | 48.9 (1.9) | 67.2 (2.9) | 127.7 (7.6) | 65.7 (2.3) |
Data are presented as % (standard error).
Data presented as mean (standard error). Standard errors of 0.0 had values <0.1.
Measured among 12- to 19-year-old participants only.
For the analyses examining mediation by BMI z-score for the relationship between MVPA adherence and the metabolic risk score in Table 2, only MVPA adherence was significantly associated (std. β=− 0.291, P=0.039) with the metabolic risk score (C path). There were no significant associations for the A or B paths or mediation by BMI z-score. In parallel analyses examining mediation by WC, MVPA adherence was inversely associated (std. β=−0.304, P=0.024) with the metabolic risk score (C path). WC was positively associated (std. β=0.449, P=4.8×10−5) with the metabolic risk score (B path). There was no significant association for the A path or mediation by WC.
Table 2.
Body Mass Index z-Score or Waist Circumference Mediating the Relationship Between Moderate-to-Vigorous Physical Activity Adherence and Clustered Metabolic risk
| A path | P value | B path | P value | C path | P value | C′ path | P value | Sobel z-score | P value | % Mediated | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| BMI z-score (mediator)a,b | |||||||||||
| Metabolic risk score | −0.009 | 0.775 | 0.119 | 0.222 | −0.291* | 0.039 | −0.287* | 0.037 | −0.282 | 0.778 | 0.7 |
| Waist circumference (mediator)a,c | |||||||||||
| Metabolic risk score | −0.017 | 0.499 | 0.449* | 4.8×10−05 | −0.304* | 0.024 | −0.260* | 0.037 | −0.677 | 0.498 | 5.0 |
Data presented are standardized beta coefficients and P values, unless otherwise indicated. Significant results are indicated by an asterisk (*).
For results with body mass index (BMI) z-score as the mediator, the “A path” is moderate-to-vigorous physical activity (MVPA) adherence and relationship to BMI z-score while controlling for waist circumference, age, gender, race/ethnicity, income, total energy intake, total alcohol intake, and smoking status. The “B path” is BMI z-score and relationship to metabolic risk factors, controlling for waist circumference, age, gender, race/ethnicity, income, total energy intake, total alcohol intake, and smoking status. The “C path” is MVPA adherence and relationship to metabolic risk factors, controlling for waist circumference, age, gender, race/ethnicity, income, total energy intake, total alcohol intake, and smoking status. The “C’ path” is identical to the C path but has the addition of BMI z-score as a covariate.
For results with waist circumference as the mediator, the analyses were identical to the previous footnote except that waist circumference is the mediator and BMI z-score is the covariate.
For the analyses examining mediation by BMI z-score for the relationship between minutes of MVPA and each of the metabolic risk factors in Table 3, mean daily minutes of MVPA were significantly associated with SBP (std. β=−0.018, P=0.033, C path) and HDL-C (std. β=4.3×10−4, P=0.006, C path). BMI z-score was positively associated (std. β=0.616, P=0.017) with SBP and inversely associated (std. β=− 0.665, P=0.045) with DBP (B path). There was no significant association for the A paths or mediation by BMI z-score.
Table 3.
Regression Series for Body Mass Index z-Score Mediating the Relationship Between Moderate-to-Vigorous Physical Activity and Metabolic Risk Factors
| A path | P value | B path | P value | C path | P value | C′ path | P value | Sobel z-score | P value | % Mediated | R2 | Partial R2for MVPA | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Metabolic risk factor (n=2,155), 6–19 years olda | |||||||||||||
| Systolic blood pressure (mmHg) | 7.0×10−4 | 0.107 | 0.616* | 0.017 | −0.018* | 0.033 | −0.013* | 0.025 | 1.387 | 0.165 | 5.6 | 0.198 | 0.010 |
| Diastolic blood pressure (mmHg) | 7.0×10–4 | 0.107 | −0.665* | 0.045 | −0.004 | 0.610 | −0.002 | 0.735 | −1.301 | 0.193 | 33.0 | 0.100 | 8.6×10−5 |
| C-reactive protein (mg/dL) | 7.0×10–4 | 0.107 | −0.009 | 0.138 | −1.4×10–4 | 0.467 | −1.3×10–4 | 0.499 | −1.122 | 0.262 | 8.1 | 0.058 | 1.2×10−4 |
| Total cholesterol (mmol/L) | 7.0×10–4 | 0.107 | −0.001 | 0.972 | 0.001 | 0.093 | 0.001 | 0.093 | −0.035 | 0.972 | 0.1 | 0.024 | 0.003 |
| High-density lipoprotein cholesterol (mmol/L) | 7.0×10–4 | 0.107 | −0.005 | 0.613 | 4.3×10–4* | 0.006 | 4.3×10–4* | 0.005 | −0.488 | 0.625 | 1.3 | 0.178 | 0.004 |
| Glycohemoglobin (%)b | 2.4×10–4 | 0.612 | −0.011 | 0.252 | 0.000 | 0.070 | 0.000 | 0.077 | −0.469 | 0.639 | 1.6 | 0.044 | 0.001 |
| Metabolic risk factor (n=1,860), fasting 12- to 19-year-old subsamplea | |||||||||||||
| Fasting triglycerides (mmol/L) | −0.001 | 0.362 | 0.016 | 0.507 | 4.5×10–4 | 0.467 | 4.7×10–4 | 0.461 | −0.543 | 0.587 | 4.591 | 0.127 | 0.001 |
| Fasting low-density lipoprotein cholesterol (mmol/L) | −0.001 | 0.362 | −0.006 | 0.889 | 0.001 | 0.122 | 0.001 | 0.123 | 0.139 | 0.889 | 0.648 | 0.079 | 0.006 |
| Plasma glucose (mmol/L) | −0.001 | 0.362 | 0.025 | 0.417 | 5.6×10–5 | 0.926 | 9.0×10–5 | 0.882 | −0.615 | 0.538 | 27.382 | 0.067 | 2.5×10−5 |
| Fasting insulin (pmol/L) | −0.001 | 0.362 | −0.866 | 0.103 | −0.027 | 0.328 | −0.012 | 0.285 | 0.810 | 0.418 | 8.721 | 0.439 | 0.002 |
Data presented are standardized beta coefficients and P values, unless otherwise indicated. Significant results are indicated by an asterisk (*). The “A path” is moderate-to-vigorous physical activity (MVPA; min/day) and relationship to body mass index (BMI) z-score while controlling for waist circumference, age, gender, race/ethnicity, income, total energy intake, total alcohol intake, and smoking status. The “B path” is BMI z-score and relationship to metabolic risk factors, controlling for waist circumference, age, gender, race/ethnicity, income, total energy intake, total alcohol intake, and smoking status. The “C path” is MVPA and relationship to metabolic risk factors, controlling for waist circumference, age, gender, race/ethnicity, income, total energy intake, total alcohol intake, and smoking status. The “C’ path” is identical to the C path but has the addition of BMI z-score as a covariate.
Glycohemoglobin was measured on 12- to 19-year-old participants only.
In parallel analyses examining mediation by WC for the relationship between minutes of MVPA and each of the metabolic risk factors in Table 4, mean daily minutes of MVPA were significantly associated with SBP (std. β=−0.019, P=0.013, C path), HDL (std. β=0.001, P=0.001, C path), and WC (std. β=−0.016, P=0.001, A path). WC was inversely associated with HDL-C (std. β=−0.004, P=7.4×10−7) and positively associated with CRP (std. β=0.005, P=5.5×10−6), glycohemoglobin (std. β=−0.002, P=0.024), fasting TG (std. β=0.004, P=0.023), and fasting insulin (std. β=0.070, P=7.4×10−8) (B path). WC significantly mediated the relationship between minutes of MVPA and CRP (Sobel z=−3.0; P=0.003; % mediated=56.9%) and HDL-C (Sobel z=3.1; P=0.002; % mediated=23.1%).
Table 4.
Regression Series for Waist Circumference Mediating the Relationship Between Moderate-to-Vigorous Physical Activity and Metabolic Risk Factors
| A path | P value | B path | P value | C path | P value | C′ path | P value | Sobel z-score | P value | % Mediated | R2 | Partial R2for MVPA | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Metabolic risk factor (n=2155), 6–19 years olda | |||||||||||||
| Systolic blood pressure (mmHg) | −0.016* | 0.001 | 0.027 | 0.185 | −0.019* | 0.013 | −0.016* | 0.025 | −1.268 | 0.205 | 5.4 | 0.198 | 0.010 |
| Diastolic blood pressure (mmHg) | −0.016* | 0.001 | 0.049 | 0.052 | −0.005 | 0.490 | −0.002 | 0.735 | −1.756 | 0.079 | 48.9 | 0.100 | 8.6×10–5 |
| C-reactive protein (mg/dL) | −0.016* | 0.001 | 0.005* | 5.5×10–6 | −2.7×10–4 | 0.184 | −1.3×10–4 | 0.499 | −2.986* | 0.003 | 56.9 | 0.058 | 1.2×10–4 |
| Total cholesterol (mmol/L) | −0.016* | 0.001 | 0.003 | 0.242 | 0.001 | 0.120 | 0.001 | 0.093 | −1.131 | 0.258 | 8.5 | 0.024 | 0.003 |
| High-density lipoprotein cholesterol (mmol/L) | −0.016* | 0.001 | −0.004* | 7.4×10–7 | 0.001* | 0.001 | 4.3×10–4* | 0.005 | 3.086* | 0.002 | 23.1 | 0.178 | 0.004 |
| Glycohemoglobin (%)b | −0.009 | 0.127 | 0.002* | 0.024 | 2.7×10–4 | 0.101 | 3.0×10–4 | 0.077 | −1.312 | 0.190 | 9.8 | 0.044 | 0.001 |
| Metabolic risk factor (n=1,860), fasting 12- to 19-year-old subsamplea | |||||||||||||
| Fasting triglycerides (mmol/L) | 0.002 | 0.879 | 0.004* | 0.023 | 4.7×10–4 | 0.471 | 4.7×10–4 | 0.461 | 0.154 | 0.878 | 2.1 | 0.127 | 0.001 |
| Fasting low-density lipoprotein cholesterol (mmol/L) | 0.002 | 0.879 | 0.004 | 0.178 | 0.001 | 0.123 | 0.001 | 0.123 | 0.153 | 0.878 | 0.8 | 0.079 | 0.006 |
| Plasma glucose (mmol/L) | 0.002 | 0.879 | −6.5×10−5 | 0.982 | 9.8×10–5 | 0.870 | 9.0×10–5 | 0.882 | −0.023 | 0.982 | 0.2 | 0.067 | 2.5×10–5 |
| Fasting insulin (pmol/L) | 0.002 | 0.879 | 0.070* | 7.4×10–8 | −0.027 | 0.388 | −0.002 | 0.285 | 0.154 | 0.878 | 8.5 | 0.439 | 0.002 |
Data presented are standardized beta coefficients and P-values, unless otherwise indicated. Significant results are indicated by an asterisk (*). The “A path” is moderate-to-vigorous physical activity (MVPA; min/day) and relationship to waist circumference while controlling for body mass index (BMI) z-score, age, gender, race/ethnicity, income, total energy intake, total alcohol intake, and smoking status. The “B path” is waist circumference and relationship to metabolic risk factors, controlling for BMI z-score, age, gender, race/ethnicity, income, total energy intake, total alcohol intake, and smoking status. The “C path” is MVPA and relationship to metabolic risk factors, controlling for BMI z-score, age, gender, race/ethnicity, income, total energy intake, total alcohol intake, and smoking status. The “C’ path” is identical to the C path but has the addition of waist circumference as a covariate.
Glycohemoglobin was measured on 12- to 19-year-old participants only.
Discussion
In extensive analyses among a large U.S. pediatric sample that considered BMI z-score, WC, and MVPA, WC appeared to be the more important correlate of pediatric metabolic risk. WC was both independently associated with the metabolic risk score and with multiple individual metabolic risk factors, in addition to partially mediating the relationship between MVPA and two individual metabolic risk factors—CRP and HDL-C.
For pediatric clustered metabolic risk, MVPA adherence was inversely associated and WC was positively associated with the metabolic risk score. In contrast, BMI z-score was not associated with the metabolic risk score. Because WC and BMI z-score are highly correlated, when WC was the independent variable of interest, BMI z-score was a covariate and vice versa. This analytic approach, which generally was not used in previous reports, uniquely allowed us to examine the independent association of BMI z-score or WC in the analyses. Neither WC nor BMI z-score mediated the relationship between MVPA adherence and the metabolic risk score. The findings for pediatric clustered metabolic risk were consistent with and build upon a cross-sectional study that compared BMI and WC on a metabolic risk score using data from a biracial sample from the Bogalusa Heart Study.7 In that study, which did not consider MVPA, BMI and WC as continuous variables did not have independent effects on a metabolic risk score.7
WC was independently associated with several metabolic risk factors, including higher CRP, glycohemoglobin, fasting TG, fasting insulin, and lower HDL-C. These findings were supported by previous studies that have reported stronger relationships between WC versus BMI z-score for children's metabolic risk,5,6 although these studies lacked objective data on physical activity. In contrast, the association between BMI z-score and metabolic risk was mixed: BMI z-score was positively associated with SBP but negatively associated with DBP. WC also partially mediated the relationship between minutes of MVPA and CRP (% mediated=56.9%) or HDL-C (% mediated=23.1%), whereas BMI z-score mediated no relationships. WC was a stronger correlate of metabolic risk than BMI z-score likely because WC directly reflects visceral fat accumulation,6 which has deleterious metabolic consequences.36–38
Participants with more minutes of MVPA had lower SBP and higher HDL-C, similar to a previous NHANES study that reported an inverse association with HDL-C.39 These results also mirror findings in which hypertension was positively associated, whereas HDL-C was inversely associated with atherosclerosis in the aorta and coronary arteries from a sample of children and young adults.40 Altogether, the results corroborate that physical activity policies and interventions aimed at increasing pediatric physical activity are important for chronic disease prevention.
Limitations include: (1) The cross-sectional design, which precludes drawing causal relationships; (2) a large number of participants with missing or incomplete data who were excluded and the attendant threat of selection bias, although most exclusions were based on insufficient valid accelerometry data for estimating habitual physical activity, as in previous NHANES accelerometry studies2,20; (3) the calculation of the metabolic risk score based on age- and gender-adjusted z-scores was specific to this sample of NHANES; and (4) pubertal status was not assessed in NHANES, which may confound relationships with adiposity and metabolic risk.
Conclusions
In contrast to general adiposity (BMI z-score), greater central adiposity (WC) appeared to be consistently related to greater metabolic risk among a large sample of U.S. children and adolescents. Likewise, lower MVPA was associated with greater metabolic risk. Clinicians and public health professionals should objectively assess children's MVPA and WC. Programs and policies aimed at increasing MVPA and achieving energy balance should be a national priority for obesity and chronic disease prevention among US youth.
Acknowledgments
The first author is grateful to the faculty of the 2007 Physical Activity and Public Health Research Course (Hilton Head, SC) for helpful preliminary discussion of this project. We thank David Berrigan, Ph.D., for discussing the approach for the NHANES accelerometer data and analysis. Any errors in this manuscript are solely those of the authors.
This study was funded by The National Cancer Institute (1K07CA131178) and the United States Department of Agriculture/Agricultural Research Service (USDA/ARS) Cooperative Agreement No. 58-6250-6001. The contents of this publication do not necessarily reflect the views or policies of the National Cancer Institute, USDA, or Baylor College of Medicine, nor does mention of trade names, commercial products, or organizations imply endorsement from the U.S. government or Baylor College of Medicine.
Author Disclosure Statement
The authors report that no competing financial interests exist. The authors alone are responsible for the content and writing of the paper.
References
- 1.Physical Activity Guidelines Advisory Committee. Physical Activity Guidelines Advisory Committee Report. Washington, DC: US Department of Health and Human Services; 2008. [Google Scholar]
- 2.Troiano RP. Berrigan D. Dodd KW, et al. Physical activity in the United States measured by accelerometer. Med Sci Sports Exerc. 2008;40:181–188. doi: 10.1249/mss.0b013e31815a51b3. [DOI] [PubMed] [Google Scholar]
- 3.Grundy SM. Brewer HB., Jr Cleeman JI, et al. Definition of metabolic syndrome: Report of the National Heart, Lung, and Blood Institute/American Heart Association conference on scientific issues related to definition. Circulation. 2004;109:433–438. doi: 10.1161/01.CIR.0000111245.75752.C6. [DOI] [PubMed] [Google Scholar]
- 4.Steele RM. Brage S. Corder K, et al. Physical activity, cardiorespiratory fitness and the metabolic syndrome in youth. J Appl Physiol. 105:342–251. doi: 10.1152/japplphysiol.00072.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Camhi SM. Kuo J. Young DR. Identifying adolescent metabolic syndrome using body mass index and waist circumference. Prev Chronic Dis. 2008;5:A115. [PMC free article] [PubMed] [Google Scholar]
- 6.Lee S. Bacha F. Gungor N, et al. Waist circumference is an independent predictor of insulin resistance in black and white youths. J Pediatr. 2006;148:188–194. doi: 10.1016/j.jpeds.2005.10.001. [DOI] [PubMed] [Google Scholar]
- 7.Janssen I. Katzmarzyk PT. Srinivasan SR, et al. Combined influence of body mass index and waist circumference on coronary artery disease risk factors among children and adolescents. Pediatrics. 2005;115:1623–1630. doi: 10.1542/peds.2004-2588. [DOI] [PubMed] [Google Scholar]
- 8.Lawlor DA. Benfield L. Logue J, et al. Association between general, central adiposity in childhood, change in these, with cardiovascular risk factors in adolescence: prospective cohort study. BMJ. 2010:341. doi: 10.1136/bmj.c6224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Schubert CM. Cook S. Sun SS, et al. Additive utility of family history and waist circumference to body mass index in childhood for predicting metabolic syndrome in adulthood. J Pediatr. 2009;155:S6.e9–S6.e13. doi: 10.1016/j.jpeds.2009.05.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.MacKinnon DP. Lockwood CM. Hoffman JM, et al. A comparison of methods to test mediation and other intervening variable effects. Psychol Methods. 2002;7:83–104. doi: 10.1037/1082-989x.7.1.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Centers for Disease Control and Prevention, 2003–2004 Current National Health and Nutrition Examination Survey (NHANES) Hyattsville, MD: National Center for Health Statistics; [Nov 26;2011 ]. [Google Scholar]
- 12.Pan Y. Pratt CA. Metabolic syndrome and its association with diet and physical activity in US adolescents. J Am Dietetic Asson. 2008;108:276–286. doi: 10.1016/j.jada.2007.10.049. [DOI] [PubMed] [Google Scholar]
- 13.Kuczmarski RJ. Ogden CL. Guo SS, et al. CDC Growth Charts for the United States: Methods and development. Vital Health Stat 11. 2000;2002:1–190. [PubMed] [Google Scholar]
- 14.Third Report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III) Final Report. Circulation. 2002;106:3143. [PubMed] [Google Scholar]
- 15.Helfand M. Buckley DI. Freeman M, et al. Emerging risk factors for coronary heart disease: A summary of systematic reviews conducted for the U.S. Preventive Services Task Force. Ann Int Med. 2009;151:496–507. doi: 10.7326/0003-4819-151-7-200910060-00010. [DOI] [PubMed] [Google Scholar]
- 16.Eisenmann J. On the use of a continuous metabolic syndrome score in pediatric research. Cardiovascular Diabetology. 2008;7(1):17. doi: 10.1186/1475-2840-7-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.de Ferranti SD. Gauvreau K. Ludwig DS, et al. Prevalence of the metabolic syndrome in American adolescents: Findings from the Third National Health and Nutrition Examination Survey. Circulation. 2004;110:2494–2497. doi: 10.1161/01.CIR.0000145117.40114.C7. [DOI] [PubMed] [Google Scholar]
- 18.Zimmet P. Alberti G. Kaufman F, et al. The metabolic syndrome in children, adolescents. Lancet. 2007;369:2059–2061. doi: 10.1016/S0140-6736(07)60958-1. [DOI] [PubMed] [Google Scholar]
- 19.Puyau MR. Adolph AL. Vohra FA, et al. Validation and calibration of physical activity monitors in children. Obes Res. 2002;10:150–157. doi: 10.1038/oby.2002.24. [DOI] [PubMed] [Google Scholar]
- 20.Belcher BR. Berrigan D. Dodd KW, et al. Physical activity in US youth: Impact of race/ethnicity, age, gender, & weight status. Med Sci Sports Exerc. 2010;42:2211–2221. doi: 10.1249/MSS.0b013e3181e1fba9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mendoza JA. Watson K. Nguyen N, et al. Active commuting to school and association with physical activity and adiposity among US youth. J Phys Act Health. 2011;8:488–495. doi: 10.1123/jpah.8.4.488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Freedson P. Pober D. Janz KF. Calibration of accelerometer output for children. Med Sci Sports Exerc. 2005;37:S523–S530. doi: 10.1249/01.mss.0000185658.28284.ba. [DOI] [PubMed] [Google Scholar]
- 23.Trost SG. Loprinzi PD. Moore R, et al. Comparison of accelerometer cut-points for predicting activity intensity in youth. Med Sci Sports Exerc. 2010;43:1360–1368. doi: 10.1249/MSS.0b013e318206476e. [DOI] [PubMed] [Google Scholar]
- 24.Evenson KR. Catellier DJ. Gill K, et al. Calibration of two objective measures of physical activity for children. J Sports Sci. 2008;26:1557–1565. doi: 10.1080/02640410802334196. [DOI] [PubMed] [Google Scholar]
- 25.National Cancer Institute. Risk factor monitoring and methods: SAS programs for analyzing NHANES 2003–2004 accelerometer data. 2007. http://riskfactor.cancer.gov/tools/nhanes_pam/ [Nov 26;2011 ]. http://riskfactor.cancer.gov/tools/nhanes_pam/
- 26.Dwyer J. Ellwood K. Leader NP, et al. Integration of the continuing survey of food intakes by individuals and the National Health And Nutrition Examination Survey. J Am Diet Assoc. 2001;101:1142–1143. doi: 10.1016/s0002-8223(01)00279-6. [DOI] [PubMed] [Google Scholar]
- 27.Siegel MB. Naimi TS. Cremeens JL, et al. Alcoholic beverage preferences and associated drinking patterns and risk behaviors among high school youth. Am J Prev Med. 2011;40:419–426. doi: 10.1016/j.amepre.2010.12.011. [DOI] [PubMed] [Google Scholar]
- 28.Alkerwi AA. Boutsen M. Vaillant M, et al. Alcohol consumption, the prevalence of metabolic syndrome: A meta-analysis of observational studies. Atherosclerosis. 2009;204:624–635. doi: 10.1016/j.atherosclerosis.2008.10.036. [DOI] [PubMed] [Google Scholar]
- 29.Nakashita Y. Nakamura M. Kitamura A, et al. Relationships of cigarette smoking and alcohol consumption to metabolic syndrome in Japanese men. J Epidemiol. 2010;20:391–397. doi: 10.2188/jea.JE20100043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Eaton DK. Kann L. Kinchen S, et al. Youth risk behavior surveillance—United States, 2007. MMWR. 2008;57:1–131. [PubMed] [Google Scholar]
- 31.Wilsgaard T. Jacobsen BK. Lifestyle factors and incident metabolic syndrome: The Tromsø Study 1979—2001. Diabetes Res Clin Pract. 2007;78:217–224. doi: 10.1016/j.diabres.2007.03.006. [DOI] [PubMed] [Google Scholar]
- 32.Benowitz NL. Bernert JT. Caraballo RS, et al. Optimal serum cotinine levels for distinguishing cigarette smokers and nonsmokers within different racial/ethnic groups in the United States between 1999 and 2004. Am J Epidemiol. 2009;169:236–248. doi: 10.1093/aje/kwn301. [DOI] [PubMed] [Google Scholar]
- 33.Weitzman M. Cook S. Auinger P, et al. Tobacco smoke exposure is associated with the metabolic syndrome in adolescents. Circulation. 2005;112:862–869. doi: 10.1161/CIRCULATIONAHA.104.520650. [DOI] [PubMed] [Google Scholar]
- 34.Fritz MS. MacKinnon DP. Required sample size to detect the mediated effect. Psycholog Sci. 2007;18:233–239. doi: 10.1111/j.1467-9280.2007.01882.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Baron R. Kenny D. The moderator-mediator variable distinction in social psychological research: Conceptual, strategic and statistical considerations. J Personality Soc Psychol. 1986;51:1173–1182. doi: 10.1037//0022-3514.51.6.1173. [DOI] [PubMed] [Google Scholar]
- 36.Weiss R. Dufour S. Taksali SE, et al. Prediabetes in obese youth: A syndrome of impaired glucose tolerance, severe insulin resistance, and altered myocellular and abdominal fat partitioning. Lancet. 2003;362:951–957. doi: 10.1016/S0140-6736(03)14364-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Caprio S. Hyman LD. McCarthy S, et al. Fat distribution and cardiovascular risk factors in obese adolescent girls: importance of the intraabdominal fat depot. Am J Clin Nutr. 1996;64:12–17. doi: 10.1093/ajcn/64.1.12. [DOI] [PubMed] [Google Scholar]
- 38.Fujioka S. Matsuzawa Y. Tokunaga K, et al. Contribution of intra-abdominal fat accumulation to the impairment of glucose and lipid metabolism in human obesity. Metabolism. 1987;36:54–59. doi: 10.1016/0026-0495(87)90063-1. [DOI] [PubMed] [Google Scholar]
- 39.LeBlanc AG. Janssen I. Dose-response relationship between physical activity and dyslipidemia in youth. Can J Cardiol. 2010;26:e201–e205. doi: 10.1016/s0828-282x(10)70400-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Berenson GS. Srinivasan SR. Bao W, et al. Association between multiple cardiovascular risk factors and atherosclerosis in children and young adults. N Engl J Med. 1998;338:1650–1656. doi: 10.1056/NEJM199806043382302. [DOI] [PubMed] [Google Scholar]