Abstract
Background & Aims
The incidence of intraductal papillary mucinous neoplasm (IPMN) is believed to be increasing; we investigated whether this is the result of increasing burden of disease or more diagnostic scrutiny.
Methods
In a retrospective cohort study, we calculated a trend in reported incidence of IPMN using data collected from Olmsted County, Minnesota from 1985 to 2005. Total IPMN cases from the Olmsted database were identified through keyword and ICD-9 search using a database from the Rochester Epidemiology Project, with all cases verified by subsequent chart review. The subsequent rate of IPMN-related carcinoma was calculated using data from the national SEER-9 database, reflecting trends from 1982 to 2007. Cases of IPMN-related carcinoma were identified in the SEER database by limiting the search to histology codes for non-invasive and invasive IPMN.
Results
Between 1985 and 2005, there was a 14-fold increase in the age and sex-adjusted incidence of IPMN, from 0.31 to 4.35 per 100,000 persons. From 2000 to 2001, the rate of reported carcinoma increased from .008 to .032 per 100,000 persons, but stabilized afterward, with a rate of .06 per 100,000 persons in 2007. Mortality from all causes of pancreatic cancer was stable between 1975 and 2007 (approximately 11 deaths per 100, 000 individuals).
Conclusion
The incidence of IPMN has increased in the absence of a rise in IPMN-related or overall pancreatic cancer-related mortality, so it likely results from an increase in diagnostic scrutiny, rather than greater numbers of patients with clinically relevant disease.
Keywords: pancreas, cyst, imaging, detection
Introduction
Over the last two decades, the diagnosis and number of surgical resections for intraductal papillary mucinous neoplasm (IPMN) have increased dramatically.1 Although the factors driving the increased recognition and surgical intervention for IPMN are not fully understood, it is likely that increased recognition of incidental pancreatic cystic lesions due to expansive utilization of high-resolution cross-sectional imaging is, at least in part, driving this trend. When evaluating these trends and practices, it is important to consider the potential overall disease burden of IPMN as well as its natural history.
Pancreatic cystic pathology has been reported to be present in up to 25% of examined cadavers in a single autopsy series, although the vast majority (97%) of these lesions were benign.2 Furthermore, identification of incidental pancreatic cystic pathology has been reported in up to 2.6% of people undergoing cross-sectional imaging for indication other than known or suspected pancreatic disease.3 Extrapolating from such data, one would estimate that amongst the nearly 80 million people in the United States over the age of 50, there are approximately 2 million people with some form of pancreatic cystic disease. Our clinical experience however dictates that only a fraction of this disease is, or ever will be, clinically relevant.
With the increasing recognition of pancreatic cystic lesions, there has been a mounting challenge for clinicians to determine which of these lesions warrants surgical intervention, cytologic sampling, ongoing surveillance, or simple reassurance. Particularly of interest is whether the increasing recognition of IPMN represents increasing identification of clinically meaningful disease, or instead identification of lesions which are never destined to result in disease-related morbidity or mortality, in which case they might be more aptly termed “pseudo-disease”.
We aimed to evaluate with best currently available data the estimated incidence of malignant IPMN relative to total IPMN diagnoses to better understand if our increasing recognition of IPMN is translating to an increasing recognition of clinically meaningful disease. We hypothesized that the vast majority of IPMN lesions have a natural history which is likely not clinically important, yet still drive specialty referral, surveillance, and patient and provider anxiety.
Methods
Currently no national database exists which accurately reflects the incidence of IPMN in the United States. Given this, data from a previously published well defined population study was used to estimate the national incidence of IPMN cases as previously described.4 In brief, the Rochester epidemiology project (REP) index includes the records of virtually all medical providers that care for the residents of Olmsted County, Minnesota. The REP has accumulated comprehensive records since the early 1900s including information related to clinic and emergency department visits, inpatient visits, nursing home care, and autopsy data for hospitals and private practice practitioners in Olmsted County. Using an exhaustive initial search of up to 26 ICD-9 codes as well as free text search using the experimental Mayo Clinic Life Sciences medical text search engine and REP database, records of patients were reviewed from the years 1976 to 2005. This search, which was restricted to residents of Olmsted County, resulted in 357 initial potential matches.
Each case was subsequently independently reviewed including radiographic and clinical data, as well as pathologic data when available (56 patients), by 2 independent pathologists with interest in IPMN. The diagnosis of IPMN was confirmed using criteria as set forth by the World Health Organization.5 Any cases with evidence of chronic pancreatitis, pseudocysts, pancreatic adenocarcinoma, or any cystic pathology other than IPMN were excluded. The incidence rate of IPMN was subsequently estimated as the number of incident IPMN cases divided by the number of person-years at risk for the population, directly standardized to the age and sex distribution of the 2000 US white population. Due to the relatively small number of incident cases per each individual year, the incidence rate was averaged over 5 year intervals in an effort to reduce variability but allow for limited comparison of trends over time.
The national rate of IPMN-related carcinoma was then calculated using data from the national Surveillance Epidemiology and End Results (SEER-9) database. The SEER-9 cancer registry database was evaluated between the years 1982 to 2007 from 9 geographic areas within the United States including Atlanta, Connecticut, Detroit, Hawaii, Iowa, New Mexico, San Francisco-Oakland, Seattle-Puget Sound, and Utah. Search criteria was restricted to ICD-0-3 histology codes 8453-2 and 8453–3, corresponding to non-invasive and invasive intraductal papillary mucinous carcinoma respectively. The national incidence rate of IPMN-related carcinoma was recorded as the number of incident cases per year 100,000 persons at risk.
The incidence rate of IMPN-related carcinoma was subsequently compared to the estimated total incidence rate of IPMN for overlapping time periods.
Results
Data interrogation from the Rochester Epidemiology Project for the years 1973 to 2007 resulted in the identification of 28 cases of IPMN, with the first identified case occurring in 1984. The principal reason for diagnosis in this series was incidental finding on an abdominal imaging study in an asymptomatic patient (n=17, 63%). 8 patients presented with abdominal pain, 1 with increasing abdominal girth, and 2 presented with jaundice. The mean cyst size was 17.4mm (SD, 12.6mm). Pathology was available for 10 of these 28 patients, 9 of whom proceeded to surgery. Of the 9 patients who proceeded to surgery 5 had a simple adenoma, 2 borderline lesions, and 3 with carcinoma.
Between 1985 and 2005 a 14-fold increase in age and sex-adjusted incidence rate of IPMN was observed with an increase from 0.31 to 4.35 cases per 100,000 persons (Figure 1). Because of the small number of cases represented over 20 years, statistical analysis was not conducted as the number of cases available does not allow for appropriately powered evaluation of trend over time.
Figure 1.
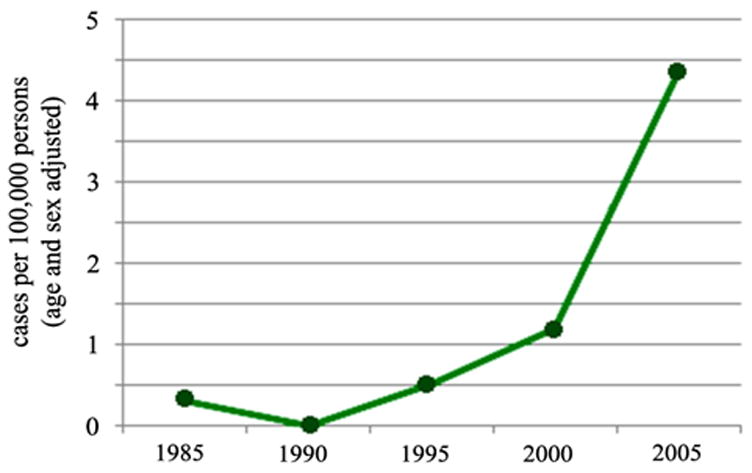
Incidence of total IPMN per 100,000 person-years as reflected by the Rochester Epidemiology Project (Green Line)
The SEER-9 database does not reflect any cases of IPMN-related carcinoma prior to the year 2000. From 2000 to 2005 there was a 7.5-fold increase in reported rates of malignant-IPMN with an increase in case-rate from .008 to .06 cases per 100,000 persons including both invasive carcinoma and carcinoma in-situ (Figure 2). Although there was a sharp rise in reported cases between 2000 and 2001 (.008 to .032 cases per 100,000 persons), there was only a 1.9x increase in incidence between 2001 and 2005. Cases of invasive carcinoma and carcinoma in-situ were nearly equally divided over the observed time period. At the most recent time point in 2005, cases of invasive and non-invasive carcinoma were represented with rates of .022 and .024 cases per 100,000 persons respectively.
Figure 2.

Incidence of IPMN-related carcinoma per 100,000 persons at risk between 2000 and 2007 as reflected by the SEER-9 database (Violet – Total Carcinoma ; Orange – Carcinoma In-Situ ; Blue – Invasive Carcinoma)
Using the most recent shared data point of 2005, direct comparison of the incidence rates of total IPMN diagnosis to malignant-IPMN was performed, demonstrating an incidence rate ratio of 68.5 (Figure 3).
Figure 3.
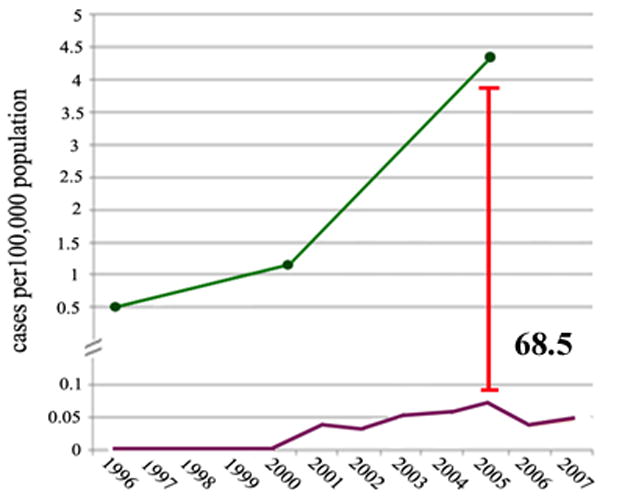
Comparison of the incidence rate of total IPMN (Green) versus IPMN-related carcinoma (Violet) per 100,000 persons at risk between the years 2000 and 2005
Discussion
The current study demonstrates a rising rate in IPMN diagnosis over the last two decades, as has previously been reported. When comparing the total IPMN rate to the malignant-IPMN rate however, it is striking that the incidence rate of malignant-IPMN is far less than that for total IPMN, nearly 70-fold less. This finding clearly has important implications as it suggests that the majority of IPMN in fact have low overall malignant potential.
Data from early literature derived predominantly from retrospective analysis of surgical series suggests evidence of invasive carcinoma or carcinoma-in-situ in as much as 60-70% of resected IPMN specimens in the presence of main duct disease, 25% with branch duct limited disease.6,7 This literature, while critically important and representative of some of the seminal work in the evaluation of IPMN, is potentially biased with overrepresentation of malignant IPMN given the nature of retrospective evaluation of surgical specimens in patients that are more likely to have symptomatic disease or larger lesions.
It has been well established that in patients with side-branch limited IPMN less than 3cm in maximal diameter without concerning cytology or radiographic features, that the risk of malignancy is much lower, as is the risk for disease progression.7–11 In asymptomatic patients with SB-IPMN less than 3cm and absence of mural nodules, the risk of malignancy is in fact quite small, on the order of 0-5%.12,13 As an increasing number of pancreatic cystic lesions are identified due to growing use of high-resolution imaging technology, one would expect a shift towards increased recognition of smaller and asymptomatic lesions. In this context, it would seem likely that we are recognizing an increasing number of pancreatic cystic lesions whose natural history may not be clinically important, yet still drive specialty referral, surveillance imaging, invasive testing, and possibly surgical intervention given heightened concern for malignant potential of mucinous cystic neoplasms of the pancreas.
Importantly, data exists demonstrating that the majority of incidentally identified pancreatic lesions will not meet current criteria for resection at the time of diagnosis.14 For such lesions current guidelines recommend frequent surveillance using high-resolution imaging without consideration however of the clinical efficacy or cost-efficacy of this practice. Moreover, we recognize currently that the identification of even small asymptomatic pancreatic cysts is driving significant amounts of referral, invasive evaluation, and at times surgical intervention due to heightened concern surrounding the malignant potential of IPMN.
A recent published case series reported evidence of malignancy in up to 25% of asymptomatic pancreatic lesions that were surgically resected. 94% of these represented solid lesions however with note that invasive cancer was identified in only 1.7% of incidentally identified cystic pancreatic lesions. This same group reports seeing more cystic lesions than ever however, reflecting up to 62% of asymptomatic pancreatic lesion referrals, while operating on only one third of cases.15 Similar data exists from alternative series with report that pancreatic cysts 2cm or less were malignant in only 3.5% of resected incidental cystic lesions.16 Such studies confirm that the prevalence of malignant-IPMN in small incidentally identified pancreatic cysts is very low. Data from these series as well as additional studies which demonstrate that the risk of subsequent malignant transformation of such lesions is similarly quite low call into question the aggressive universal surveillance practices for small asymptomatic IPMN.7–11
Further complicating questions surrounding the optimal management of IPMN remains the fact that the natural history of IPMN, even frankly malignant IPMN, is not yet well understood.12,14,17 It has been demonstrated that the 5-year survival for even invasive IPMN is significantly better than that of traditional ductal adenocarcinoma.18 While survival is frequently the outcome of highest priority in most medical care models, in a patient population that is older and likely to harbor significant medical co-morbidities, a careful risk to benefit analysis needs to be performed on a case-by-case basis taking into consideration the likely impact IPMN will have on a given patient’s life versus morbidity and costs related to intervention or life-long surveillance.
The current analysis is not meant to undermine the importance of identifying and treating IPMN that are high risk to harbor or develop malignancy. The presented data does highlight however the need for effective and efficient tools to differentiate IPMN that warrant surveillance or intervention from those that may be labeled as low risk and followed no further. While the Sendai criteria have reliably identified high risk lesions, the majority of incidentally identified pancreatic cystic lesions will in fact not meet criteria for resection and our current predictive tools regarding future malignant potential are limited. For instance, even in the best of hands, the ability of EUS to predict the presence or absence of malignancy is less than ideal with a reported accuracy of only 51%, no better than a flip of the coin.19
Finally, there are limitations of the above analysis that warrant discussion. Most important, data regarding the incidence rates of total IPMN and malignant IPMN are derived from different databases and patient populations. Unfortunately there is no national database currently which accurately reflects the incidence of IPMN. This study therefore utilized data from a single well-defined population study and extrapolated results from this sub-population with assumption that this data is representative of national trends. It is possible however that the sampled population is in fact not representative of the national trend which would reduce accuracy of any further comparisons. Moreover, the number of cases reflecting the incidence of IPMN from the Rochester Epidemiology Project is small and the number of years followed limited, adding to potential sampling error. Finally, without surgical pathology data for all patients to determine the presence or absence of ovarian stroma, clinically distinguishing between SB-IPMN and MCN may be challenging. As is the case in clinical practice, this is accomplished predominantly through imaging to evaluate for the presence or absence of communication with the pancreatic duct, although the possibility of diagnostic error remains.
Similarly, our analysis makes the assumption that the SEER database effectively captures the majority of cases of IPMN-related malignancy for dedicated regions and is again representative of national trend. Additionally, the assumption is made that misclassification bias is minimal in the SEER database and there is high fidelity between reported diagnosis and actual pathology. Finally, while the cited studies provide ample evidence surrounding the low malignant potential of small side-branch IPMN, it is worth noting that concern has been raised previously about the possibility of a field defect in patients with IPMN suggesting an increased risk not just for IPMN-related carcinoma, but traditional ductal carcinoma as well. Such considerations add further to the complexity of guidelines surrounding surveillance practices of the incidentally identified pancreatic cystic lesion.
Acknowledging the above limitations, the current data is provocative and underscores the need for a prospective national or international database which will allow for better understanding of the true incidence and prevalence of IPMN as well as its natural history. Such data will further help guide management practices regarding both therapeutic intervention as well as surveillance practices of IPMN in the future.
Acknowledgments
Grant Support: Dr. Gardner is supported in part by NIH grant 1K23DK088832-01 Dr. Reid-Lombardo is supported in part by NIH grant 1UL1 RR024150
Abbreviations
- EUS
Endoscopic Ultrasound
- ICD
International Classification of Diseases
- IPMN
Intraductal Papillary Mucinous Nesoplasm
- SEER
Surveillance Epidemiology and End Results
Footnotes
Financial Disclosures: None
Writing Assistance: None
Author Contributions: Study concept and design: All
Acquisition of data: All
Analysis and interpretation of data: Klibansky, Reid-Lombardo, Gardner
Drafting of the manuscript: All
Critical revision of the manuscript for important intellectual content: All
Statistical analysis: Klibansky, Gardner
Obtained funding: NA
Administrative, technical, or material support: NA
Study supervision: Gardner
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Sohn TA, Yeo CJ, Camern JL, et al. Intraductal Papillary Mucinous Neoplasms of the Pancreas. An Updated Experience. Ann Sug. 2004;239:788–799. doi: 10.1097/01.sla.0000128306.90650.aa. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kimura W, Nagai H, Kuroda A, et al. Analysis of small cystic lesions of the pancreas. Int J Pancreatol. 1995;18:197–206. doi: 10.1007/BF02784942. [DOI] [PubMed] [Google Scholar]
- 3.Laffan TA, Horton KM, Klein AP, et al. Prevalence of unsuspected pancreatic cysts on MDCT. AJR. 2008;191:802–807. doi: 10.2214/AJR.07.3340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Reid-Lomardo K, Sauver J, Li Z, et al. Incidence, Prevalence, and Management of Intraductal Papillary Mucinous Neoplasm in Olmsted County, Minnesota, 1984–2005: A population study. Pancreas. 2008;37:139–144. doi: 10.1097/MPA.0b013e318162a10f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Longnecker DS, Adler G, Hruban R, et al. Intraductal Papillary Mucinous neoplasms of the pancreas. In: Hamilton SR, Analtonem LA, editors. World Health Organization Classification of Tumors. Tumors of the Digestive System. Oxford: IARC Press; 2000. pp. 237–240. [Google Scholar]
- 6.Salvia R, Fernandez-del Castillo C, Bassi C, et al. Main-duct Intraductal Papillary Mucinous Neoplasms of the Pancreas. Clinical predictors of malignancy and long-term survival following resection. Ann Surg. 2004;239:678–687. doi: 10.1097/01.sla.0000124386.54496.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tanaka M, Chari S, Adsay V, et al. International Consensus Guidelines for Management of Intraductal Papillary Mucinous Neoplasms and Mucinous Cystic Neoplasms of the Pancreas. Pancreatology. 2006;6:17–32. doi: 10.1159/000090023. [DOI] [PubMed] [Google Scholar]
- 8.Pelaez-Luna M, Chari ST, Smyrk TC, et al. Do consensus indications for resection in branch duct intraductal papillary mucinous neoplasm predict malignancy? Am J Gastroenterol. 2007;102:1759–1764. doi: 10.1111/j.1572-0241.2007.01224.x. [DOI] [PubMed] [Google Scholar]
- 9.Rodriguez JR, Salvia R, Crippa S, et al. Branch-duct intraductal papillary mucinous neoplasms and mucinous cystic neoplasms: observations in 145 patients who underwent resection. Gastroenterology. 2007;133:72–79. doi: 10.1053/j.gastro.2007.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tang RS, Weinberg B, Dawson DW, et al. Evaluation of the guidelines for management of pancreatic branch-duct intraductal papillary mucinous neoplasm. Clin Gastroenterol Hepatol. 2008;6:815–819. doi: 10.1016/j.cgh.2008.04.005. [DOI] [PubMed] [Google Scholar]
- 11.Cone M, Rea JD, Diggs BS, et al. Predicting malignant intraductal papillary mucinous neoplasm: a single-center review. Am J Surg. 2011;201:575–579. doi: 10.1016/j.amjsurg.2011.01.003. [DOI] [PubMed] [Google Scholar]
- 12.Matsumoto T, Aramaki M, Yada K, et al. Optimal management of the branch duct type of the intraductal papillary neoplasms of the pancreas. J Clin Gastroenterol. 36:261–265. doi: 10.1097/00004836-200303000-00014. [DOI] [PubMed] [Google Scholar]
- 13.Sugiyama M, Izumisato Y, abe N, et al. predictive factors for malignancy in intraductal papillary mucinous tumours of the pancreas. Br J Surg. 90:1244–1249. doi: 10.1002/bjs.4265. [DOI] [PubMed] [Google Scholar]
- 14.Bassi C, Sarr MG, Lillemore KD, et al. Natural History of Intraductal Papillary Mucinous Neoplasms (IPMN): Current Evidence and Implications for Management. J Gastrintest Surg. 2008;12:645–650. doi: 10.1007/s11605-007-0447-x. [DOI] [PubMed] [Google Scholar]
- 15.Sachs T, Pratt WB, Callery MP, et al. The Incidental Asymptomatic Pancreatic Lesion: Nuisance or Threat? J Gastrointest Surg. 2009:405–415. doi: 10.1007/s11605-008-0788-0. [DOI] [PubMed] [Google Scholar]
- 16.Fernandez-del Castillo C, Targarona J, Thayer SP, et al. Incidental Pancreatic Cysts. Clinicopathologic characteristics and comparison with symptomatic patients. Arch Surg. 2003;138:427–434. doi: 10.1001/archsurg.138.4.427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ball CG, Howard TJ. Natural history of intraductal papillary mucinous neoplasia: How much do we really know? World J Gastrointest Surg. 2010:368–372. doi: 10.4240/wjgs.v2.i10.368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Poultsides GA, Reddy S, Cameron JL, et al. Histopathologic basis for the favorable survival after resection of intraductal papillary mucinous neoplasm-associated invasive adenocarcinom of the pancreas. Ann Surg. 2010;251:470–476. doi: 10.1097/SLA.0b013e3181cf8a19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Brugge WR, Lewandrowski K, Lee-Lewandrowski, et al. Gastroenterology. 2004;126:1330–1336. doi: 10.1053/j.gastro.2004.02.013. [DOI] [PubMed] [Google Scholar]


