Abstract
A key characteristic of hematopoietic stem cells (HSC) is the ability to self-renew. Several genes and signaling pathways control the fine balance between self-renewal and differentiation in HSC and potentially also in leukemic stem cells. Besides pathways such as Wnt signaling, Hedgehog signaling and Notch signaling, transcription factors (FoxOs) and cell fate determinants may also play a role in stem cells. While some of these pathways seem to be dispensable for maintenance of adult HSC, there may be a distinct requirement in leukemia stem cells for leukemic self-renewal. Here we will focus on self-renewal related signaling in myeloid leukemia stem cells and its therapeutic relevance.
Keywords: Leukemia stem cell, Self-renewal, Hedgehog, Wnt, Notch, FoxO
1 Myeloid leukemia stem cells
More than a decade ago, leukemia initiating cells were described as a subpopulation of acute myeloid leukemia (AML) cells that have extensive self-renewal capacity. These cells were able to serially transplant human AML into immunocompromised mice [1]. Based on these initial xenotransplantation studies and subsequent studies assessing both human and mouse leukemias, AML is believed to be organized in a hierarchy with cells possessing properties such as extensive self-renewal proliferative capacity at the apex [2, 3]. These leukemia initiating cells or leukemia stem cells (LSC) arise either from corrupted hematopoietic stem cells (HSC) or more differentiated and committed progenitors that acquire self-renewal potential [4–7]. They possess properties of normal HSC, such as self-renewal capacity and long-term growth potential. This distinguishes LSC from the bulk of leukemia cells and suggests that novel targeted strategies might be developed to target these cells and thus eradicate disease.
For patients with AML, relapse of previously treated disease remains an unsolved problem. Yet, more than 50% of all patients with AML eventually experience relapse [8–10]. Residual leukemic cells are not eradicated by chemotherapy treatment and give rise to relapse after discontinuation of treatment. Thus, new approaches that target these residual cells are desperately needed, and targeting the pathways responsible for the extensive self-renewal properties found in LSC is a potential new approach to this problem.
In contrast to AML, chronic myelogenous leukemia (CML) is a myeloproliferative neoplasia that is characterized by unlimited expansion of mature myeloid cells. The majority of patients with CML can be treated successfully using the tyrosine kinase inhibitor imatinib. However, upon discontinuation of treatment, an increase in disease burden becomes evident in the majority of cases [11–13] and relapse occurs due to residual leukemic cells that reestablish disease. Development of CML is well characterized in model systems, and is caused by corruption of HSC by the oncogenic fusion kinase BCR-ABL. BCR-ABL cannot transform committed progenitors [5] and therefore depends on self-renewal properties inherent in HSC. This may offer therapeutic options if one could take advantage of potential ‘synthetic lethality’ where the BCR-ABL expressing LSC might be dependent on pathways that are not necessary for normal HSC.
In both, AML and CML, aberrant activation of various signaling pathways that stimulate proliferation or inhibit apoptosis are known to influence disease initiation and development of leukemia. However, the property of self-renewal is unique to normal and cancer stem cells. Targeting this unique property of LSC—self-renewal capacity—is therefore thought to be a promising way to eradicate disease if one can determine which pathways are critical for LSC, but not HSC. We will focus on pathways that are known to be involved in regulation of self-renewal capacity of myeloid LSC in murine models and human disease, and point out where they might be uniquely required in leukemia.
2 Self-renewal associated signaling and transcription
2.1 Hedgehog pathway
The Hedgehog (Hh) pathway is a highly conserved developmental pathway which regulates the proliferation, migration and differentiation of cells during development [14, 15]. It is typically active during development, but silenced in adult tissues, except during tissue regeneration and injury repair [16, 17]. Three distinct ligands, Sonic (Shh), Indian (Ihh) and Desert (Dhh) Hedgehog exist in humans. Upon ligand binding to the receptor patched (Ptch), inhibition of smoothened (Smo) receptor is relieved. Smo then activates members of the Gli family of zinc-finger transcription factors, which translocate to the nucleus to regulate the transcription of Hh target genes including Gli1, Gli2, Ptch and regulators of cell proliferation and survival [18–20] (Fig. 1). As expected, abrogating such a critical developmental pathway is embryonic lethal: Smo−/− mouse embryos did not survive past 9.5 days post-coitum [21], exhibiting ventral cyclopia and holoprosencephaly, which is also observed in the Shh knockout. To study the role of Smo in the hematopoietic systems and to avoid the embryonic lethality of Smo−/−, investigators have used transplanted Smo−/− and Ptch+/− fetal liver cells [22] and conditional deletion of Smo [23–25]. The results have been somewhat conflicting, depending on the experimental system utilized, which may reflect the importance of these pathways during specific periods of development. Transplanted Smo−/− fetal liver cells had decreased colony-forming potential in cellular replating assays, while Ptch+/− cells, in which Smo was activated, showed enhanced serial replating [22]. In vivo, there was improved engraftment of Ptch+/− cells in mice when compared with wild-type under homeostatic conditions or during acute regeneration implying an important function for the Hh pathway in HSC. In SmoloxP/loxP mice crossed with vav-cre mice, which conditionally deletes Smo early in embryogenesis in the hematopoietic, and a limited number of other compartments, no acute effect was seen in the hematopoietic populations, but a defect in long-term HSC function was uncovered in primary and secondary transplants [25]. In contrast to these studies, two recent papers that used SmoloxP/loxP mice crossed with Mx-cre mice, which conditionally deletes Smo in hematopoietic cells after interferon stimulation, demonstrated that the Hh pathway is dispensable for adult HSC function and hematopoiesis [23, 24]. Pharmacological inhibition of Hh signaling using a small molecule had no effect on normal hematopoiesis in adult mice. In addition, gene expression profiling showed that an HSC-specific gene expression signature was preserved in the Smo-deficient HSC and a Smo gain-of-function model (SmoM2) did not lead to expansion of HSC.
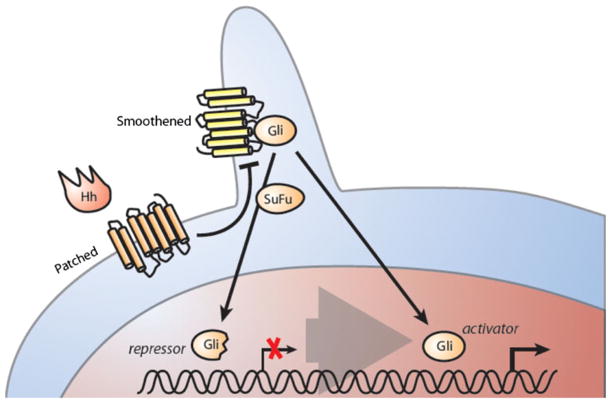
Fig. 1.
Hedgehog signaling has been implicated in regulation of leukemia stem cells with different oncogenic background. The transmembrane proteins patched (Ptch) and smoothened (Smo) are located on the plasma membrane. Various kinases phosphorylate the transcription factor Gli, creating a repressor form of this transcription factor. SuFu prevents the active form of Gli from transactivating Hh-responsive genes. Upon binding of Hh ligand Patched Gli activates Smo, which itself translocates to the plasma membrane. The active form of Gli then regulates the expression of Hedgehog target genes
The role of Hh signaling in hematopoietic malignancies has also been studied, with most data on chronic myeloid leukemia (CML) models. When bone marrow from vav-cre; SmoloxP/loxP mice [25] was transduced with BCR-ABL retrovirus and transplanted into irradiated primary and secondary recipients, a CML-like disease occurred in only 47% of recipients, and with an increased latency, compared to control Smo+/+ cells, which had disease in 94% of recipients. The Smo−/− CML mice showed a significant decrease in the population of Lin− Sca-1+ c-Kit+ (LSK) cells, which contains the LSC in this model, while activated SmoM2, expressed by vav-cre, showed an increase in LSK cells and acceleration of CML progression. Hh inhibition by cyclopamine, like the Smo−/− cells, caused increased latency of disease and a decrease in the LSK population, even in CML with the imatinib (Novartis Pharmaceuticals, Basel, Switzerland)-resistant T315I mutation.
Supporting the previous work in another model, Smo−/− fetal liver cells, when transduced with BCR-ABL retrovirus and transplanted into mice, caused CML-like disease in only 60% of recipients [22], again with longer latency, compared with control Smo+/+ fetal liver cells, where all recipient mice were affected rapidly. In the secondary recipients, no mice receiving Smo−/− cells developed disease, even after 9 months of observation. A combination of cyclopamine and the BCR-ABL inhibitor nilotinib (Novartis) had an additive effect in reducing colony-forming units in vitro, and mice with CML took longer to relapse after discontinuation of therapy than those treated with Nilotinib alone. Although only published in abstract form [26, 27], the Smo antagonist NVP-LDE225 (Novartis) caused a significant reduction in secondary colony formation and replating efficacy in a dose-dependant manner in vitro in primary CML cells. A combination of NVP-LDE225 and nilotinib reduced replating efficiency even further and also improved survival after drug discontinuation in mice, similar to the cyclopamine results. Another recent abstract reported that the novel Smo antagonist, PF-04449913 (Pfizer, New York City, NY, USA), when combined with dasatinib (Bristol-Myers Squibb, New York City, NY, USA) [28], showed decreases in primary CML cell engraftment in Rag2−/−gc−/− mice and eradication of the cells’ ability to form myeloid sarcomas.
On the other hand, Mx-cre deleted Smo was shown to be dispensable in an MLL-AF9 induced murine AML model [24] and a Notch-inducted T-cell acute lymphoblastic leukemia (T-ALL) model [23]. In the AML model, no difference in phenotype was noted as compared to the control Smo+/+ cells when assessed by in vitro serial plating assays, in vivo AML disease penetrance or latency, in contrast to the findings in CML models. In the T-ALL model, the kinetics of disease onset was unchanged by genetic deletion or pharmacological inhibition of Smo. Secondary transplants were similarly unaffected.
The conflicting data on the role of the Hh pathway in hematopoiesis are likely attributed to differences in the experimental designs used in the studies, particularly the timing of the deletion of Smo, with reports that abrogated Hh in embryogenesis more likely to observe a deleterious stem cell phenotype than those that abrogate Hh in adulthood. A murine experiment conditionally knocking out the Hh pathway at various points in primitive and definitive hematopoiesis could help clarify if this is indeed the case.
In hematopoietic malignancies, several reports now support an effect of Hh inhibition on CML stem cells, using genetic models and inhibition by a number of small molecules, although several of these reports were in abstract form only. As this preclinical data matures, the role of Hh in CML will be clarified; however, several pharmaceutical companies with Smo inhibitors have already used this data to justify opening clinical trials, as described below.
2.2 Canonical Wnt pathway
Canonical Wnt signaling is involved in self-renewal of stem cells. β-catenin (Ctnnb1), the pathway’s central effector molecule, is negatively regulated via phosphorylation by a multiprotein complex including APC, Axin, GSK-3β and casein kinase [29, 30]. When bound in this complex, Ctnnb1 is phosphorylated, consecutively ubiquitinylated and undergoes proteasomal degradation. Activation of the pathway occurs by binding of its physiological ligand Wnt to the cysteine-rich domain of receptors of the Frizzled (Fz) and LRP (low-density lipoprotein receptor-related) family (LRP 5 or LRP6) [31–33]. The protein dissheveled (DVL) consecutively binds and inhibits GSK3. This leads to inhibition of Ctnnb1 phosphorylation [34] (Fig. 2). Unphosphorylated Ctnnb1 is released and shuttles to the nucleus, to initiate transcription of its target genes via transcription factors such as TCF or LEF. In mouse models, straight knockout of Ctnnb1 is embryonic lethal due to lack of mesoderm formation and defects of the ectodermal cell layer [35]. In hematopoiesis, Wnt pathway activity is required in the bone marrow niche to regulate HSC proliferation and preserve self-renewal capacity [36]. The canonical Wnt pathway has also been shown to be necessary for appropriate HSC development [37] using the vav1-Cre system. In this model, Ctnnb1−/− bone marrow cells are deficient in long-term HSC maintenance and compete poorly against wild-type cells. However, experiments in adult HSC revealed that Ctnnb1 is dispensable for HSC maintenance in fully developed HSC [38]. This indicates differential requirements for self-renewal pathways in development versus maintenance of HSC, a mechanism similar to canonical Hedgehog signaling.
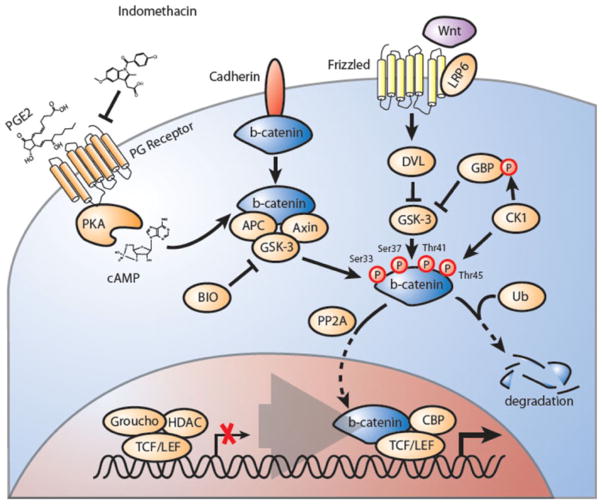
Fig. 2.
The Wnt/Ctnnb1 signaling pathway is involved in self-renewal of leukemic stem cells. In the absence of Wnt, Ctnnb1 (beta-catenin) associates with an inhibitory complex including axin (axis inhibitor) and APC (adenomatous polyposis coli) and GSK3β (glycogen-synthase kinase 3β). Ctnnb1 is phosphorylated in this complex by CK1 (casein kinase 1) and GSK3β. This phosphorylation leads to its ubiquitylation and degradation by the proteasome. Members of the TCF/LEF transcription factor family are bound in the nucleus by repressors that belong to the GRG (groucho-related gene) family and are therefore inactive. Wnt is known to bind to its receptor (Frizzled), through the cysteine-rich domain of the receptor, and co-receptors such as LRP5 (low-density lipoprotein receptor-related protein 5) or LRP6. Binding of WNT to the receptor complex results in the inactivation of GSK3β by dissheveled. The unphosphorylated version of Ctnnb1 is able to migrate to the nucleus, where it binds TCFs to activate target genes. Prostaglandin signaling has been shown to negatively regulate Wnt-Ctnnb1 via PKA/cAMP signaling in normal and leukemic stem cells
The role of Ctnnb1 has also been studied in CML using various mouse models. Inactivation of Ctnnb1 during HSC development (using vav1-Cre) resulted in a decreased development of retrovirally induced BCR-ABL driven CML [37]. However, development of ALL was still detectable in this model. When Ctnnb1 was deleted contemporaneously with activation of BCR-ABL using retroviral infection and transformation of HSC, CML-LSC failed to engraft in secondary recipient mice [39]. These experiments clearly indicate a pivotal role of Wnt signaling in CML-LSC development. More recently, Ctnnb1 has been investigated in the maintenance of already engrafted CML-LSC. In this clinically relevant setting, pharmacologic or genetic inactivation of Ctnnb1 after onset of the myeloproliferative disease acted synergistically with imatinib, reduced LSC numbers, and improved survival in a bone marrow transplant model [40]. Thus, despite its dispensability for adult HSC, CML-LSCs seem to retain dependency on canonical Ctnnb1 to maintain self-renewal capacity. In human disease, Ctnnb1 activation via the canonical Wnt pathway has been shown to occur in CML-blast crisis LSCs, which phenotypically resemble granulocyte macrophage progenitors (GMP) [41]. Aberrant splicing of GSK3 appears to contribute to this hyperactivation in blast crisis samples [42]. Thus, there is growing evidence that canonical Wnt signaling is an attractive target pathway in the treatment of CML-LSC.
Certain oncogenes are dependent on self-renewal properties, such as BCR-ABL or the combination of a Hox-Gene (e.g., HoxA9) with its cofactor Meis1. Other mostly translocation-associated oncogenes can confer ‘stemness’ and self-renewal properties to more differentiated cells such as GMP [4–6]. Using a mouse model of AML induced by MLL-rearrangements, it has been shown that this self-renewal capacity is mediated at least in part by Ctnnb1 [43, 44]. Constitutively expressed Ctnnb1 enabled progenitor cells to form leukemia with a similar efficacy as the corresponding stem cell controls [43]. Inversely, genetic deletion of Ctnnb1 led to reduction of LSC and thus to decreased leukemia formation. Interestingly, these effects could be mimicked using pharmacologic treatment. Interference of prostaglandin signaling has been shown before to target the Wnt/β-catenin axis in HSC [45, 46]. Abrogation of Ctnnb1 by the cyclooxygenase inhibitor indomethacin led to a 100-fold decrease in AML initiating cells in secondary recipients [43]. Moreover, indomethacin treatment of fully developed MLL-AF9 induced leukemia led to reduction of Ctnnb1 levels and caused reduction of LSC frequency. These data indicate that certain subtypes of AML-LSC retain dependency on Ctnnb1 and suggest that self-renewal pathways can be selective therapeutic targets for leukemia stem cells [47].
2.3 Forkhead O pathway
Forkhead O (FoxO) transcription factors regulate cell cycle, stress resistance, differentiation and long-term regenerative potential of HSC [48], and protect integrity of the stem cell pool. FoxO members are known to be effectors of the PI3K/AKT pathway, which is frequently mutated or hyperactivated in hematologic malignancies, and are abundantly expressed in the hematopoietic system. Loss of FoxO transcription factors (by using conditional alleles for FoxO1, FoxO3 and FoxO4) led to a marked reduction of LT-HSC numbers and repopulation capacity. They play an important role in response to oxidative stress and thereby mediate increased regenerative potential and self-renewal capacity [49]. As FoxO3a knockout mice reveal a rather discreet deficiency in repopulation capacity upon serial transplantation [50], there seems to be a certain degree of functional redundancy. However, FoxO3a−/− mice showed a reduction of LSC after serial transplantation in a BCR-ABL driven CML-mouse model [51]. This effect was even more pronounced using the tyrosine kinase inhibitor imatinib. As imatinib does not affect CML stem cells, these data suggest an impact of FoxO3a deficiency on stem cell properties, especially self-renewal. Using an AML model induced by MLL-AF9, one of the most recent reports has shown that ablation of FoxOs leads to decreased disease burden, prolonged survival and selective reduction of leukemic stem cells (LSC) in vivo [52]. This suggests that FoxOs are important regulators of MLL-AF9 induced leukemogenesis and may provide a novel molecular target in AML.
FoxOs play an important role in the homeostasis of reactive oxygen species (ROS) levels in normal HSC. The findings in malignant stem cells (as described above) suggest that self-renewal properties of LSC may be affected by elevated ROS levels. One putative mechanism would include oxidative stress-enhanced binding of FoxOs to Ctnnb1, which may eventually protect HSC self-renewal from detrimental action of ROS [48]. This could potentially indicate a cross talk between canonical Wnt signaling and FoxO transcription factors, with FoxOs intensifying Ctnnb1-mediated self-renewal in leukemia stem cells. Therefore, targeting FoxO transcription factors via influencing upstream PI3K-AKT signaling could offer a potential therapeutic approach to reduce LSC burden.
2.4 Notch pathway
Another pathway that has been shown to have cross talk with other major ‘stemness-pathways’ described above in regulation of self-renewal is Notch signaling. Notch receptors are an evolutionarily conserved family of trans-membrane receptors that are known to be expressed and activated in HSC [53, 54]. Binding of its physiological ligands (of the Delta and Serrata families) leads to separation of an intracellular portion of Notch. This fragment is capable of entering the nucleus where it binds the transcriptional repressor CBF-1. Interconnection of Notch, CBF-1 and the co-factor MAML-1 (mastermind-like-1) leads to transcriptional activation of target genes [53, 55–57] (Fig. 3). Constitutively active Notch is able to mediate multilineage potential in vivo [58]. Differentiation of cells leads conversely to downregulation of Notch [54]. Furthermore, there appears to be an interaction between Notch signaling and Wnt-mediated maintenance of HSC, suggesting a role in regulation of self-renewal capacity [53]. Although, the relevance of Notch signaling in self-renewal of AML-LSC has not been convincingly demonstrated, the Wnt–Notch cross talk suggests a potential influence on Wnt-dependent AMLs (as described above). In AML cell lines and primary patient blasts, down-regulation of Notch-1 expression was associated with a decrease in PU.1-mediated differentiation capacity, indicating a pivotal role in maintenance of an immature state [59]. In CML, Notch-associated self-renewal has not been clearly demonstrated per se either. However, the Notch target Hes1 was found to be highly expressed in CML-blast crisis. In a murine model, retroviral co-expression of Hes1 with BCR-ABL resulted in generation of an aggressive acute leukemia [60].
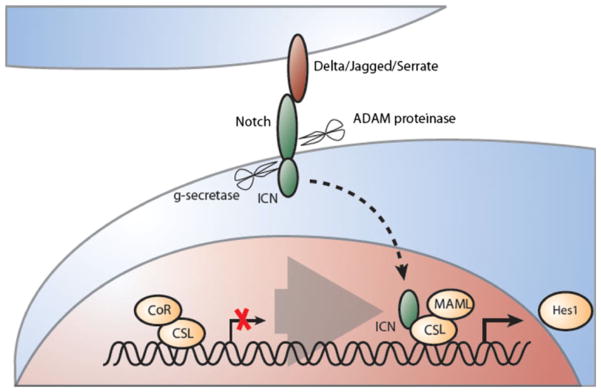
Fig. 3.
Canonical Notch signaling has context-dependent influence on stem cell self-renewal. Activation of the Notch receptor leads to a cascade of proteolytic events: (1) extracellular (ADAM proteinase) and (2) intracellular (gamma-secretase). These result in translocation of the intracellular fragment of Notch (ICN) to the nucleus causing exchange of a repressor complex by an activator complex. Notch-dependent target gene expression such as Hes-transcription factors and Hes-related genes is initiated via binding of ICN to the activation complex (containing CSL and MAML)
Recently, inactivating mutations of Notch signaling have been described in patients with chronic myelomonocytic leukemia (CMML). Investigation of Notch-inhibition in HSC led to accumulation of aberrant myeloid progenitors and a CMML-like disease in mice [61]. Taken together, the role of Notch signaling in myeloid leukemia stem cells has not yet been examined adequately and seems to be context dependent. Several factors indicate a potential role in stem cell signaling, but its role in self-renewal properties of LSC will have to be the goal of future investigations.
2.5 Cell fate determinant pathways
Cell fate determinants, such as RNA-binding proteins or polarity regulators, have been recently described as effectors in stem cell engraftment and repopulation [62]. RNA-interference led to enhanced (Prox1) or decreased (Pard6a, Prkcz, Msi2) repopulation potential in vivo. Of these candidate genes, Musashi-2 (Msi-2) has been investigated recently in leukemia mouse models [63, 64]. Initially described as a regulator of asymmetric cell division in drosophila [65], Msi-2 decrease was shown to be associated with a reduction in both, symmetric division [64] and progression of CML in mouse models [63]. Increased expression of Msi2 was associated with aggressive disease and an immature phenotype of human AML and CML [63, 64]. Although these reports do not clearly prove an effect of Msi2 on self-renewal capacity, it is tempting to speculate that its influence on symmetric versus asymmetric stem cell divisions could influence self-renewal. An increase in symmetric versus asymmetric division by, e.g., Msi2 could potentially result in distribution of ‘stemness-mediating determinants’ into both daughter cells and therefore expand the stem cell pool. Several of these cell fate determinants are currently investigated with regard to their influence on stem cell properties.
3 Therapeutic outlook
Targeting cancer stem cells by pharmacologic interference with self-renewal mediating pathways may be a promising approach to leukemia. ‘Small molecules that target self-renewal pathways are being tested in clinical trials, and thus we may soon know if this approach will have a significant impact clinically. Several small molecule Hh pathway inhibitors have already been developed for use in solid tumors, including GDC0449 (Genentech, San Francisco, CA, USA), NVP-LDE225, NVP-LEQ-506 (Novartis), IPI-926 (Infinity, Cambridge, MA, USA), BMS-833923 (Exelixis, San Francisco, CA, USA) and PF-04449913 (Pfizer). The preclinical data regarding Hh signaling in CML has justified the opening of 2 phase I clinical trials of Smo inhibitors with the BCR-ABL inhibitor dastinib: one in combination with BMS-833923, and the other in combination with PF-04449913 [66]. These trials are currently recruiting and results will be available in early 2013.
Activation of canonical Wnt signaling is known to be an important oncogenic event in solid tumors, especially in colon carcinomas and long-term treatment with COX-inhibitors/non-steroidal anti-inflammatory drugs (NSAID) has been found to reduce the relative risk for colon carcinoma development [67, 68]. Recently, reduction of tumor incidence has been reported for long-term use of the COX-inhibitor acetylsalicylic acid (aspirin) for a variety of cancers [69], suggesting that dysregulated Wnt signaling may be a common feature of many malignancies. The more selective COX-2 inhibitor celecoxib has been approved for treatment of familial adenomatous polyposis. Other rather nonspecific drugs that influence canonical Wnt signaling include vitamins such as retinoids and polyphenols. Both groups lack specificity and the exact mechanisms of action remain so far elusive. Retinoids may induce nuclear vitamin receptors that compete with TCFs for Ctnnb1 binding [70]. Polyphenols, such as curcumin or resveratrol, also target other signaling pathways [71, 72]. Only few molecules that target this pathway have made it into clinical trials, likely due to a lack of enzyme targets in the pathway. Examples include an inhibitor of creb-binding protein (CBP), ICG-001, which is currently being investigated in a phase 1 clinical trial [73]. A small molecule named XAV939 was recently discovered and stabilizes axin by inhibition of the enzymes tankyrase 1 and 2, thereby promoting degradation of Ctnnb1 in a colon cancer model [74]. Future biologics being developed are antibodies against Wnt proteins [75, 76] or the corresponding surface (Fz- or LRP-) receptors as well as recombinant proteins [77] and RNA-interference.
Inhibitors of Notch signaling have already been developed and tested in leukemia. Gain-of-function mutations are frequent in T-cell acute lymphoblastic leukemia (T-ALL). Therefore, the gamma-secretase inhibitors MK-0752 (Merck, Whitehouse Station, NJ, USA) and PF03084014 have been tested in this disease entity [66]. Other gamma-secretase inhibitors such as R04929097 (Roche, Basel, Switzerland) or LY450139 (Eli Lilly, Indianapolis, IN, USA) are under investigation in solid cancers [66]. Other targeted therapies such as monoclonal Notch-receptor antibodies [78], Notch soluble receptor decoys or RNA-interference are still in pre-clinical stages.
In summary, targeting self-renewal associated signaling is currently under preclinical and clinical development. So far, only few compounds are available for myeloid leukemia stem cells. However, given the rising evidence for the importance and dependency of myeloid leukemia stem cells on these pathways to maintain self-renewal capacity, development of targeted drugs for these signaling nodes will come increasingly into focus.
Contributor Information
Florian H. Heidel, Email: florian.h.heidel@gmail.com, Division of Hematology/Oncology, Children’s Hospital, Boston, MA, USA. Department of Pediatric Oncology, Dana-Farber-Cancer Institute, Harvard Medical School, Boston, MA, USA. Department of Hematology/Oncology, Otto-von-Guericke University Magdeburg, Magdeburg, Germany
Brenton G. Mar, Division of Hematology/Oncology, Children’s Hospital, Boston, MA, USA. Department of Pediatric Oncology, Dana-Farber-Cancer Institute, Harvard Medical School, Boston, MA, USA
Scott A. Armstrong, Division of Hematology/Oncology, Children’s Hospital, Boston, MA, USA. Department of Pediatric Oncology, Dana-Farber-Cancer Institute, Harvard Medical School, Boston, MA, USA. Harvard Stem Cell Institute, Boston, MA, USA
References
- 1.Lapidot T, et al. A cell initiating human acute myeloid leukaemia after transplantation into SCID mice. Nature. 1994;367(6464):645–8. doi: 10.1038/367645a0. [DOI] [PubMed] [Google Scholar]
- 2.Bonnet D, Dick JE. Human acute myeloid leukemia is organized as a hierarchy that originates from a primitive hematopoietic cell. Nat Med. 1997;3(7):730–7. doi: 10.1038/nm0797-730. [DOI] [PubMed] [Google Scholar]
- 3.Hope KJ, Jin L, Dick JE. Acute myeloid leukemia originates from a hierarchy of leukemic stem cell classes that differ in self-renewal capacity. Nat Immunol. 2004;5(7):738–43. doi: 10.1038/ni1080. [DOI] [PubMed] [Google Scholar]
- 4.Cozzio A, et al. Similar MLL-associated leukemias arising from self-renewing stem cells and short-lived myeloid progenitors. Genes Dev. 2003;17(24):3029–35. doi: 10.1101/gad.1143403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Huntly BJ, et al. MOZ-TIF2, but not BCR-ABL, confers properties of leukemic stem cells to committed murine hematopoietic progenitors. Cancer Cell. 2004;6(6):587–96. doi: 10.1016/j.ccr.2004.10.015. [DOI] [PubMed] [Google Scholar]
- 6.Krivtsov AV, et al. Transformation from committed progenitor to leukaemia stem cell initiated by MLL-AF9. Nature. 2006;442(7104):818–22. doi: 10.1038/nature04980. [DOI] [PubMed] [Google Scholar]
- 7.Somervaille TC, Cleary ML. Identification and characterization of leukemia stem cells in murine MLL-AF9 acute myeloid leukemia. Cancer Cell. 2006;10(4):257–68. doi: 10.1016/j.ccr.2006.08.020. [DOI] [PubMed] [Google Scholar]
- 8.Lowenberg B, et al. Mitoxantrone versus daunorubicin in induction-consolidation chemotherapy—the value of low-dose cytarabine for maintenance of remission, and an assessment of prognostic factors in acute myeloid leukemia in the elderly: final report. European Organization for the Research and Treatment of Cancer and the Dutch-Belgian Hemato-Oncology Cooperative Hovon Group. J Clin Oncol. 1998;16(3):872–81. doi: 10.1200/JCO.1998.16.3.872. [DOI] [PubMed] [Google Scholar]
- 9.Stone RM, et al. Granulocyte-macrophage colony-stimulating factor after initial chemotherapy for elderly patients with primary acute myelogenous leukemia. N Engl J Med. 1995;332(25):1671–7. doi: 10.1056/NEJM199506223322503. [DOI] [PubMed] [Google Scholar]
- 10.Mayer RJ, et al. Intensive postremission chemotherapy in adults with acute myeloid leukemia. N Engl J Med. 1994;331(14):896–903. doi: 10.1056/NEJM199410063311402. [DOI] [PubMed] [Google Scholar]
- 11.Cortes J, et al. Front-line and salvage therapies with tyrosine kinase inhibitors and other treatments in chronic myeloid leukemia. J Clin Oncol. 2011;29(5):524–31. doi: 10.1200/JCO.2010.31.3619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mahon FX, et al. Discontinuation of imatinib in patients with chronic myeloid leukaemia who have maintained complete molecular remission for at least 2 years: the prospective, multi-centre Stop Imatinib (STIM) trial. Lancet Oncol. 2010;11(11):1029–35. doi: 10.1016/S1470-2045(10)70233-3. [DOI] [PubMed] [Google Scholar]
- 13.Rousselot P, et al. Imatinib mesylate discontinuation in patients with chronic myelogenous leukemia in complete molecular remission for more than 2 years. Blood. 2007;109(1):58–60. doi: 10.1182/blood-2006-03-011239. [DOI] [PubMed] [Google Scholar]
- 14.Ingham PW, McMahon AP. Hedgehog signaling in animal development: paradigms and principles. Genes Dev. 2001;15(23):3059–87. doi: 10.1101/gad.938601. [DOI] [PubMed] [Google Scholar]
- 15.Nusslein-Volhard C, Wieschaus E. Mutations affecting segment number and polarity in Drosophila. Nature. 1980;287(5785):795–801. doi: 10.1038/287795a0. [DOI] [PubMed] [Google Scholar]
- 16.Ahn S, Joyner AL. In vivo analysis of quiescent adult neural stem cells responding to Sonic hedgehog. Nature. 2005;437(7060):894–7. doi: 10.1038/nature03994. [DOI] [PubMed] [Google Scholar]
- 17.Beachy PA, Karhadkar SS, Berman DM. Tissue repair and stem cell renewal in carcinogenesis. Nature. 2004;432(7015):324–31. doi: 10.1038/nature03100. [DOI] [PubMed] [Google Scholar]
- 18.Lee J, et al. Gli1 is a target of Sonic hedgehog that induces ventral neural tube development. Development. 1997;124(13):2537–52. doi: 10.1242/dev.124.13.2537. [DOI] [PubMed] [Google Scholar]
- 19.Ikram MS, et al. GLI2 is expressed in normal human epidermis and BCC and induces GLI1 expression by binding to its promoter. J Invest Dermatol. 2004;122(6):1503–9. doi: 10.1111/j.0022-202X.2004.22612.x. [DOI] [PubMed] [Google Scholar]
- 20.Teglund S, Toftgard R. Hedgehog beyond medulloblastoma and basal cell carcinoma. Biochim Biophys Acta. 2010;1805(2):181–208. doi: 10.1016/j.bbcan.2010.01.003. [DOI] [PubMed] [Google Scholar]
- 21.Zhang XM, Ramalho-Santos M, McMahon AP. Smoothened mutants reveal redundant roles for Shh and Ihh signaling including regulation of L/R symmetry by the mouse node. Cell. 2001;106(2):781–92. [PubMed] [Google Scholar]
- 22.Dierks C, et al. Expansion of Bcr-Abl-positive leukemic stem cells is dependent on Hedgehog pathway activation. Cancer Cell. 2008;14(3):238–49. doi: 10.1016/j.ccr.2008.08.003. [DOI] [PubMed] [Google Scholar]
- 23.Gao J, et al. Hedgehog signaling is dispensable for adult hematopoietic stem cell function. Cell Stem Cell. 2009;4(6):548–58. doi: 10.1016/j.stem.2009.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hofmann I, et al. Hedgehog signaling is dispensable for adult murine hematopoietic stem cell function and hematopoiesis. Cell Stem Cell. 2009;4(6):559–67. doi: 10.1016/j.stem.2009.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhao C, et al. Hedgehog signalling is essential for maintenance of cancer stem cells in myeloid leukaemia. Nature. 2009;458(7239):776–9. doi: 10.1038/nature07737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Irvine DA, et al. Combination of Hedgehog pathway inhibitor LDE225 and Nilotinib eliminates chronic myeloid leukemia stem and progenitor cells. Blood (ASH) 2009 (abstract no. 1428) [Google Scholar]
- 27.Zhang B, et al. Inhibition of chronic myeloid leukemia stem cells by the combination of the Hoedgehog pathway inhibitor LDE225 with Nilotinib. Blood (ASH) 2010:abstract no. 514. [Google Scholar]
- 28.Schairer A, et al. Human blast crisis leukemia stem cell inhibition with a novel smoothened antagonist. Blood (ASH) 2010:abstract no. 1223. [Google Scholar]
- 29.Behrens J, et al. Functional interaction of an axin homolog, conductin, with beta-catenin, APC, and GSK3beta. Science. 1998;280(5363):596–9. doi: 10.1126/science.280.5363.596. [DOI] [PubMed] [Google Scholar]
- 30.Rubinfeld B, et al. Binding of GSK3beta to the APC-beta-catenin complex and regulation of complex assembly. Science. 1996;272(5264):1023–6. doi: 10.1126/science.272.5264.1023. [DOI] [PubMed] [Google Scholar]
- 31.Tamai K, et al. LDL-receptor-related proteins in Wnt signal transduction. Nature. 2000;407(6803):530–5. doi: 10.1038/35035117. [DOI] [PubMed] [Google Scholar]
- 32.Mao J, et al. Low-density lipoprotein receptor-related protein-5 binds to Axin and regulates the canonical Wnt signaling pathway. Mol Cell. 2001;7(4):801–9. doi: 10.1016/s1097-2765(01)00224-6. [DOI] [PubMed] [Google Scholar]
- 33.Mao B, et al. LDL-receptor-related protein 6 is a receptor for Dickkopf proteins. Nature. 2001;411(6835):321–5. doi: 10.1038/35077108. [DOI] [PubMed] [Google Scholar]
- 34.Nelson WJ, Nusse R. Convergence of Wnt, beta-catenin, and cadherin pathways. Science. 2004;303(5663):1483–7. doi: 10.1126/science.1094291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Haegel H, et al. Lack of beta-catenin affects mouse development at gastrulation. Development. 1995;121(11):3529–37. doi: 10.1242/dev.121.11.3529. [DOI] [PubMed] [Google Scholar]
- 36.Fleming HE, et al. Wnt signaling in the niche enforces hematopoietic stem cell quiescence and is necessary to preserve self-renewal in vivo. Cell Stem Cell. 2008;2(3):274–83. doi: 10.1016/j.stem.2008.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zhao C, et al. Loss of beta-catenin impairs the renewal of normal and CML stem cells in vivo. Cancer Cell. 2007;12(6):528–41. doi: 10.1016/j.ccr.2007.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Koch U, et al. Simultaneous loss of beta- and gamma-catenin does not perturb hematopoiesis or lymphopoiesis. Blood. 2008;111(1):160–4. doi: 10.1182/blood-2007-07-099754. [DOI] [PubMed] [Google Scholar]
- 39.Hu Y, et al. beta-Catenin is essential for survival of leukemic stem cells insensitive to kinase inhibition in mice with BCR-ABL-induced chronic myeloid leukemia. Leukemia. 2009;23(1):109–16. doi: 10.1038/leu.2008.262. [DOI] [PubMed] [Google Scholar]
- 40.Heidel FH, et al. Beta-catenin (Ctnnb1) suppression targets imatinib resistant leukemia stem cells in mice with BCR-ABL induced myeloproliferative disease. Blood (ASH) 2010 (annual meeting abstracts) [Google Scholar]
- 41.Jamieson CH, et al. Granulocyte-macrophage progenitors as candidate leukemic stem cells in blast-crisis CML. N Engl J Med. 2004;351(7):657–67. doi: 10.1056/NEJMoa040258. [DOI] [PubMed] [Google Scholar]
- 42.Abrahamsson AE, et al. Glycogen synthase kinase 3beta missplicing contributes to leukemia stem cell generation. Proc Natl Acad Sci USA. 2009;106(10):3925–9. doi: 10.1073/pnas.0900189106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wang Y, et al. The Wnt/beta-catenin pathway is required for the development of leukemia stem cells in AML. Science. 2010;327(5973):1650–3. doi: 10.1126/science.1186624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Yeung J, et al. beta-Catenin mediates the establishment and drug resistance of MLL leukemic stem cells. Cancer Cell. 2010;18(6):606–18. doi: 10.1016/j.ccr.2010.10.032. [DOI] [PubMed] [Google Scholar]
- 45.North TE, et al. Hematopoietic stem cell development is dependent on blood flow. Cell. 2009;137(4):736–48. doi: 10.1016/j.cell.2009.04.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Goessling W, et al. Genetic interaction of PGE2 and Wnt signaling regulates developmental specification of stem cells and regeneration. Cell. 2009;136(6):1136–47. doi: 10.1016/j.cell.2009.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Eaves CJ, Humphries RK. Acute myeloid leukemia and the Wnt pathway. N Engl J Med. 2010;362(24):2326–7. doi: 10.1056/NEJMcibr1003522. [DOI] [PubMed] [Google Scholar]
- 48.Tothova Z, Gilliland DG. FoxO transcription factors and stem cell homeostasis: insights from the hematopoietic system. Cell Stem Cell. 2007;1(2):140–52. doi: 10.1016/j.stem.2007.07.017. [DOI] [PubMed] [Google Scholar]
- 49.Tothova Z, et al. FoxOs are critical mediators of hematopoietic stem cell resistance to physiologic oxidative stress. Cell. 2007;128(2):325–39. doi: 10.1016/j.cell.2007.01.003. [DOI] [PubMed] [Google Scholar]
- 50.Miyamoto K, et al. Foxo3a is essential for maintenance of the hematopoietic stem cell pool. Cell Stem Cell. 2007;1(1):101–12. doi: 10.1016/j.stem.2007.02.001. [DOI] [PubMed] [Google Scholar]
- 51.Naka K, et al. TGF-beta-FOXO signalling maintains leukaemia-initiating cells in chronic myeloid leukaemia. Nature. 2010;463(7281):676–80. doi: 10.1038/nature08734. [DOI] [PubMed] [Google Scholar]
- 52.Sykes SM, et al. The AKT/FOXO signaling pathway is required for the maintenance of acute myeloid leukemia. Cell. 2011 (in press) [Google Scholar]
- 53.Duncan AW, et al. Integration of Notch and Wnt signaling in hematopoietic stem cell maintenance. Nat Immunol. 2005;6(3):314–22. doi: 10.1038/ni1164. [DOI] [PubMed] [Google Scholar]
- 54.Wu M, et al. Imaging hematopoietic precursor division in real time. Cell Stem Cell. 2007;1(5):541–54. doi: 10.1016/j.stem.2007.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Allman D, Aster JC, Pear WS. Notch signaling in hematopoiesis and early lymphocyte development. Immunol Rev. 2002;187:75–86. doi: 10.1034/j.1600-065x.2002.18707.x. [DOI] [PubMed] [Google Scholar]
- 56.Artavanis-Tsakonas S, Matsuno K, Fortini ME. Notch signaling. Science. 1995;268(5208):225–32. doi: 10.1126/science.7716513. [DOI] [PubMed] [Google Scholar]
- 57.Wu L, et al. MAML1, a human homologue of Drosophila mastermind, is a transcriptional co-activator for NOTCH receptors. Nat Genet. 2000;26(4):484–9. doi: 10.1038/82644. [DOI] [PubMed] [Google Scholar]
- 58.Varnum-Finney B, et al. Immobilization of Notch ligand, Delta-1, is required for induction of notch signaling. J Cell Sci. 2000;113(Pt 23):4313–8. doi: 10.1242/jcs.113.23.4313. [DOI] [PubMed] [Google Scholar]
- 59.Chen PM, et al. Down-regulation of Notch-1 expression decreases PU.1-mediated myeloid differentiation signaling in acute myeloid leukemia. Int J Oncol. 2008;32(6):1335–41. doi: 10.3892/ijo_32_6_1335. [DOI] [PubMed] [Google Scholar]
- 60.Nakahara F, et al. Hes1 immortalizes committed progenitors and plays a role in blast crisis transition in chronic myelogenous leukemia. Blood. 2010;115(14):2872–81. doi: 10.1182/blood-2009-05-222836. [DOI] [PubMed] [Google Scholar]
- 61.Klinakis A, et al. A novel tumour-suppressor function for the Notch pathway in myeloid leukaemia. Nature. 2011;473(7346):230–3. doi: 10.1038/nature09999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Hope KJ, et al. An RNAi screen identifies Msi2 and Prox1 as having opposite roles in the regulation of hematopoietic stem cell activity. Cell Stem Cell. 2010;7(1):101–13. doi: 10.1016/j.stem.2010.06.007. [DOI] [PubMed] [Google Scholar]
- 63.Ito T, et al. Regulation of myeloid leukaemia by the cell-fate determinant Musashi. Nature. 2010;466(7307):765–8. doi: 10.1038/nature09171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Kharas MG, et al. Musashi-2 regulates normal hematopoiesis and promotes aggressive myeloid leukemia. Nat Med. 2010;16(8):903–8. doi: 10.1038/nm.2187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Okabe M, et al. Translational repression determines a neuronal potential in Drosophila asymmetric cell division. Nature. 2001;411(6833):94–8. doi: 10.1038/35075094. [DOI] [PubMed] [Google Scholar]
- 66.NIH. http://www.clinicaltrials.gov.
- 67.Gupta RA, Dubois RN. Colorectal cancer prevention and treatment by inhibition of cyclooxygenase-2. Nat Rev Cancer. 2001;1(1):11–21. doi: 10.1038/35094017. [DOI] [PubMed] [Google Scholar]
- 68.Rothwell PM, et al. Long-term effect of aspirin on colorectal cancer incidence and mortality: 20-year follow-up of five randomised trials. Lancet. 2010;376(9754):1741–50. doi: 10.1016/S0140-6736(10)61543-7. [DOI] [PubMed] [Google Scholar]
- 69.Rothwell PM, et al. Effect of daily aspirin on long-term risk of death due to cancer: analysis of individual patient data from randomised trials. Lancet. 2011;377(9759):31–41. doi: 10.1016/S0140-6736(10)62110-1. [DOI] [PubMed] [Google Scholar]
- 70.Shah S, et al. Trans-repression of beta-catenin activity by nuclear receptors. J Biol Chem. 2003;278(48):48137–45. doi: 10.1074/jbc.M307154200. [DOI] [PubMed] [Google Scholar]
- 71.Jaiswal AS, et al. Beta-catenin-mediated transactivation and cell–cell adhesion pathways are important in curcumin (diferuylmethane)-induced growth arrest and apoptosis in colon cancer cells. Oncogene. 2002;21(55):8414–27. doi: 10.1038/sj.onc.1205947. [DOI] [PubMed] [Google Scholar]
- 72.Roccaro AM, et al. Resveratrol exerts antiproliferative activity and induces apoptosis in Waldenstrom’s macroglobulinemia. Clin Cancer Res. 2008;14(6):1849–58. doi: 10.1158/1078-0432.CCR-07-1750. [DOI] [PubMed] [Google Scholar]
- 73.Takahashi-Yanaga F, Kahn M. Targeting Wnt signaling: can we safely eradicate cancer stem cells? Clin Cancer Res. 2010;16(12):3153–62. doi: 10.1158/1078-0432.CCR-09-2943. [DOI] [PubMed] [Google Scholar]
- 74.Huang SM, et al. Tankyrase inhibition stabilizes axin and antagonizes Wnt signalling. Nature. 2009;461(7264):614–20. doi: 10.1038/nature08356. [DOI] [PubMed] [Google Scholar]
- 75.You L, et al. An anti-Wnt-2 monoclonal antibody induces apoptosis in malignant melanoma cells and inhibits tumor growth. Cancer Res. 2004;64(15):5385–9. doi: 10.1158/0008-5472.CAN-04-1227. [DOI] [PubMed] [Google Scholar]
- 76.You L, et al. Inhibition of Wnt-1 signaling induces apoptosis in beta-catenin-deficient mesothelioma cells. Cancer Res. 2004;64(10):3474–8. doi: 10.1158/0008-5472.CAN-04-0115. [DOI] [PubMed] [Google Scholar]
- 77.DeAlmeida VI, et al. The soluble wnt receptor Frizzled8CRD-hFc inhibits the growth of teratocarcinomas in vivo. Cancer Res. 2007;67(11):5371–9. doi: 10.1158/0008-5472.CAN-07-0266. [DOI] [PubMed] [Google Scholar]
- 78.Hoey T, et al. DLL4 blockade inhibits tumor growth and reduces tumor-initiating cell frequency. Cell Stem Cell. 2009;5(2):168–77. doi: 10.1016/j.stem.2009.05.019. [DOI] [PubMed] [Google Scholar]